1. Introduction
In the modern world, there is a strong interest in miniaturization of a variety of systems. Small systems are often less expensive for mass production; they can have properties exceeding those of large systems; small systems may be combined in complex devices without significant increase of volume; and they may facilitate extended or improved performance of large systems (cars, for example). To drive any microsystem, there must be an engine that has to be at least not significantly larger than the system itself. This restriction is a bottleneck for many microsystems. In the macroworld, we are using mainly internal combustion engines and electromagnetic motors that have proven their efficiency and reliability. However, in the microworld, these engines are not available. Small electromagnetic motors cannot generate forces of useful magnitude because it is not possible to scale down the coil size. Small internal combustion engines loose their efficiency due to the increase of the surface-to-volume ratio, which is responsible for fast heat loss from the reaction chamber [
1,
2].
Miniaturization of engines demands also shorter working cycles, but it happens to be difficult to build an engine that is both fast and strong. This problem is well recognized in the microelectromechanical community [
3,
4]. Existing microengines (actuators) use mostly two types of forces [
3,
4,
5,
6]: electrostatic forces and forces generated by thermal expansion. The electrostatic actuators are fast, but weak. This is due to low energy density accumulated in the electric field. On the other hand, the actuators using the thermal principle are strong, but slow. Their response time is restricted by the diffusion process. Most advanced piezoelectric actuators [
7] are fast and strong, but not compatible with microtechnology, need a high voltage for actuation and have a small stroke. Actuators using electrochemical decomposition of water were also discussed in many papers [
8,
9,
10,
11,
12,
13,
14], but they are notoriously slow. One can produce a large amount of gas in a short time, but it is impossible to get rid of this gas fast, as well. The fastest electrochemical actuator reported up to date [
15] has a response time in the range of minutes.
Internal combustion engines having all three dimensions in the range of micrometers do not exist at all. The smallest size of microcombustors [
16,
17] is a little bit smaller than one millimeter with two other dimensions to be significantly larger. Due to severe heat losses for small-scale combustion systems, possible flame extinction requires a delicate balance between heat loss and heat generation [
18,
19] when designing microcombustors.
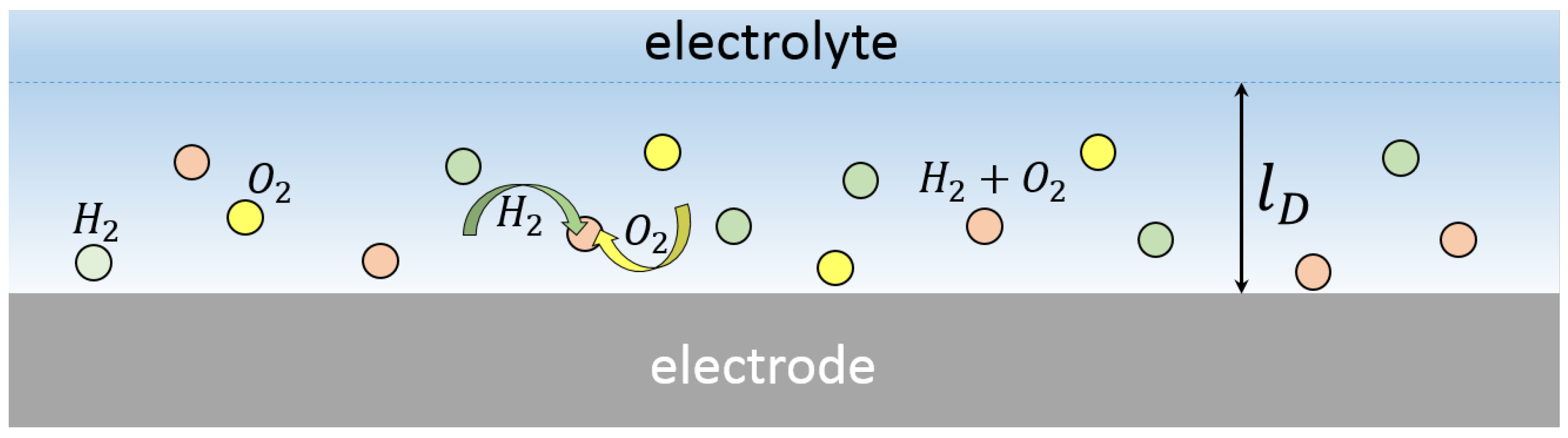
In spite of heat losses, the reaction between hydrogen and oxygen was recently observed in extremely small volumes: nanobubbles [
20]. Using a special electrochemical process of water decomposition in which the polarity of the applied voltage is alternated at frequencies of the order of
kHz, nanobubbles containing a mixture of hydrogen and oxygen were produced. The gas production was observed with a stroboscope. It was found that with the frequency increase, the production of visible bubbles is strongly reduced, while the Faraday current changes very little. When the stoichiometric balance between H
and O
production close to a single electrode was broken, the bubbles appeared in the system again. These phenomena were explained by the reaction between gases in the nanobubbles, which are produced homogeneously due to an extremely high supersaturation near the electrodes [
21].
