High Pressure Oxydesulphurisation of Coal—Effect of Oxidizing Agent, Solvent, Shear and Agitator Configuration
Abstract
:1. Introduction
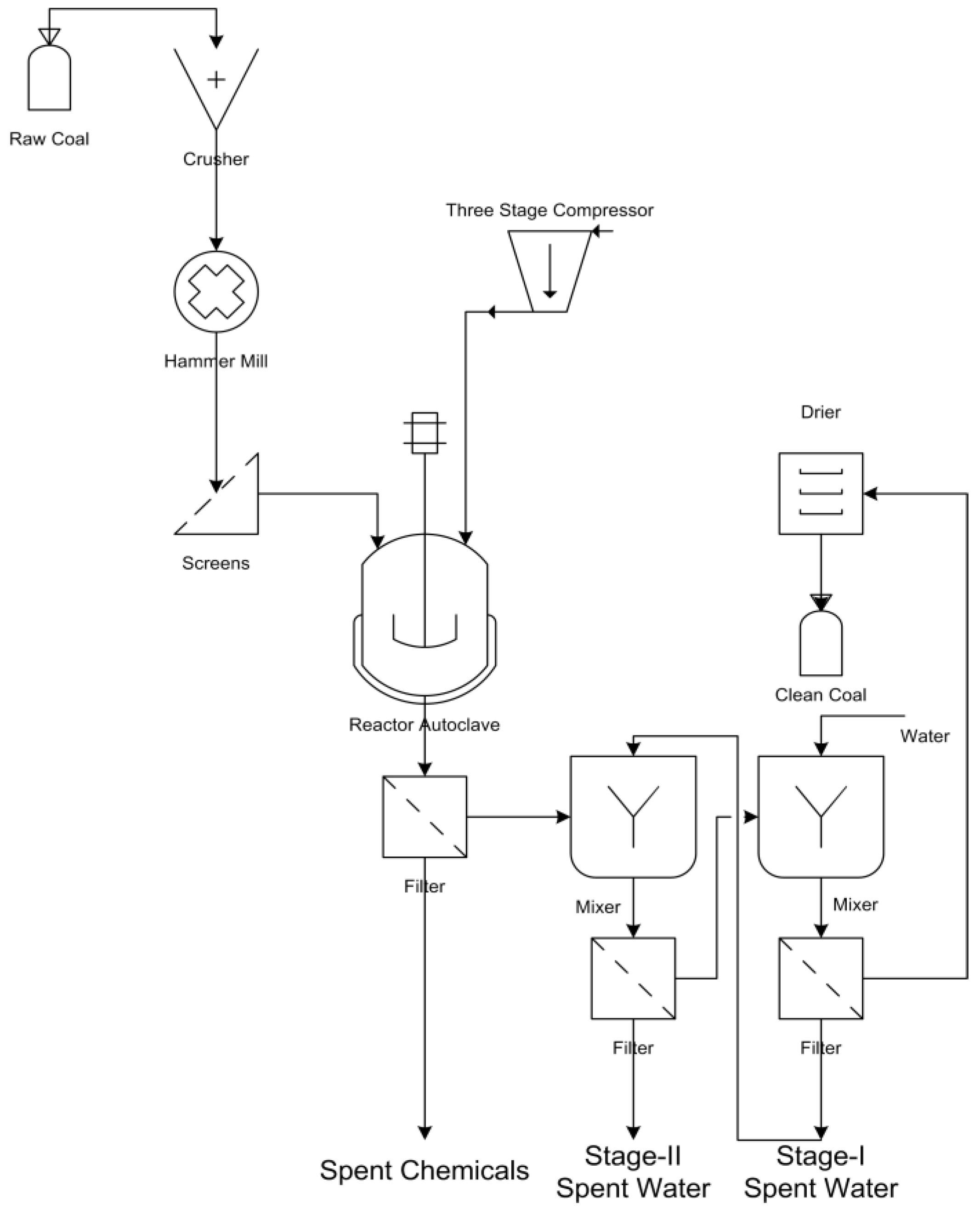
2. Materials and Methods
2.1. Reaction Mechanism with Potassium Permanganate
2.2. Materials
2.3. Method
3. Results and Discussions
