High Stable, Transparent and Conductive ZnO/Ag/ZnO Nanofilm Electrodes on Rigid/Flexible Substrates
Abstract
:1. Introduction
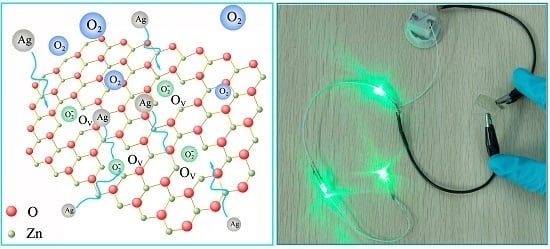
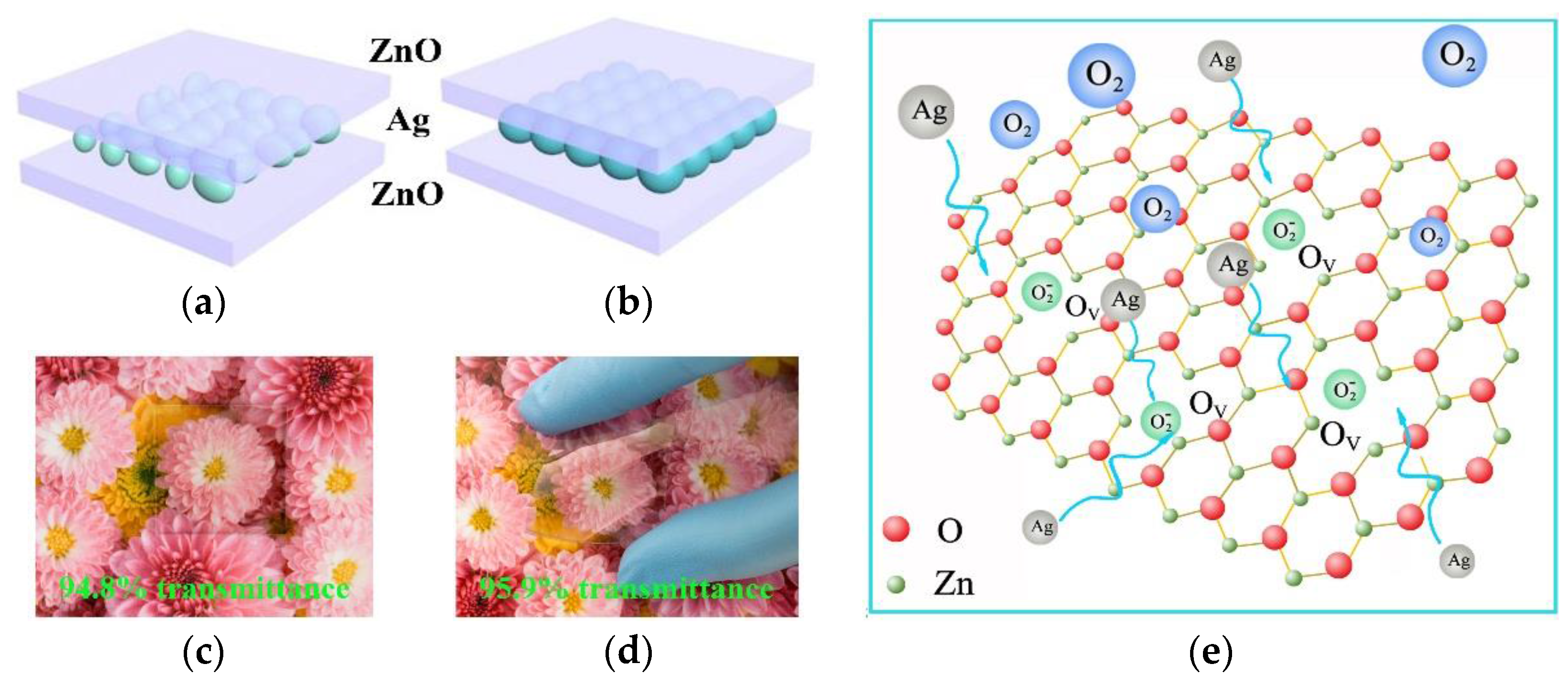
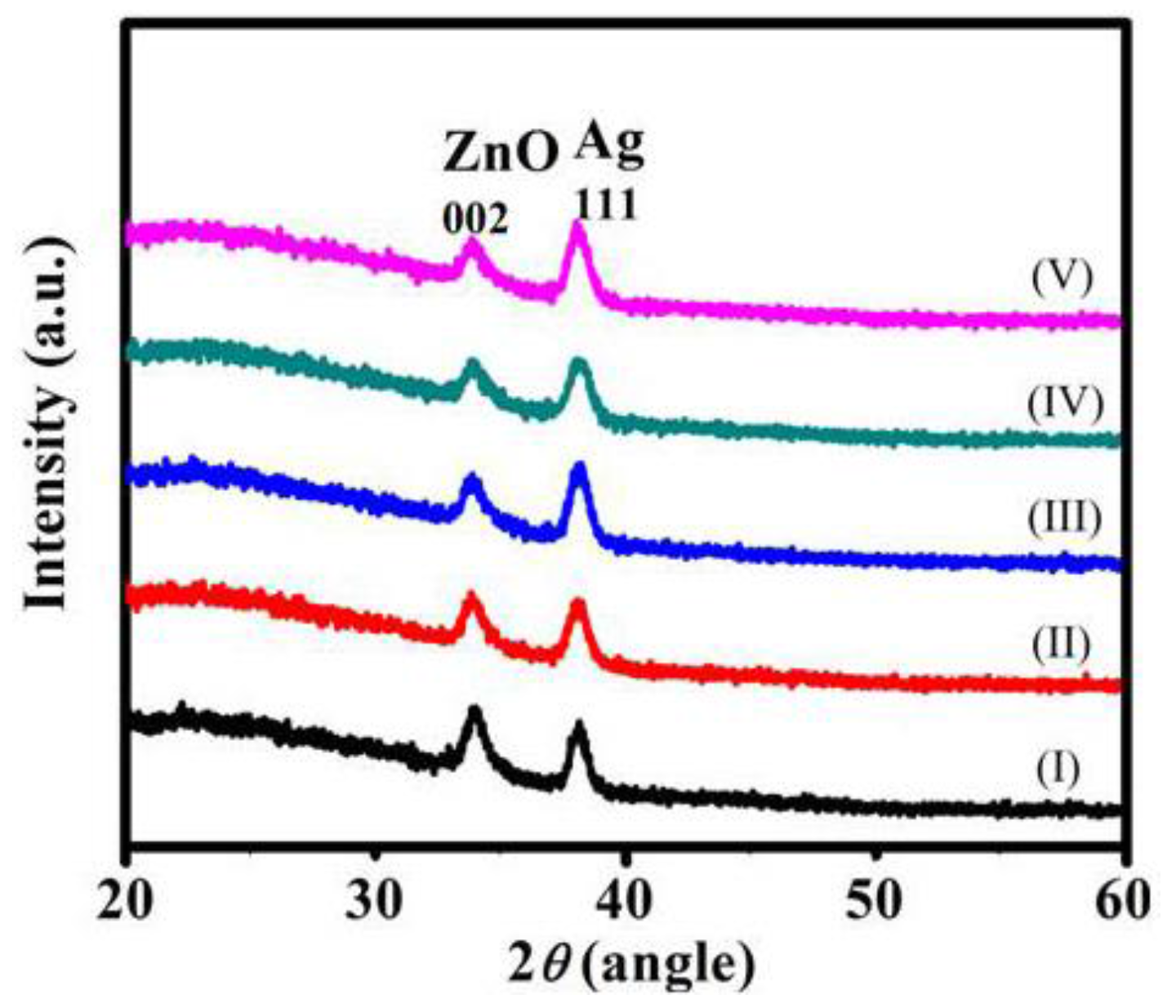
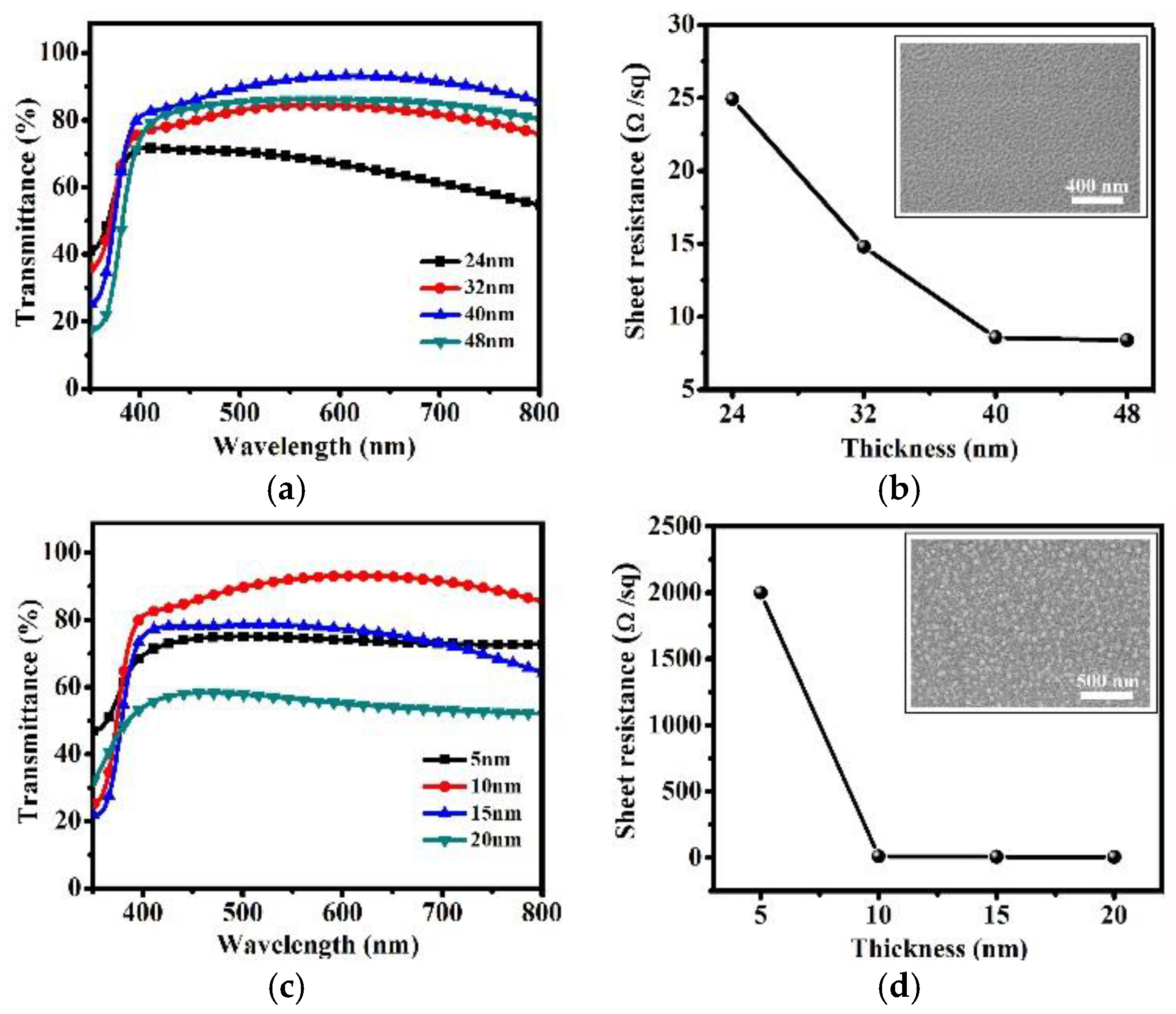
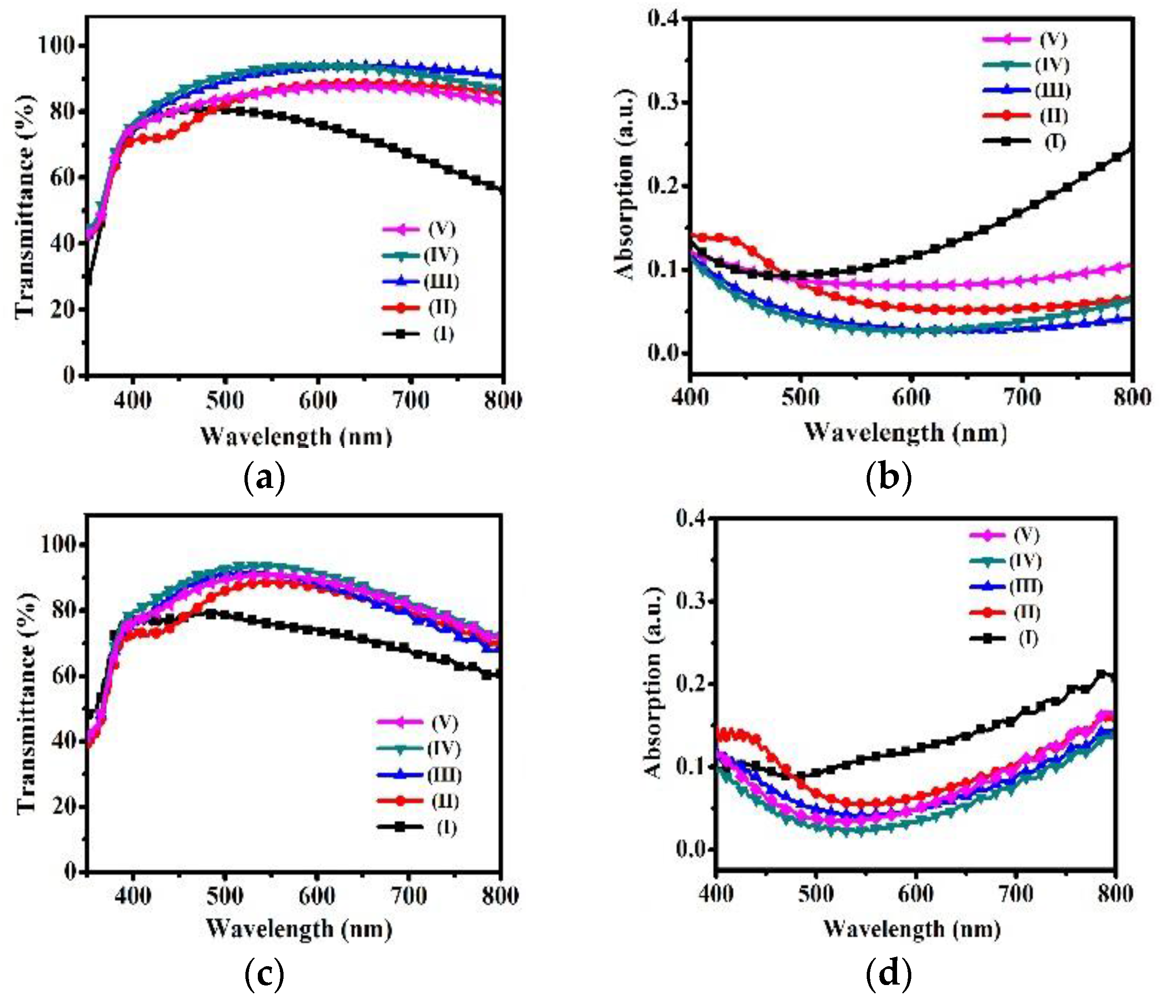
2. Results and Discussion
3. Materials and Methods
3.1. Fabrication of ZnO/Ag/ZnO Films
3.2. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zadsar, M.; Fallah, H.R.; Mahmoodzadeh, M.H.; Tabatabaei, S.V. The effect of Ag layer thickness on the properties of WO3/Ag/MoO3 multilayer films as anode in organic light emitting diodes. J. Lumin. 2012, 132, 992–997. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.S.; Kim, W.M.; Seong, T.Y.; Lee, J.; Müller-Meskamp, L.; Leo, K. Realizing the potential of ZnO with alternative nonmetallic Co-dopants as electrode materials for small molecule optoelectronic devices. Adv. Funct. Mater. 2013, 23, 3645–3652. [Google Scholar] [CrossRef]
- Ye, S.; Rathmell, A.R.; Stewart, I.E.; Ha, Y.C.; Wilson, A.R.; Chen, Z.; Wiley, B.J. A rapid synthesis of high aspect ratio copper nanowires for high-performance transparent conducting films. Chem. Commun. 2014, 50, 2562–2564. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.; Kim, S.; Jung, G.H.; Dong, W.J.; Lee, J.-L. Design of broadband transparent electrodes for flexible organic solar cells. J. Mater. Chem. A 2013, 1, 3076–3082. [Google Scholar] [CrossRef]