The gas concentration in the electrolyte produced by the alternating polarity pulses was directly measured with a vibrometer. It demonstrated oscillations [
20] that were in phase with the driving electrical pulses. Without the reaction between gases, this concentration would increase monotonously, and the oscillations would not be possible. Additionally, the surface of the electrodes was strongly modified on the nanoscale for such non-reactive materials as platinum or gold. This was explained by nanoexplosions close to the surface of the electrodes.
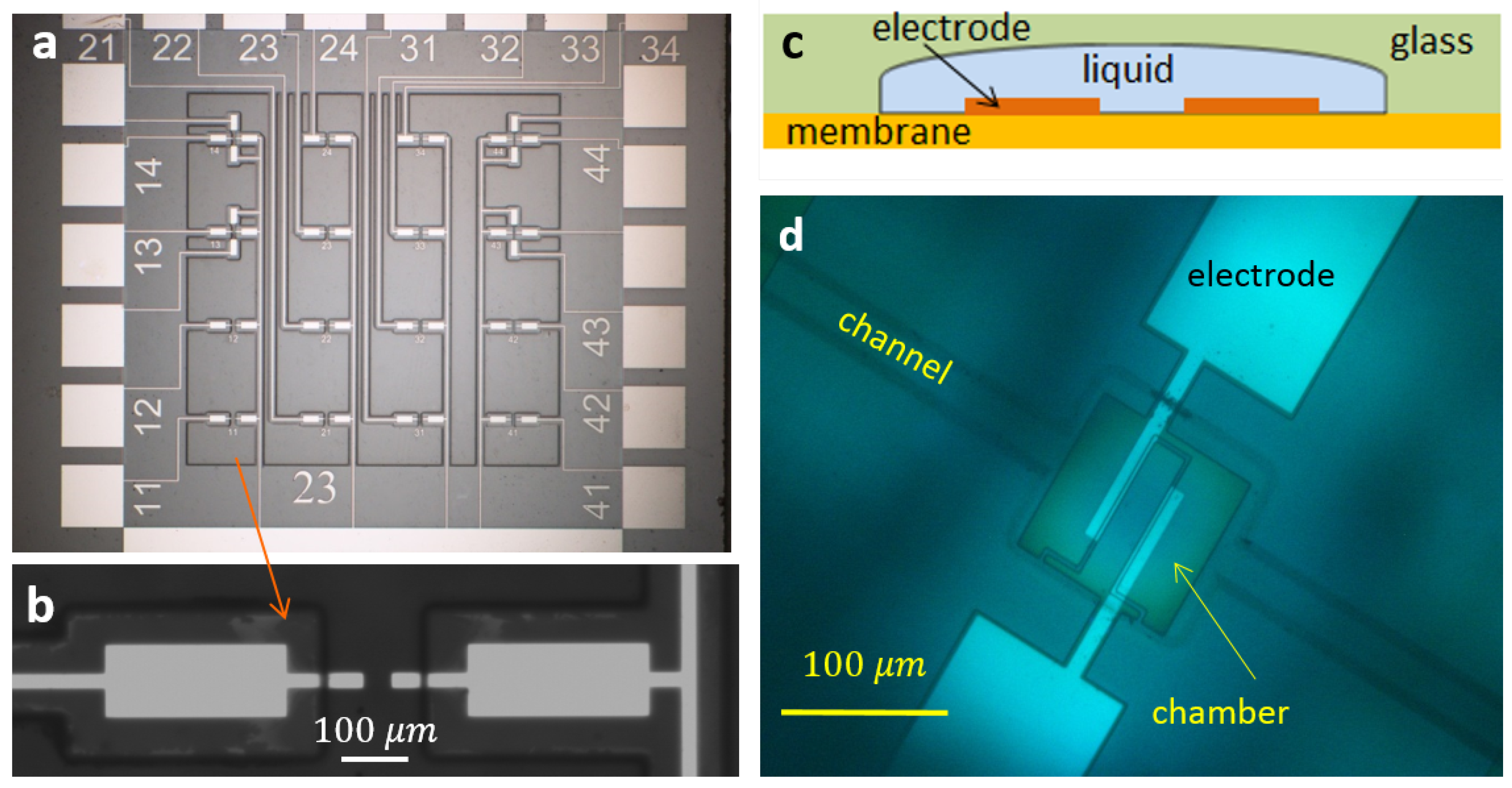
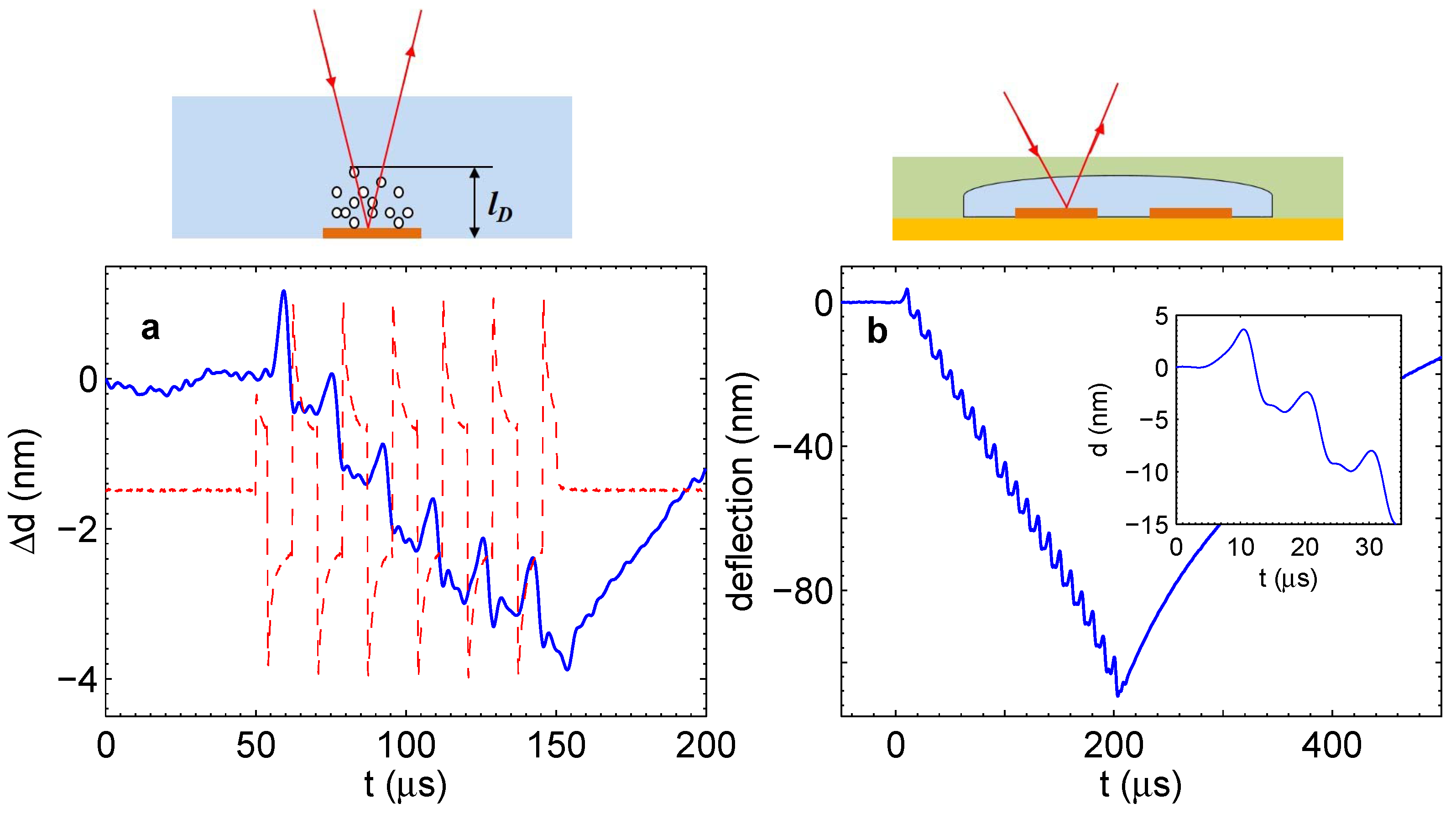
To run the process in a closed microchamber, a special microfluidic device was fabricated [
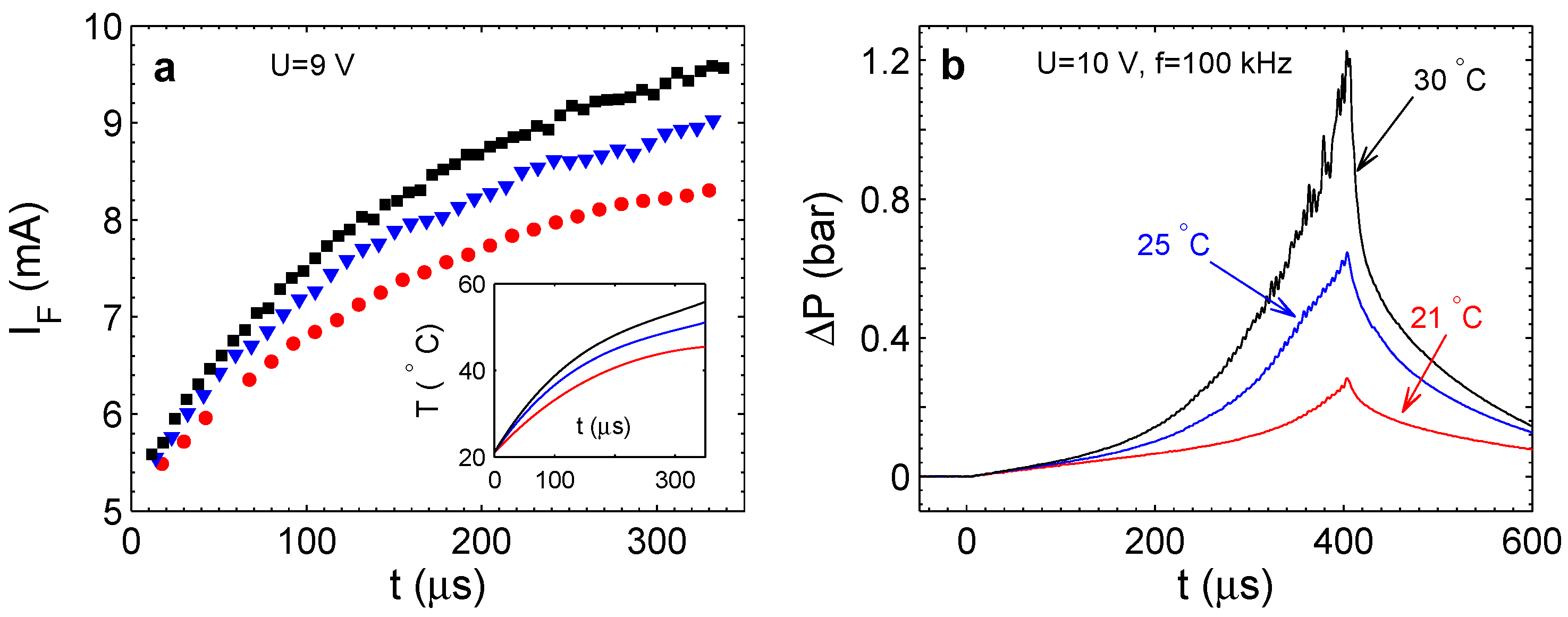
22]. The chamber containing electrodes and filled with the electrolyte was covered with a transparent flexible membrane. Pressure in the chamber was estimated by measuring the deflection of the membrane with the vibrometer. On the other hand, the small thermal mass of the membrane made it possible to measure the temperature change in the chamber. This was done using the temperature dependence