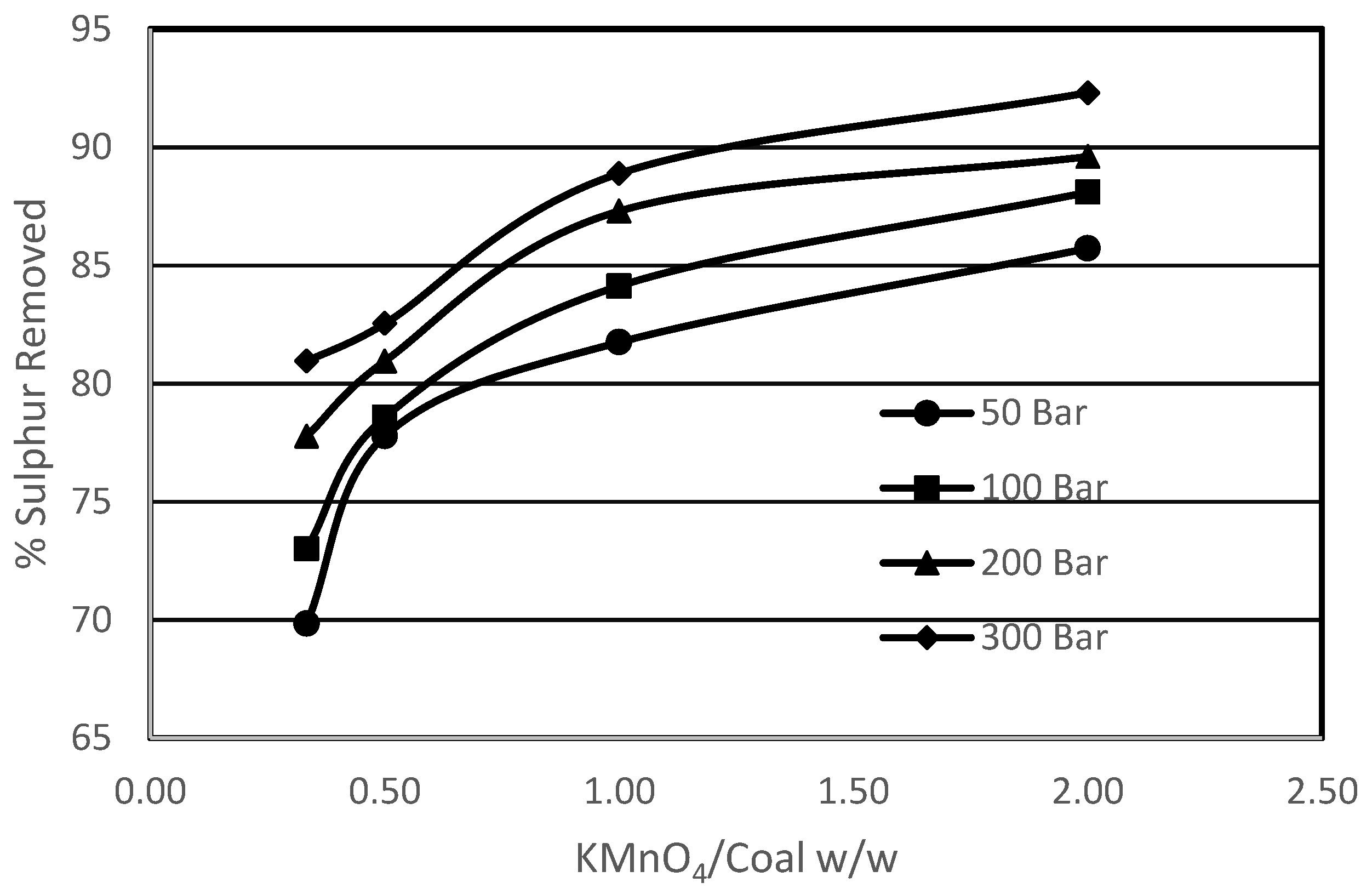
3.1. Effect of Amount of KMnO4 vs. Amount of Coal on Sulphur Removal
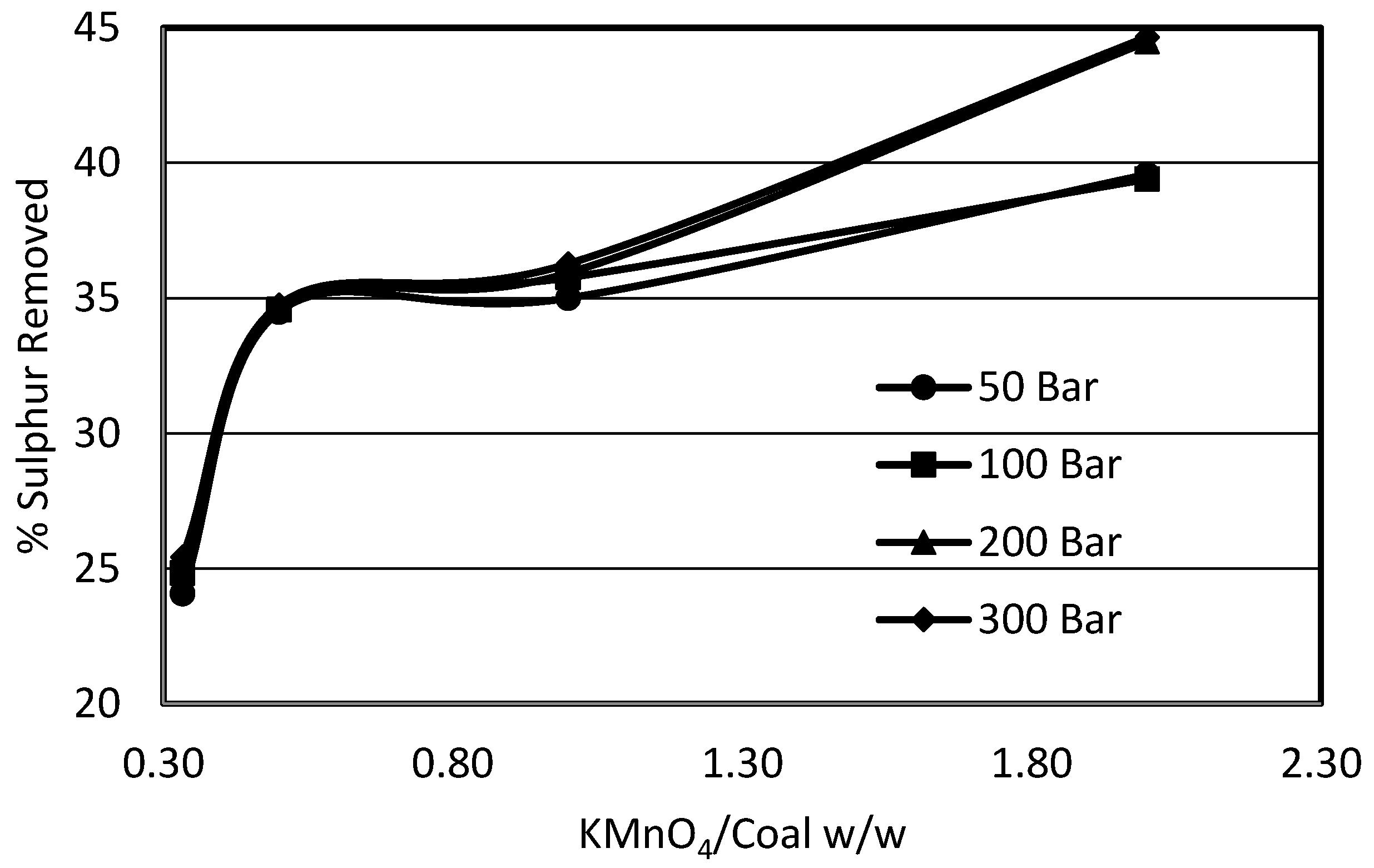
3.2. Effect of Agitation on Sulphur Removal
3.2.1. Configuration of the Agitators
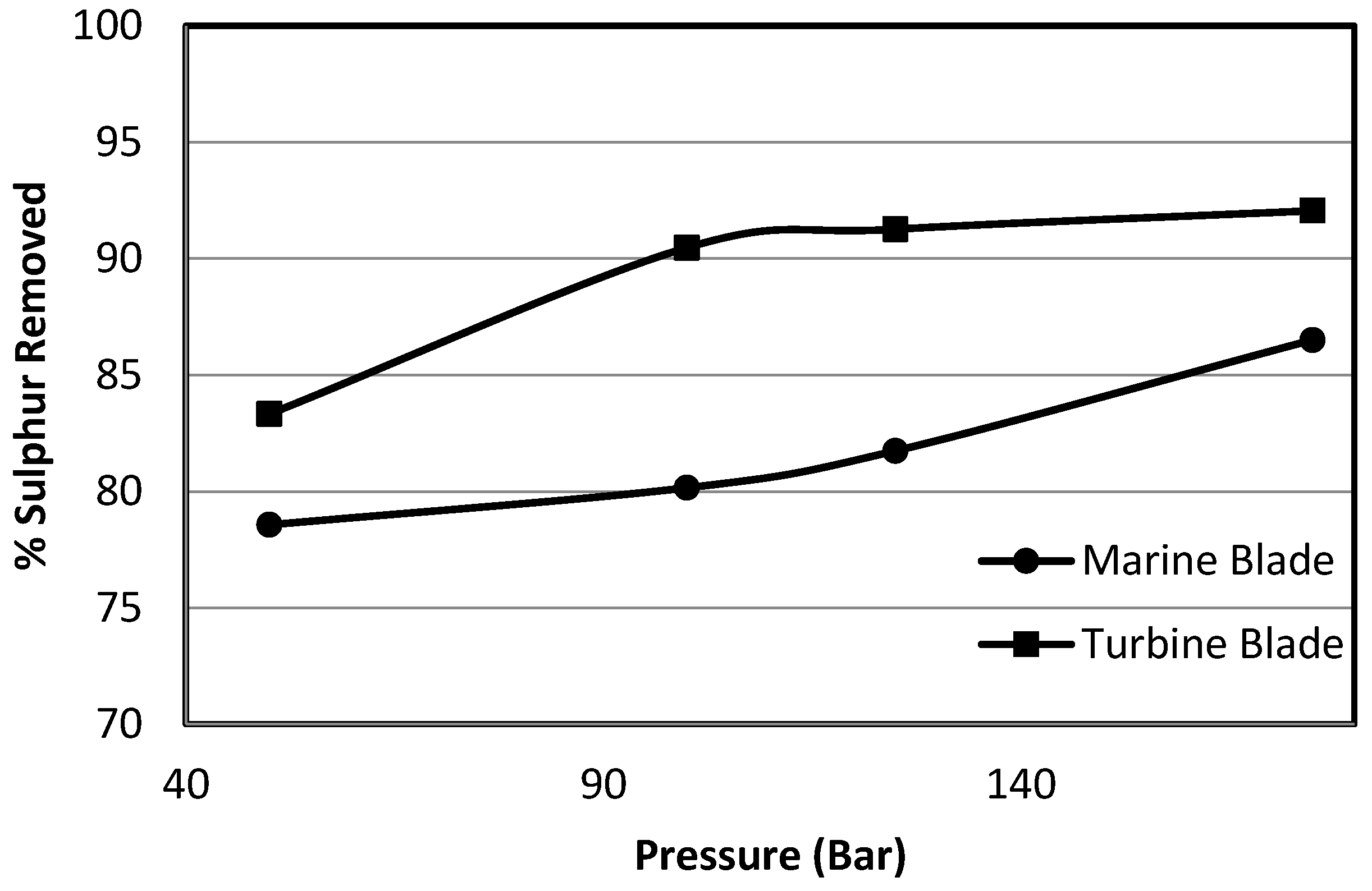
3.2.2. Effect of Marine Blade vs. Turbine Blade Agitation on Sulphur Removal