- Tenent, R.C.; Barnes, T.M.; Bergeson, J.D.; Ferguson, A.J.; To, B.; Gedvilas, L.M.; Heben, M.J.; Blackburn, J.L. Ultrasmooth, large-area, high-uniformity, conductive transparent single-walled-carbon-nanotube films for photovoltaics produced by ultrasonic spraying. Adv. Mater. 2009, 21, 3210–3216. [Google Scholar] [CrossRef]
- Hellstrom, S.L.; Vosgueritchian, M.; Stoltenberg, R.M.; Irfan, I.; Hammock, M.; Wang, Y.B.; Jia, C.; Guo, X.; Gao, Y.; Bao, Z. Strong and stable doping of carbon nanotubes and graphene by MoOx for transparent electrodes. Nano Lett. 2012, 12, 3574–3580. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Lee, D.; Lee, Y. Copper nanowire-graphene core-shell nanostructure for highly stable transparent conducting electrodes. ACS Nano 2015, 9, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Kahng, Y.H.; Kim, M.-K.; Lee, J.-H.; Kim, Y.J.; Kim, N.; Park, D.-W.; Lee, K. Highly conductive flexible transparent electrodes fabricated by combining graphene films and inkjet-printed silver grids. Sol. Energy Mater. Sol. Cells 2014, 124, 86–91. [Google Scholar] [CrossRef]
- Lei, P.-H.; Hsu, C.-M.; Fan, Y.-S. Flexible organic light-emitting diodes on a polyestersulfone (PES) substrate using Al-doped ZnO anode grown by dual-plasma-enhanced metalorganic deposition system. Org. Electron. 2013, 14, 236–249. [Google Scholar] [CrossRef]