of the electrolyte conductivity. It was found that the amount of gas produced by the measured Faraday current has to be much larger than the volume of the chamber. The fact that this gas is not observed is a strong argument in favor of the reaction. Pressure in the chamber increases with time, but then saturates at the value of a few bars. In the steady state, all of the gas produced electrochemically is consumed in the reaction.
The combustion reaction is a highly exothermic process. Energy released in the reaction has to have a measurable thermal effect. The effect is not as large as for classical combustion due to significant heat losses via the volume boundaries, but nevertheless, it was reliably separated from the background Joule heating [
22]. It was stressed also that the external temperature influences strongly the rate of pressure increase in the chamber. This effect was related to the rate of nucleation of nanobubbles.
A high concentration of gases in the electrolyte is possible due to high Laplace pressure in nanobubbles. At some conditions, the gas concentration can be large enough that the nanobubbles start to touch each other, merge and form optically visible microbubbles. This effect was observed [
23] and revealed interesting features. Microbubbles appearing on very short time scales (
s) were directly observed in the chamber by means of stroboscopic illumination. The appearance of these bubbles was accompanied by strong pressure jumps in the chamber. It was concluded that the short-lived microbubbles are formed by coalescence of H
and O
nanobubbles, igniting the reaction in a microbubble spontaneously, thereby destroying the bubble in a short time with resulting jumps in pressure.
Not all possible methods to investigate the reaction in small volumes have been explored so far. For example, the reaction between hydrogen and oxygen is based on branching, propagation and termination of H, O, OH and HO
radicals [
24]. Some of these radicals can survive in the solution long enough to be picked up with radical scavengers. It is difficult to see nanobubbles because they do not scatter visible light. However, they can be visualized by scattering UV light or neutrons [
25]. The dynamics of short-lived microbubbles can be investigated in more detail with a high speed camera. The final purpose of the investigation is to understand the mechanism of the reaction in small volumes. It is obvious that this mechanism is different from macroscopic combustion, and it has to be surface dominated.
In this paper, we review the experimental facts that are known about the reaction in nano- and micro-scopic volumes up to date. Physical interpretation of the observations is proposed. We do not discuss any specific device, but concentrate on the basic principle that allows combustion in three-dimensional microscopic volumes. Although the paper is positioned as a review, it contains a number of figures that were not published before and includes new details in the description of the physical processes.
The paper is organized as follows. In
Section 2, we describe the microchips that have been used in the experiments and explain the short-time electrochemical process. In
Section 3, the signatures of the reaction in nanobubbles are presented. Observations of the reaction in microbubbles are described in
Section 4. The discussion of the experimental results is presented in
Section 5. Our conclusions are collected in the last section.