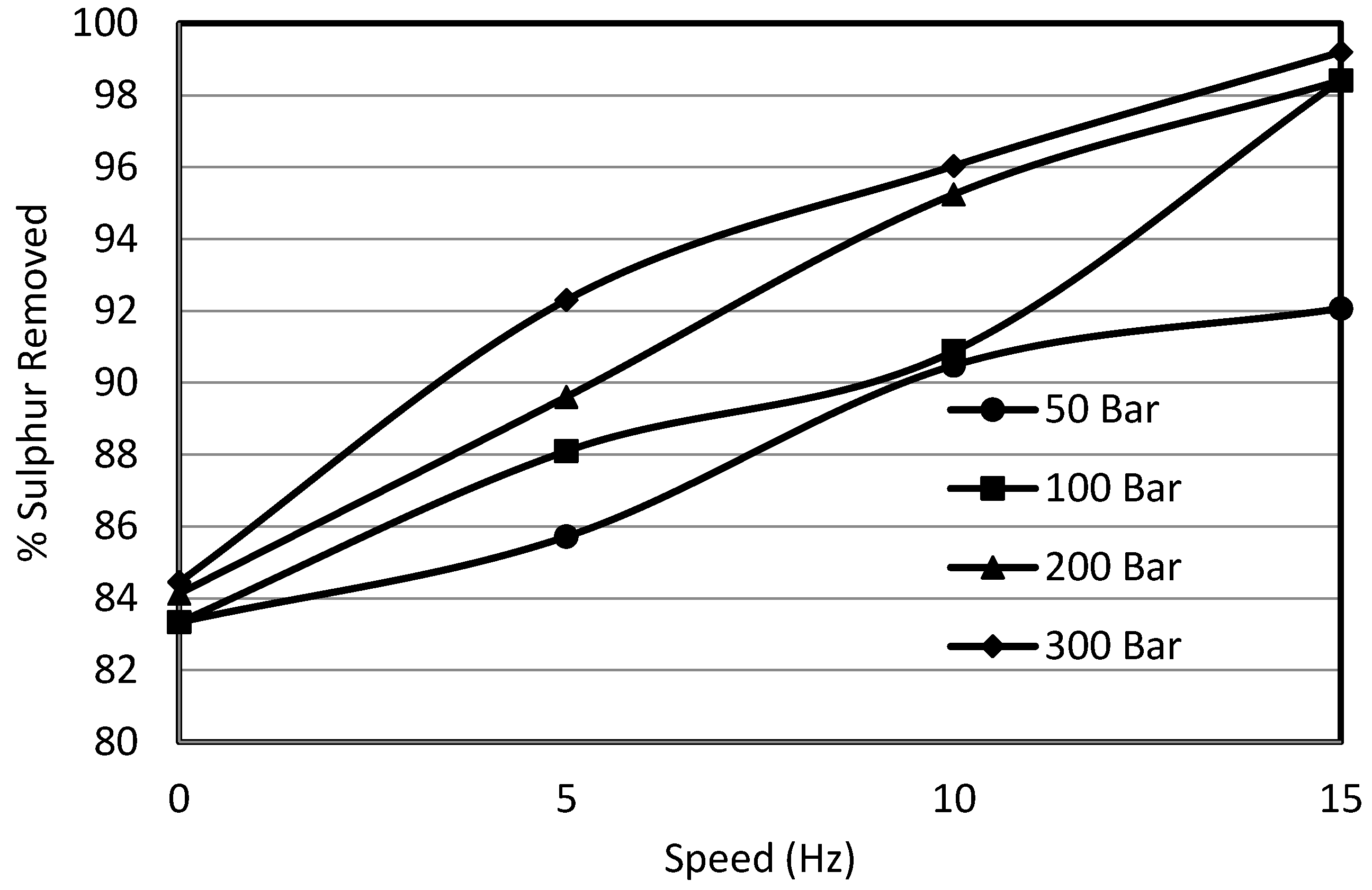
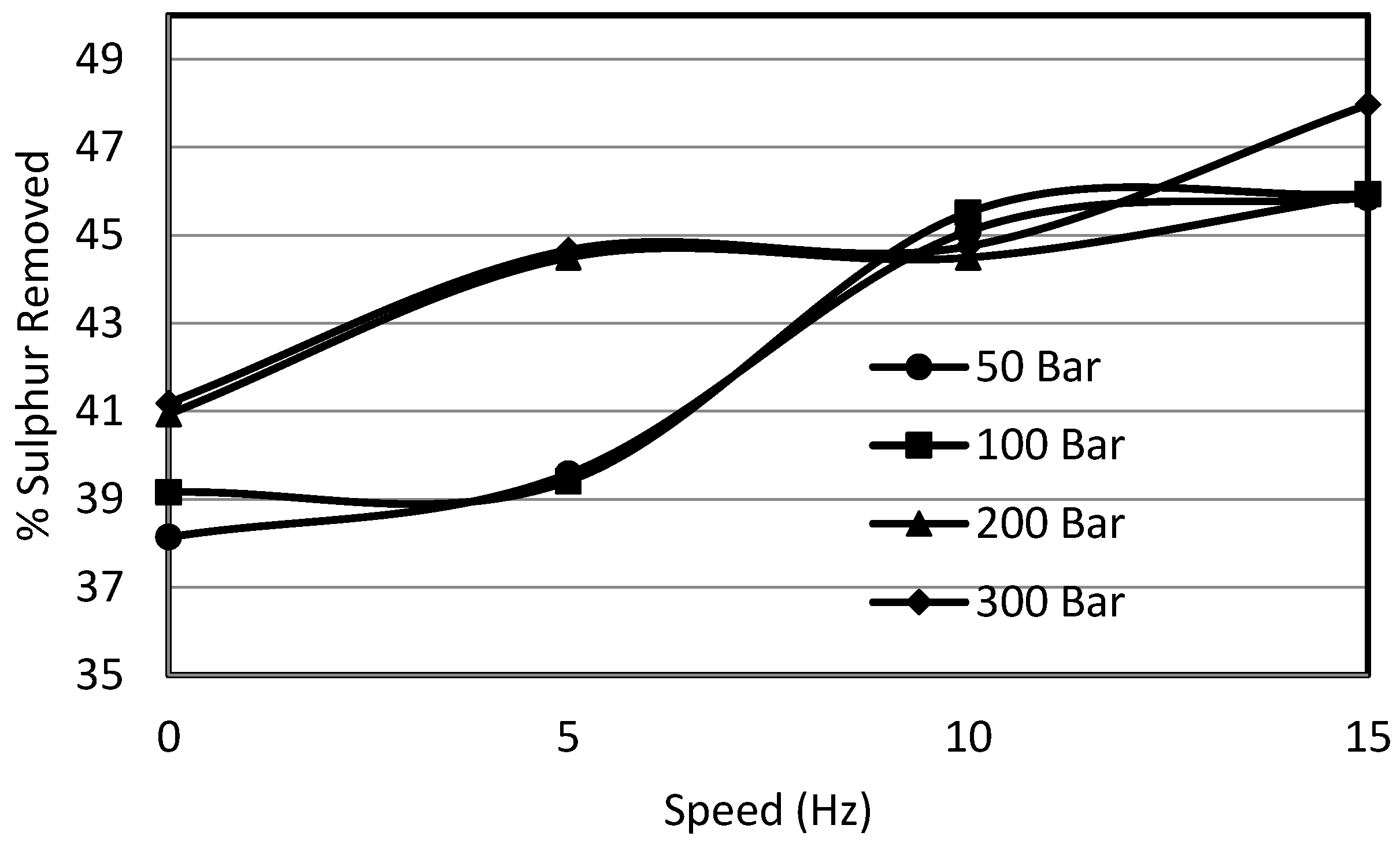
3.3. Effect of Shear on Sulphur Removal
- T = Mixing vessel diameter = 0.082 m;
- D = Impeller diameter = 0.052 m;
- N = Agitator speed, Hz;
- W = Width of blade = 0.012 m
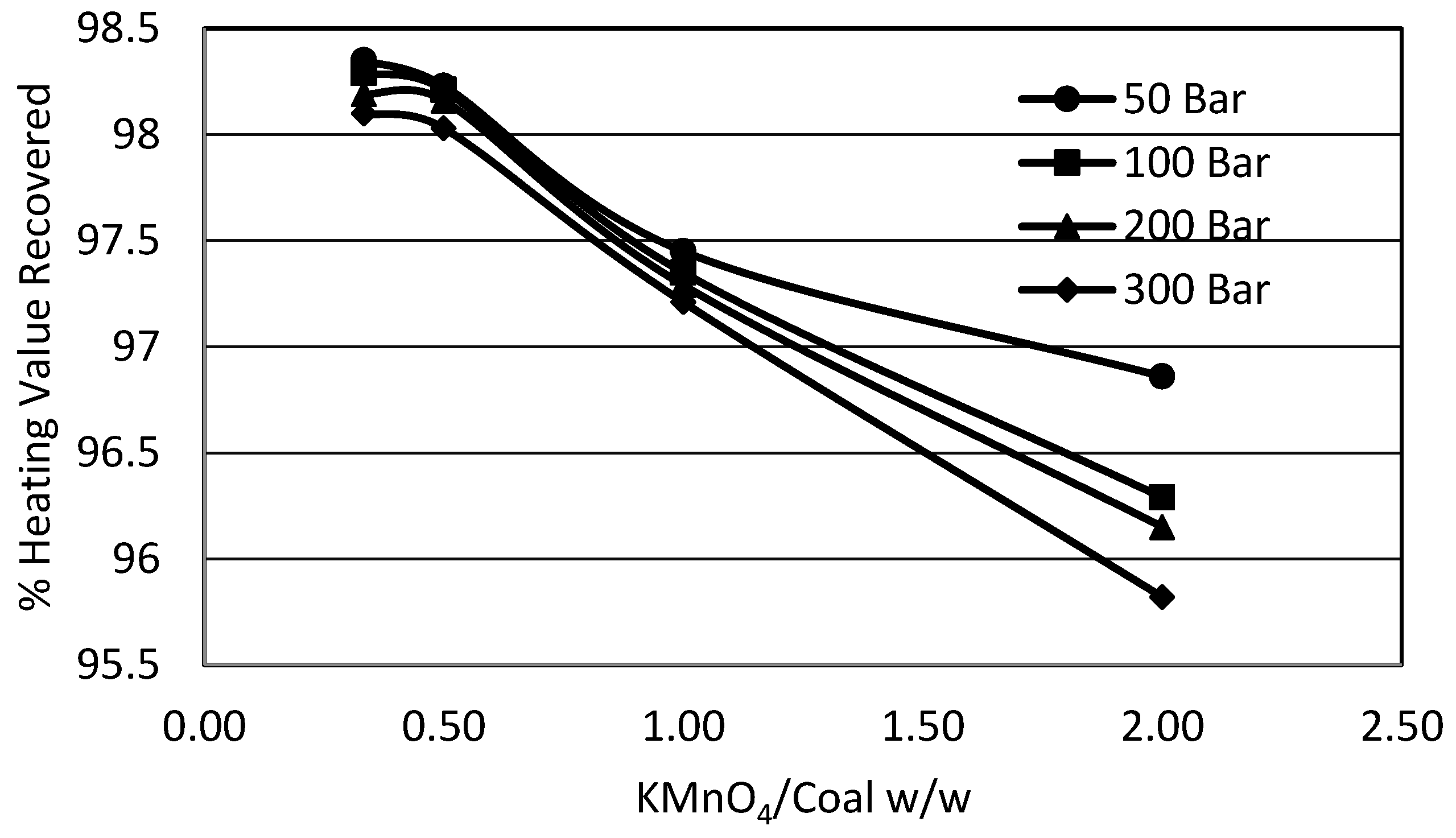