- Duan, L.; Wang, P.; Yu, X.; Han, X.; Chen, Y.; Zhao, P.; Li, D.; Yao, R. The synthesis and characterization of Ag–N dual-doped p-type ZnO: Experiment and theory. Phys. Chem. Chem. Phys. 2014, 16, 4092–4097. [Google Scholar] [CrossRef] [PubMed]
- Vosgueritchian, M.; Lipomi, D.J.; Bao, Z. Highly conductive and transparent PEDOT: PSS films with a fluorosurfactant for stretchable and flexible transparent electrodes. Adv. Funct. Mater. 2012, 22, 421–428. [Google Scholar] [CrossRef]
- Zhang, W.F.; Zhao, B.F.; He, Z.C.; Zhao, X.M.; Wang, H.T.; Yang, S.F.; Wu, H.B.; Cao, Y. High-efficiency ITO-free polymer solar cells using highly conductive PEDOT:PSS/surfactant bilayer transparent anodes. Energy Environ. Sci. 2013, 6, 1956–1964. [Google Scholar] [CrossRef]
- Feng, J.; Hong, Y.; Zhang, J.; Wang, P.; Hu, Z.; Wang, Q.; Han, L.; Zhu, Y. Novel core-shell TiO2 microsphere scattering layer, with superior surface area and scattering properties, shows superior performance in dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 1502–1508. [Google Scholar] [CrossRef]
- Koo, J.-R.; Lee, S.J.; Lee, H.W.; Lee, D.H.; Yang, H.J.; Kim, W.Y.; Kim, Y.K. Flexible bottom-emitting white organic light emitting diodes with semitransparent Ni/Ag/Ni anode. Opt. Express 2013, 21, 11086–11094. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Liu, X.; Zhang, N.; Chen, H.; Zheng, X.; Wang, H.; Guo, X. High-performance NiO/Ag/NiO transparent electrodes for flexible organic photovoltaic cells. ACS Appl. Mater. Interfaces 2014, 6, 16403–16408. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Alford, T.L. High quality transparent TiO2/Ag/TiO2 composite electrode films deposited on flexible substrate at room temperature by sputtering. APL Mater. 2013, 1. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wang, P.P.; Murakami, R.-I.; Song, X.P. Effect of an interface charge density wave on surface plasmon resonance in ZnO/Ag/ZnO thin films. Appl. Phys. Lett. 2010, 96, 233114–233117. [Google Scholar] [CrossRef]
- Zou, J.; Li, C.Z.; Chang, C.Y.; Yip, H.-L.; Jen, A.K. Interfacial engineering of ultrathin metal film transparent electrode for flexible organic photovoltaic cells. Adv. Mater. 2014, 26, 3618–3623. [Google Scholar] [CrossRef] [PubMed]
- Vedraine, S.; Hajj, A.E.; Torchio, P.; Lucas, B. Optimized ITO-free tri-layer electrode for organic solar cells. Org. Electron. 2013, 14, 1122–1129. [Google Scholar] [CrossRef]
- Wu, J.M.; Chen, Y.-R.; Lin, Y.-H. Rapidly synthesized ZnO nanowires by ultraviolet decomposition process in ambient air for flexible photodetector. Nanoscale 2011, 3, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gui, P.B.; Yu, Q.H.; Mei, J.; Wang, H.; Fang, G.J. Self-powered, visible-blind ultraviolet photodetector based on n-ZnO nanorods/i-MgO/p-GaN structure light-emitting diodes. J. Mater. Chem. C 2015, 3, 990–994. [Google Scholar] [CrossRef]
- Wüstner, W.; Menzel, D. A field emission investigation of adsorption and nucleation of silver on tungsten, and of the interaction of the deposits with oxygen. Thin Solid Films 1974, 24, 211–228. [Google Scholar] [CrossRef]