4. Reaction in Microbubbles
In this section, we describe a new regime of alternating polarity electrolysis, in which the reaction between hydrogen and oxygen happens in microbubbles containing a stoichiometric mixture of the gases [
23]. Although these microbubbles only live around
s, it is still possible to observe them optically due to their relatively large size. A short appearance of the microbubbles is accompanied by a large pressure jump in a closed chamber.
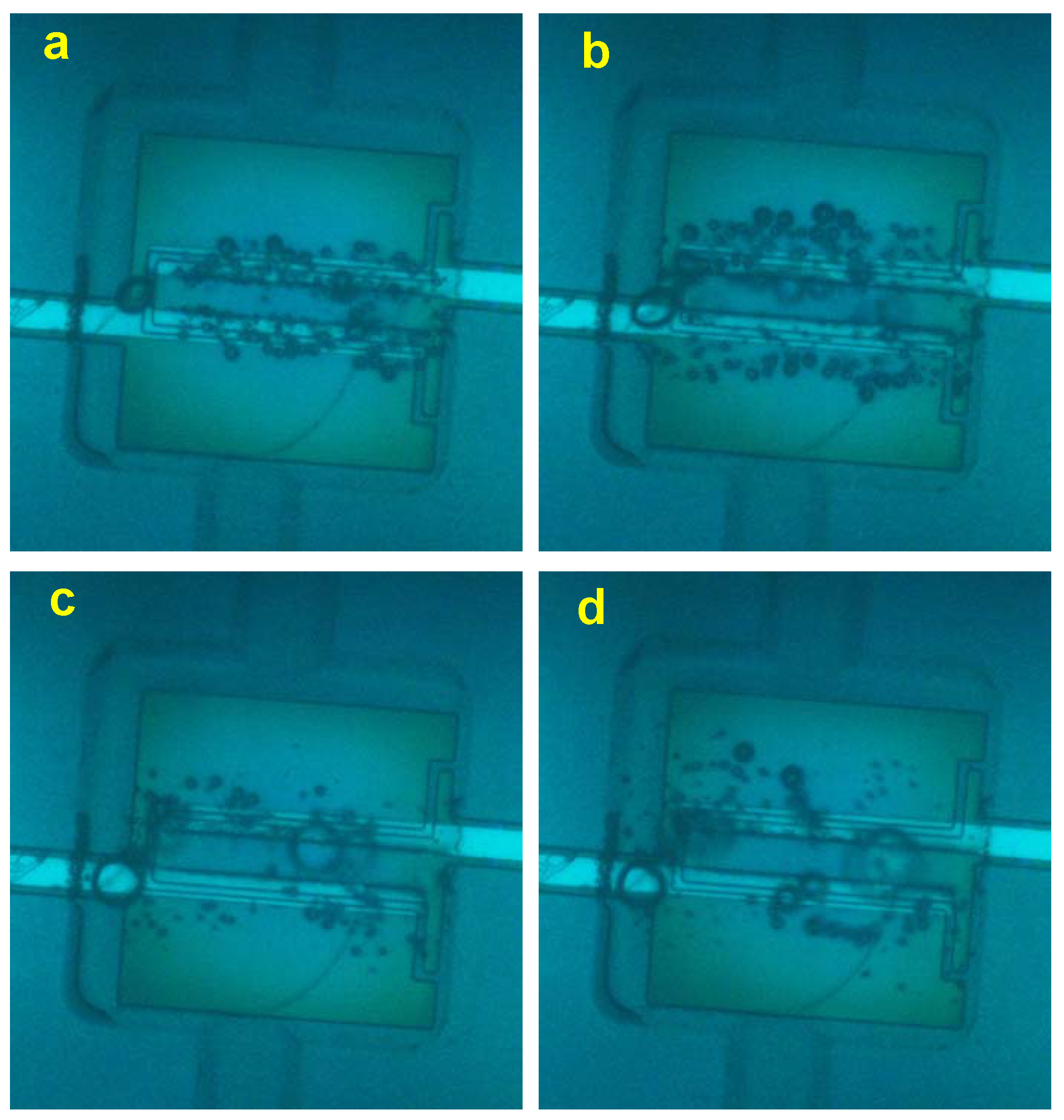
Figure 8.