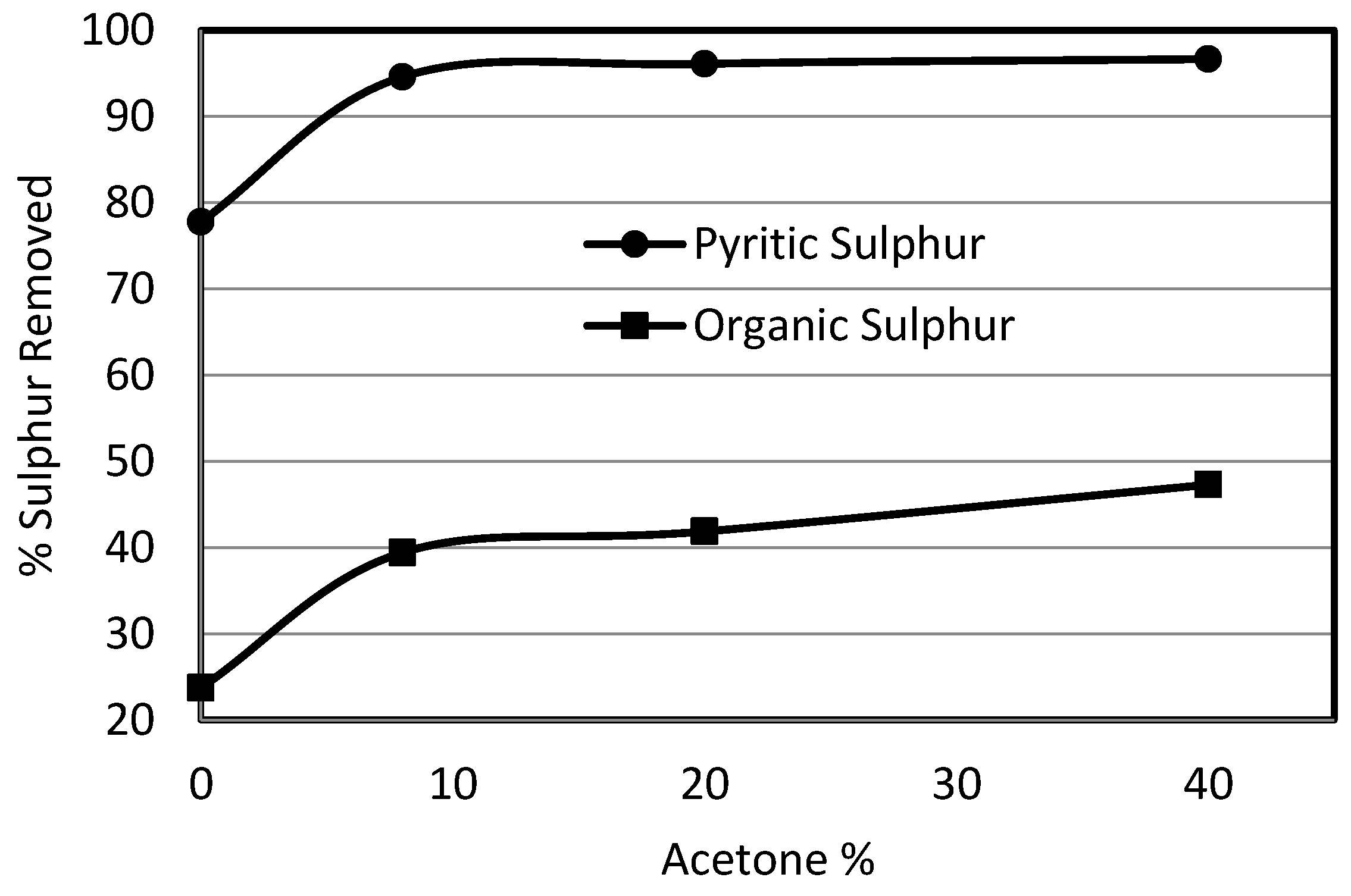
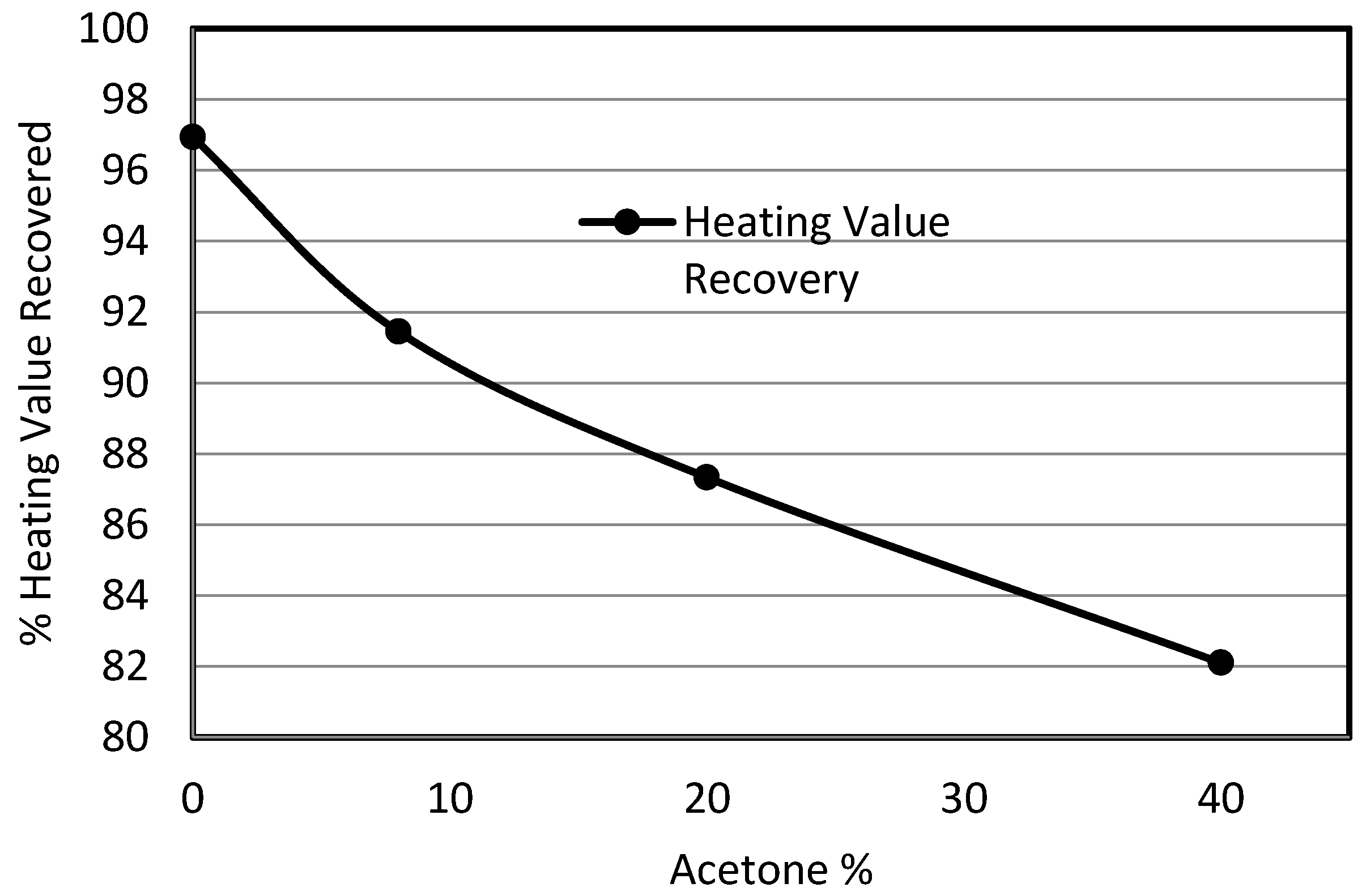
3.4. Effect of Organic Solvent (Acetone) on Sulphur Removal
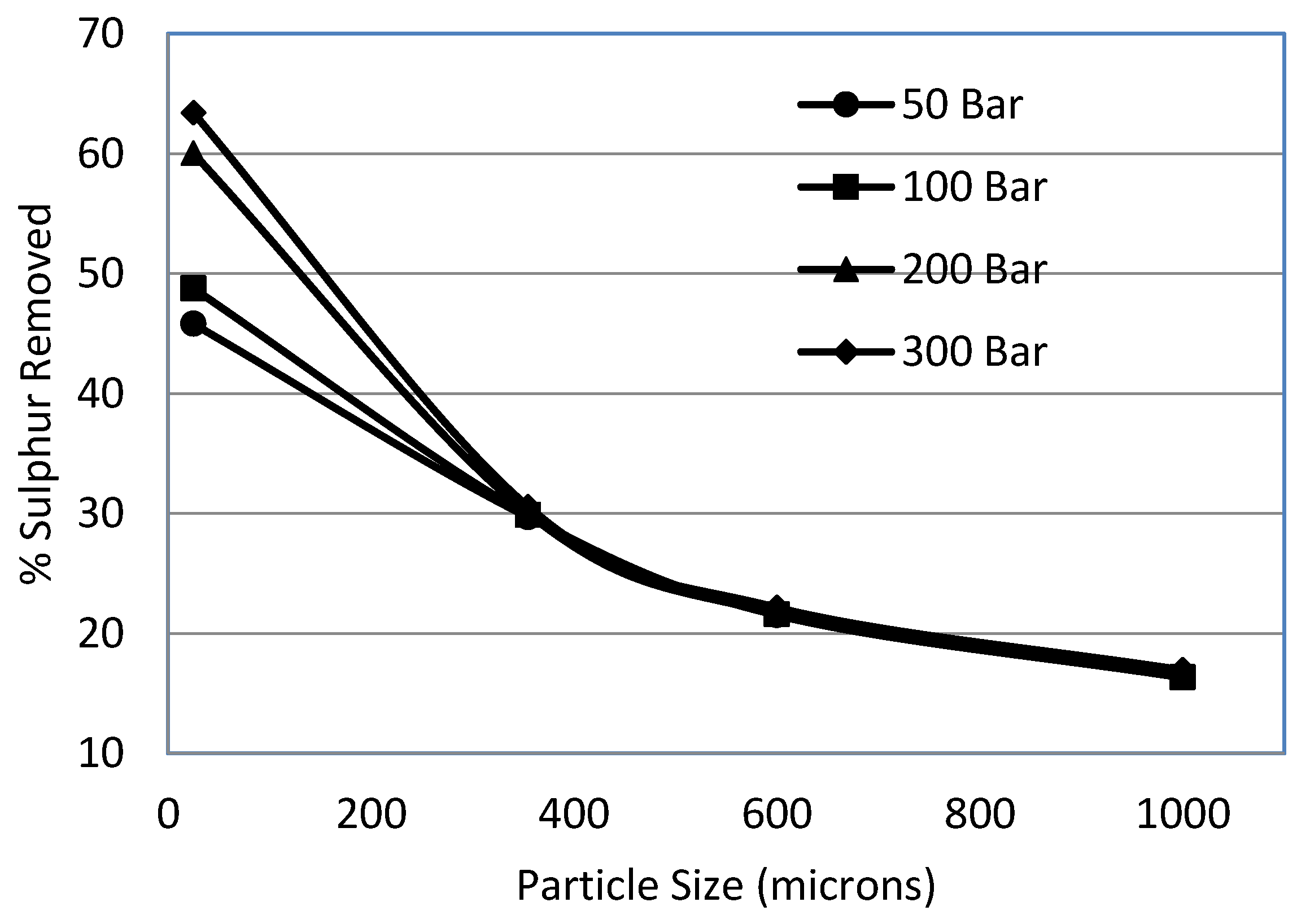
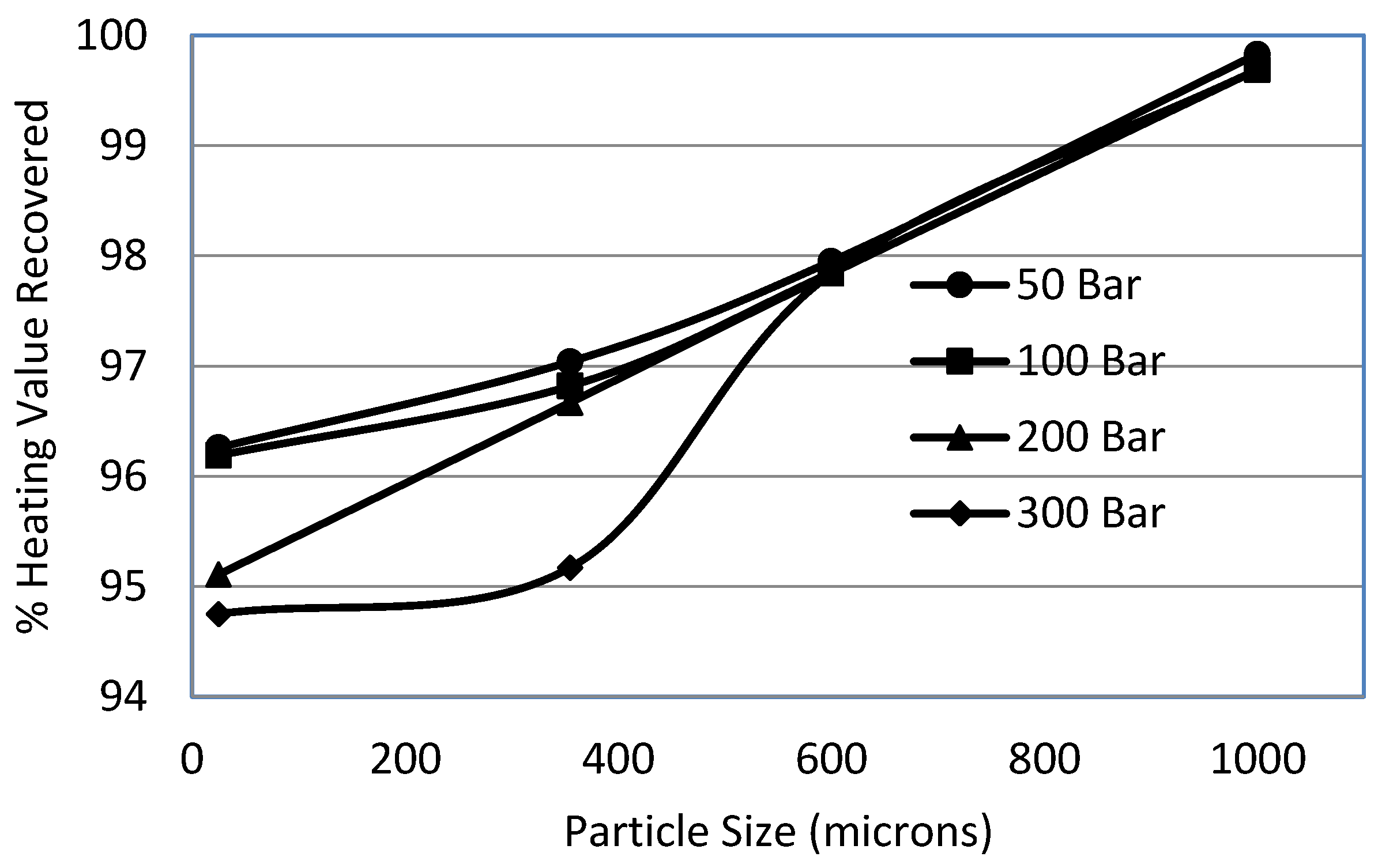
3.5. Effect of Particle Size
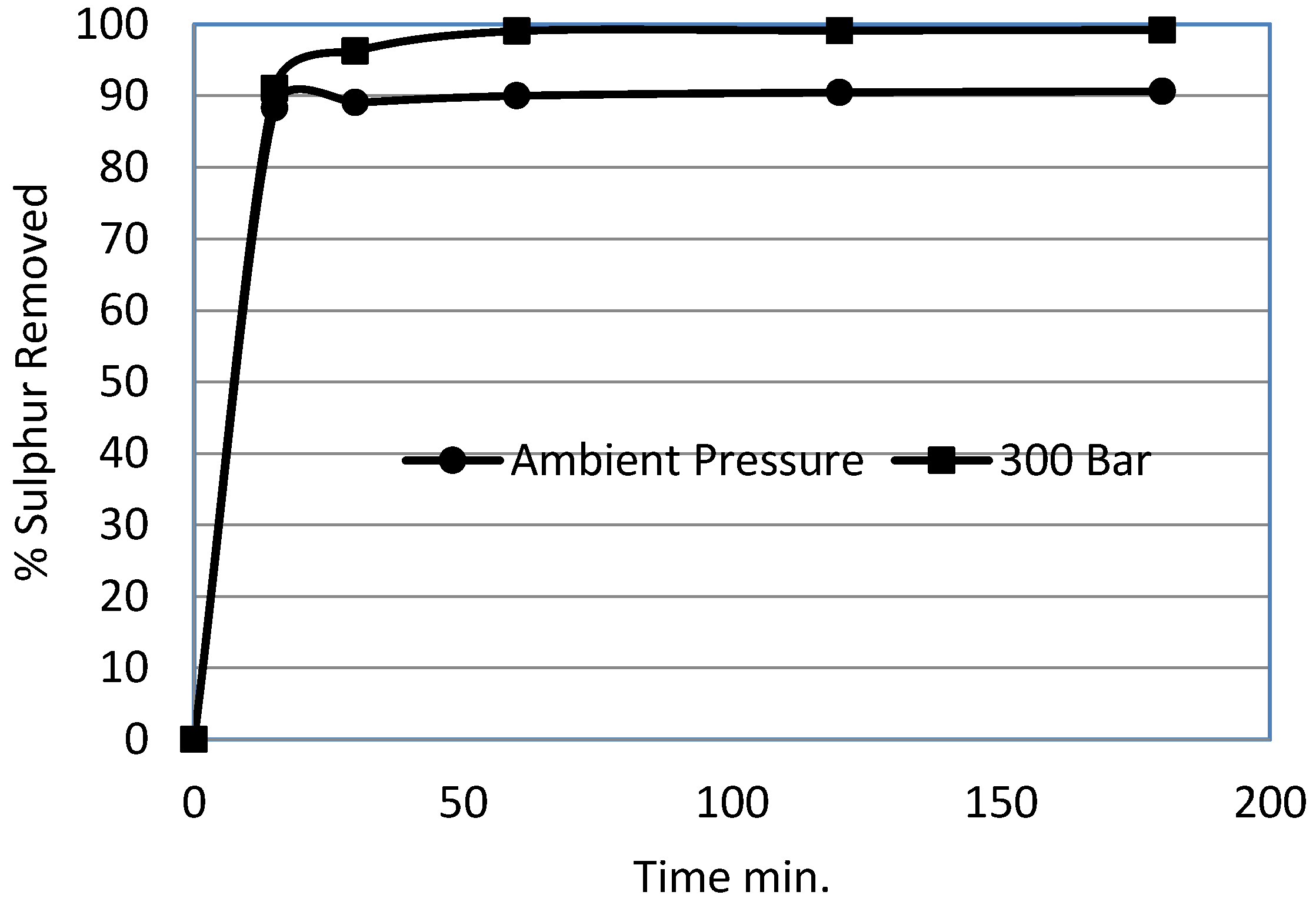
3.6. Effect of Time
- (i)
- to prevent the build up of manganese dioxide on the outside of the coal;
- (ii)
- to increase the diffusion of oxygen into the coal particle increasing the rate of access to the pyritic sulphur. Once this has been converted to the sulphate, the porosity of the coal is increased which allows access to the organic sulphur.
4. Conclusions
- A high shear turbine agitator gives a better sulphur removal than an axial flow propeller agitator for all systems investigated.
- Using pressure up to 50 bar in the case of potassium permanganate oxidation had a significant effect on the removal of organic sulphur from Prince of Wales coal from 24% to 40%.