- Formica, N.; Mantilla-Perez, P.; Ghosh, D.S.; Janner, D.; Chen, T.L.; Huang, M.; Garner, S.; Martorell, J.; Pruneri, V. An indium tin oxide-free polymer solar cell on flexible glass. ACS Appl. Mater. Interfaces 2015, 7, 4541–4548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Jung, S.; Jeong, S.; Kim, G.; Lee, K. Polymer-metal hybrid transparent electrodes for flexible electronics. Nat. Commun. 2015, 6, 6503–6509. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhang, Y.Z.; Lai, W.Y.; Chen, Y.; Zeng, J.W.; Huang, W. High-performance stretchable transparent electrodes based on silver nanowires synthesized via an eco-friendly halogen-free method. J. Mater. Chem. C 2014, 2, 10369–10376. [Google Scholar] [CrossRef]
- Gao, X.Y.; Liu, X.Y.; Liu, F.Y.; Li, H.L.; Fan, Y.; Zhang, N. Highly conductive transparent organic electrodes with multilayer structures for rigid and flexible optoelectronics. Sci. Rep. 2015, 5, 10569–10577. [Google Scholar]
- Wu, X.K.; Liu, J.; Wu, D.Q.; Zhao, Y.R.; Shi, X.D.; Wang, J.S.; Huang, J.; He, G.F. Highly conductive and uniform graphene oxide modified PEDOT:PSS electrodes for ITO-Free organic light emitting diodes. J. Mater. Chem. C 2014, 2, 4044–4050. [Google Scholar] [CrossRef]
- Haacke, G. New figure of merit for transparent conductors. J. Appl. Phys. 1976, 47, 4086–4089. [Google Scholar] [CrossRef]