Stroboscope snapshots of the short-lived bubbles. These bubbles look like they are out of focus due to motion blur. The driving amplitude is V. (a,b) kHz. The images were made at s and s, respectively. (c,d) kHz. The images correspond to the moments s and s, respectively.
Figure 8.
Stroboscope snapshots of the short-lived bubbles. These bubbles look like they are out of focus due to motion blur. The driving amplitude is V. (a,b) kHz. The images were made at s and s, respectively. (c,d) kHz. The images correspond to the moments s and s, respectively.
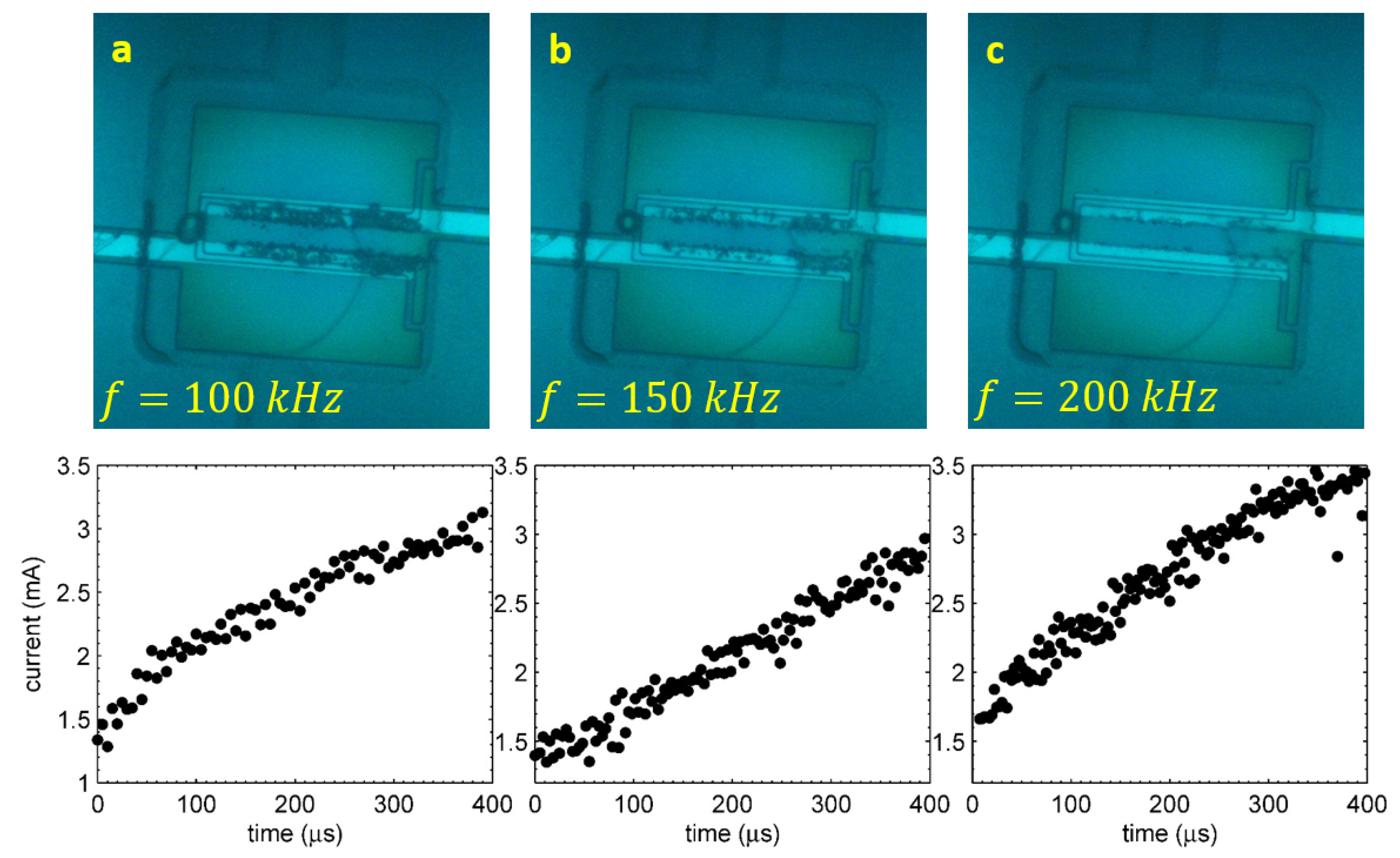
4.1. Stroboscopic Observations
When the alternating polarity process is run in a closed chamber for sufficiently long time
s at high frequencies
kHz, a new phenomenon can be observed. This phenomenon is the appearance of short-lived microbubbles in the chamber. A series of stroboscopic snapshots shown in
Figure 8 demonstrates the phenomenon. The process was run at the amplitude
V. The driving pulses for Panels (a) and (b) correspond to a frequency of
kHz, but for (c) and (d), the frequency was
kHz. The image in Panel (a) was made at
s when the short-lived microbubbles are just starting to develop. In contrast with the images in
Figure 3, one can see that the long-lived bubbles are shifted a little bit out of the electrodes, and there is a weak contrast in between the electrodes. At a longer delay of
s, it becomes more obvious as one can see in Panel (b). The contrast in between the electrodes looks like microbubbles that are out of focus. This happens due to motion blur and means that these bubbles exist in the chamber for a shorter time than the exposure time (
s). There must be a flow in the chamber related to these short-lived bubbles, because the long-lived bubbles (those that are in focus) are strongly pushed away from the electrodes.
Panels (c) and (d) show the images that were taken at s and s, respectively, for the driving frequency kHz. At higher frequencies, the short-lived bubbles have a larger size, but need a longer incubation period to appear. One can see rather large displacement of long-lived microbubbles, which assumes that rather strong local flow exist in the chamber. At frequencies below kHz, the short-lived bubbles do not appear at all.