- Increase in the potassium permanganate/coal ratio showed an increase in sulphur removal.
- A small increase in sulphur removal was attained using acetone instead of water for suspending the coal and potassium permanganate; however, the use of the solvent leached out all the volatiles from the product resulting in a loss in product recovery.
- The formation of potassium manganate does not normally occur below 70 °C, so mineral matter in the coal must catalyse the reaction.
- Decrease in sulphur removal was observed with the increase in particle size. The pressure helped to overcome the diffusional resistance for particle sizes of less than 355 μm.
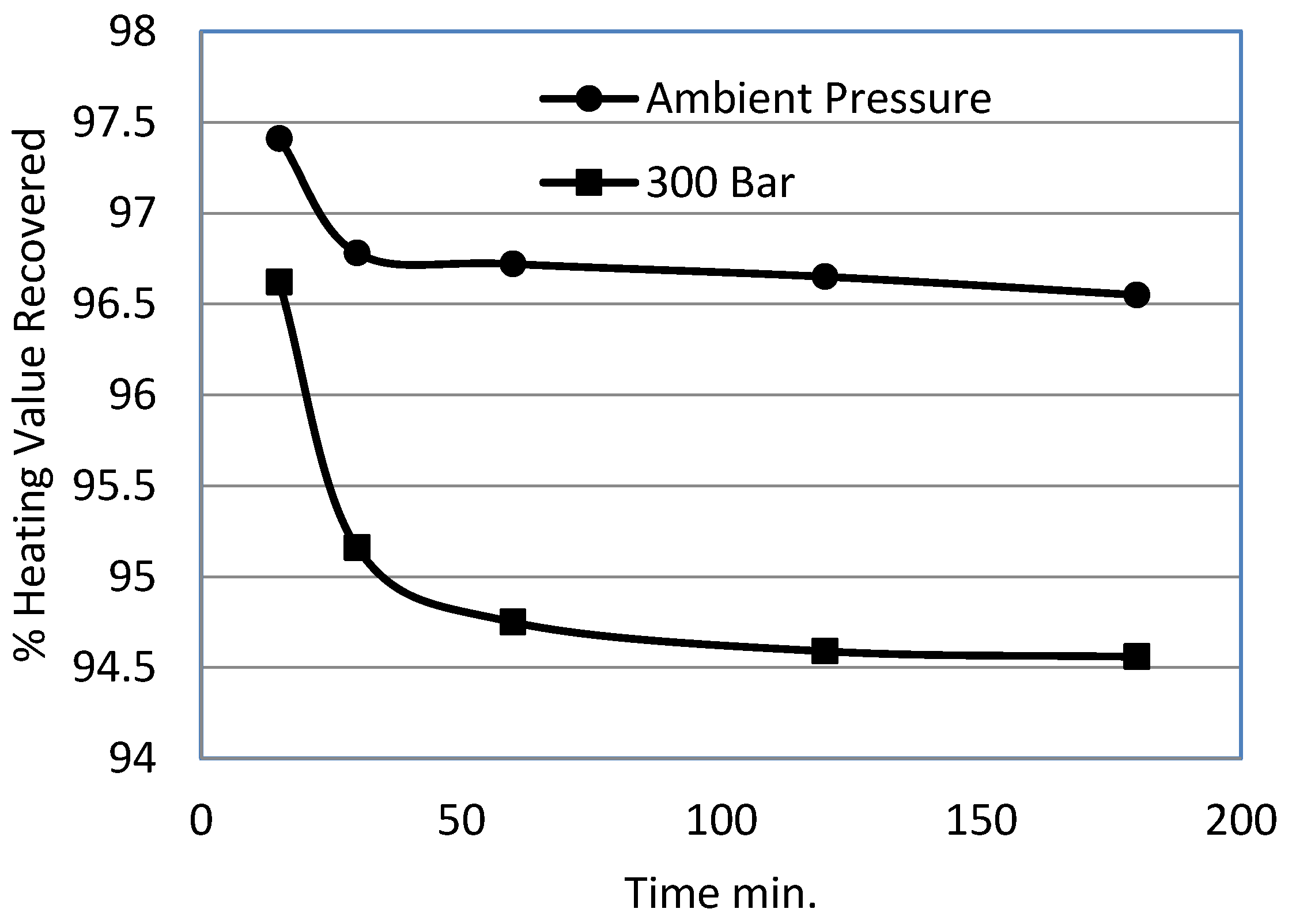
- The pyritic sulphur removal rate almost dropped to zero beyond 15 min time under ambient pressure. However, for 300 bar experiments, pyritic sulphur was removed beyond 60 min.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hippo, E.J.; Crelling, J.C.; Palmer, S.E.; Kruge, M.A. Organic sulfur compounds in coals. In Proceedings of the 14th Annual Conference on Fuel, Palo Alto, CA, USA, 18–19 May 1989; Electric Power Research Institute: Palo Alto, CA, USA, 1990; pp. 6:1–6:28. [Google Scholar]
- Chen, W.; Xu, R. Clean coal technology development in china. Energy Policy 2010, 38, 2123–2130. [Google Scholar] [CrossRef]
- Olszewska, D. Application of modified montmorillonite for desulfurization during the combustion of hard coal. Fuel Process. Technol. 2011, 92, 2412–2419. [Google Scholar] [CrossRef]
- Olszewska, D. Application of XPS method in the research into Ni ion-modified montmorillonite as a SO2 sorbent. Fuel Process. Technol. 2012, 95, 90–95. [Google Scholar] [CrossRef]
- Marcewicz Kuba, A.; Olszewska, D. The activity of SO2 removal from combustion gases by the desonox type catalyst supported on montmorillonite and zeolite. Pol. J. Chem. 2008, 82, 43–47. [Google Scholar]
- Robinson, K. Reaction engineering of direct coal liquefaction. Energies 2009, 2, 976–1006. [Google Scholar] [CrossRef]
- Vejahati, F.; Xu, Z.; Gupta, R. Trace elements in coal: Associations with coal and minerals and their behavior during coal utilization—A review. Fuel 2010, 89, 904–911. [Google Scholar] [CrossRef]
- Laskowski, J.S. Chapter 3 coal surface properties. In Developments in Mineral Processing; Elsevier: Amsterdam, The Netherlands, 2001; Volume 14, pp. 31–94. [Google Scholar]
- Zhu, B. Advanced environmental/ energy technology: Desulfurisation and fuel cell cogeneration. Fuel Cells Bull. 1999, 2, 9–12. [Google Scholar] [CrossRef]
- Shui, H.; Cai, Z.; Xu, C. Recent advances in direct coal liquefaction. Energies 2010, 3, 155–170. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Lin, Z.; Whiddon, R.; Qiu, K.; Kuang, M.; Cen, K. Transformation of nitrogen and sulphur impurities during hydrothermal upgrading of low quality coals. Fuel 2016, 164, 254–261. [Google Scholar] [CrossRef]
- Demirbas, A.; Balat, M. Coal desulfurization via different methods. Energy Sources 2004, 26, 541–550. [Google Scholar] [CrossRef]
- Chriswell, C.D.; Markuszewski, R.; Norton, G.A. Use of naoh alone vs. Naoh-koh mixtures for the removal of sulphur and ash from coal by the molten caustic leaching process. In Processing and Utilisation of High Sulphur Coals IV; Dugan, P.R., Quigley, D.R., Attia, Y.A., Eds.; Elsevier: New York, NY, USA, 1991; pp. 385–397. [Google Scholar]
- Norton, G.A.; Bluhm, D.D.; Markuszewski, R.; Chriswell, C.D. Application of microwave energy to caustic cleaning of coal. In Processing and Utilisation of High Sulphur Coals IV; Dugan, P.R., Quigley, D.R., Attia, Y.A., Eds.; Elsevier: New York, NY, USA, 1991; pp. 425–438. [Google Scholar]
- Steel, K.M.; Patrick, J.W. The production of ultra clean coal by chemical demineralisation. Fuel Energy Abstr. 2002, 43, 238. [Google Scholar] [CrossRef]
- Yaman, S.; Kücükbayrak, S. Effect of oxydesulphurization on the combustion characteristics of coal. Thermochim. Acta 1997, 293, 109–115. [Google Scholar] [CrossRef]
- Palmer, S.R.; Hippo, E.J.; Dorai, X.A. Chemical coal cleaning using selective oxidation. Fuel 1994, 73, 161–169. [Google Scholar] [CrossRef]
- Palmer, S.R.; Hippo, E.J.; Dorai, X.A. Selective oxidation pretreatments for the enhanced desulfurization of coal. Fuel 1995, 74, 193–200. [Google Scholar] [CrossRef]
- Palmer, S.R.; Hippo, E.J.; Kruge, M.A.; Crelling, J.C. Characterization and selective removal of organic sulfur from iiiinois basin coals. Coal Prep. 1992, 10, 93–106. [Google Scholar] [CrossRef]
- Demirbaş, A. Desulfurization of coal using biomass ash. Energy Sources 2002, 24, 1099–1105. [Google Scholar] [CrossRef]
- Agarwal, J.C. Chemical desulphurization of coal. Min. Congr. J. 1975, 61, 40–43. [Google Scholar]
- Mukherjee, S.; Mahiuddin, S.; Borthakur, P.C. Demineralization and desulfurization of subbituminous coal with hydrogen peroxide. Energy Fuels 2001, 15, 1418–1424. [Google Scholar] [CrossRef]
- Qin, Z.-H.; Zhang, H.-F.; Dai, D.-J.; Zhao, C.-C.; Zhang, L.-F. Study on occurrence of sulfur in different group components of xinyu clean coking coal. J. Fuel Chem. Technol. 2014, 42, 1286–1294. [Google Scholar] [CrossRef]