- Bergin, S.M.; Chen, Y.H.; Rathmell, A.R.; Charbonneau, P.; Li, Z.Y.; Wiley, B.J. The effect of nanowire length and diameter on the properties of transparent, conducting nanowire films. Nanoscale 2012, 4, 1996–2004. [Google Scholar] [CrossRef] [PubMed]



| Deposition Parameters | Conditions | ||
|---|---|---|---|
| Top ZnO | Ag | Bottom ZnO | |
| Target | ZnO (99.9%) | Ag (99.9%) | ZnO (99.9%) |
| Target diameter (inch) | 2 | 2 | 2 |
| Sputtering power (W) | 100 (RF) | 20 (DC) | 100 (RF) |
| Gas flow rate (sccm) | Ar: 63 | O2 flow rate: 0–4, Ar flow rate: 96–100 | Ar: 63 |
| Initial pressure (Pa) | 8 × 10−4 | ||
| Working pressure (Pa) | 2.0 | ||
| Substrate temperature | Room temperature (~22 °C) | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhao, Y.; Jia, Z.; Qin, Z.; Chu, L.; Yang, J.; Zhang, J.; Huang, W.; Li, X. High Stable, Transparent and Conductive ZnO/Ag/ZnO Nanofilm Electrodes on Rigid/Flexible Substrates. Energies 2016, 9, 443. https://doi.org/10.3390/en9060443
Zhang Q, Zhao Y, Jia Z, Qin Z, Chu L, Yang J, Zhang J, Huang W, Li X. High Stable, Transparent and Conductive ZnO/Ag/ZnO Nanofilm Electrodes on Rigid/Flexible Substrates. Energies. 2016; 9(6):443. https://doi.org/10.3390/en9060443
Chicago/Turabian StyleZhang, Qiaoxia, Yanghua Zhao, Zhenhong Jia, Zhengfei Qin, Liang Chu, Jianping Yang, Jian Zhang, Wei Huang, and Xing’ao Li. 2016. "High Stable, Transparent and Conductive ZnO/Ag/ZnO Nanofilm Electrodes on Rigid/Flexible Substrates" Energies 9, no. 6: 443. https://doi.org/10.3390/en9060443