- Friedman, S.; Warzinski, R.P. Chemical cleaning of coal. Eng. Power 1977, 99, 361–364. [Google Scholar] [CrossRef]
- Norton, G.A.; Markuszewski, R.; Araghi, H.G. Chemical cleaning of coal. In Fossil Fuels Utilization; American Chemical Society: Washington, DC, USA, 1986; Volume 319, pp. 63–74. [Google Scholar]
- Pecina, E.T.; Camacho, L.F.; Herrera, C.A.; MartÃ-nez, D. Effect of complexing agents in the desulphurization of coal by H2SO4 and H2O2 leaching. Miner. Eng. 2012, 29, 121–123. [Google Scholar] [CrossRef]
- Mukherjee, S. Demineralization and desulfurization of high-sulfur assam coal with alkali treatment. Energy Fuels 2003, 17, 559–564. [Google Scholar] [CrossRef]
- Kusnierova, M.; Prascakova, M.; Fecko, P.; Janakova, I. Chemical and biological desulphurization of boiler coal. J. Biotechnol. 2010, 150, 252. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, T.; Jia, J. A novel desulphurization process of coal water slurry via sodium metaborate electroreduction in the alkaline system. Fuel 2012, 96, 250–256. [Google Scholar] [CrossRef]
- Chuang, K.C.; Chen, M.C.; Greer, R.T.; Markuszewski, R.; Sun, Y.; Wheelock, T.D. Pyrite desulfurization by wet oxidation in alkaline solutions. Chem. Eng. Commun. 1980, 7, 79–94. [Google Scholar] [CrossRef]
- Markuszewski, R.; Chuang, K.C.; Wheelock, T.D. Coal desulfurization by leaching with alkaline solutions containing oxygen. In Proceedings of the Symposium on Coal Cleaning to Achieve Energy and Environmental Goals, Hollywood, FL, USA, 11–15 September 1978; p. 1039.
- Joshi, J.B.; Shah, Y.T. Kinetics of organic sulphur removal from coal by oxydesulphurization. Fuel 1981, 60, 612–614. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Chriswell, C.D. Hydrothermal pretreatment of coal before molten caustic leaching. In Proceedings of the 5th International Coal Conference, Seoul, Korea, 18–22 October 1993; Ames Laboratory: New York, NY, USA.
- Mukherjee, S.; Borthakur, P.C. Effect of leaching high sulphur subbituminous coal by potassium hydroxide and acid on removal of mineral matter and sulphur. Fuel 2003, 82, 783–788. [Google Scholar] [CrossRef]
- Syed, M.M.; Cliffe, K.R. Desulphurisation of Coal by Chemical Oxidation Using Potassium Permanganate; Institution of Chemical Engineers Research Event, University of Manchester: Manchester, UK, 1992. [Google Scholar]
- Syed, M.M.; Cliffe, K.R. Low temperature oxidation of sulphur. In Proceedings of the 5th International Conference on Processing and Utilisation of High Sulphur Coals, Lexington, KY, USA, 25–28 October 1993.
- Ghauri, M.; Inayat, A.; Bashir, M.; Ali, S.; Cliffe, K. High pressure oxydesulphurisation of coal—A parametric study. Energies 2013, 6, 1930–1943. [Google Scholar] [CrossRef]
- Ghauri, M.; Shahzad, K.; Inayat, A.; Ali, Z.; Cliffe, K. High pressure oxydesulphurisation of coal using KMnO4—Effect of coal slurry concentration, pH and alkali. Energies 2016, 9, 289. [Google Scholar] [CrossRef]
- Raask, E. Mineral Impurities in Coal Combustion; Hemisphere Publishing: Washington, WA, USA, 1985; p. 37. [Google Scholar]
- Wheelock, T.D. Oxydesulphurization of coal in alkaline solutions. Chem. Eng. Commun. 1981, 12, 137–159. [Google Scholar] [CrossRef]
- Holland, F.A.; Chapman, F.S. Liquid Mixing and Processing in Stirred Tanks; Reinhold Publishing Cooperation: New York, NY, USA, 1966; pp. 892–933. [Google Scholar]
- Meyers, R.A. Coal Desulphurisation; Marcel Dekker Inc.: New York, NY, USA, 1977; pp. 44–45. [Google Scholar]
- Bowen, R. Unraveling the mysteries of shear-sensitive mixing systems. Chem. Eng. 1986, 9, 55–63. [Google Scholar]
- Attia, Y.A.; Fung, A. Chemical desulphurisation of canadian high sulphur coal with potassium permanganate. Process. Util. High Sulphur Coals V 1993, 263–282. [Google Scholar]


| Parameter | POW * Coal |
|---|---|
| Total Sulphur % | 2.54 |
| Pyritic Sulphur % | 1.26 |
| Sulphate Sulphur % | 0.10 |
| Organic Sulphur % | 1.18 |
| Ash % | 13.03 |
| GCV ** (kJ/g) | 28.80 |
| Carbon % | 71.04 |
| Hydrogen % | 5.42 |
| Nitrogen % | 1.00 |
| Oxygen % | 4.21 |
| Particle size (μm) | 25 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghauri, M.; Shahzad, K.; Inayat, A.; Ali, Z.; Khan, W.A.; Akhtar, J.; Cliffe, K.R. High Pressure Oxydesulphurisation of Coal—Effect of Oxidizing Agent, Solvent, Shear and Agitator Configuration. Energies 2016, 9, 505. https://doi.org/10.3390/en9070505
Ghauri M, Shahzad K, Inayat A, Ali Z, Khan WA, Akhtar J, Cliffe KR. High Pressure Oxydesulphurisation of Coal—Effect of Oxidizing Agent, Solvent, Shear and Agitator Configuration. Energies. 2016; 9(7):505. https://doi.org/10.3390/en9070505
Chicago/Turabian StyleGhauri, Moinuddin, Khurram Shahzad, Abrar Inayat, Zulfiqar Ali, Waqar Ali Khan, Javaid Akhtar, and Keith R. Cliffe. 2016. "High Pressure Oxydesulphurisation of Coal—Effect of Oxidizing Agent, Solvent, Shear and Agitator Configuration" Energies 9, no. 7: 505. https://doi.org/10.3390/en9070505