4.2. Pressure Fluctuations
When the short-lived microbubbles are formed in the chamber, the membrane deflection also demonstrates peculiar features. This can be seen in
Figure 9. Panel (a) shows the membrane deflection as measured by the vibrometer for the pulses with amplitude
V and frequency
kHz. The pulses were switched on during
s. The graph shows small oscillation of the membrane superimposed on the monotonous increase of the deflection. The situation here is similar to that shown in
Figure 5b. The change of the slope happens due to the effect of internal heating. In Panel (b), the membrane response is shown for the driving frequency
kHz. One can observe qualitatively new features that appear in the response. These are high narrow peaks in the deflection. Of course, the peaks in the deflection are equivalent to the peaks in the overpressure in the chamber. These peaks appear after an incubation period of
s.
Figure 9.
Transition to the regime with pressure fluctuations. (a) Deflection of the membrane for V and kHz. Regular oscillations easily visible in the inset correspond to the periodic termination of gas in nanobubbles. (b) Deflection of the membrane for higher frequency kHz. The oscillations develop in irregular sharp and strong peaks. The inset shows details of the peaks.
Figure 9.
Transition to the regime with pressure fluctuations. (a) Deflection of the membrane for V and kHz. Regular oscillations easily visible in the inset correspond to the periodic termination of gas in nanobubbles. (b) Deflection of the membrane for higher frequency kHz. The oscillations develop in irregular sharp and strong peaks. The inset shows details of the peaks.
We observed the following properties of the phenomenon. The pressure in the chamber starts to fluctuate after some incubation time. This time increases with frequency and decreases with the voltage amplitude. The magnitude of the fluctuations also increases with frequency. When the frequency is below kHz, the fluctuations become very small and practically disappear. On the other hand, at kHz, they become so violent that they can cause the membrane to break. There is a clear correspondence between the short-lived microbubbles observed stroboscopically and the pressure jumps observed with the vibrometer.
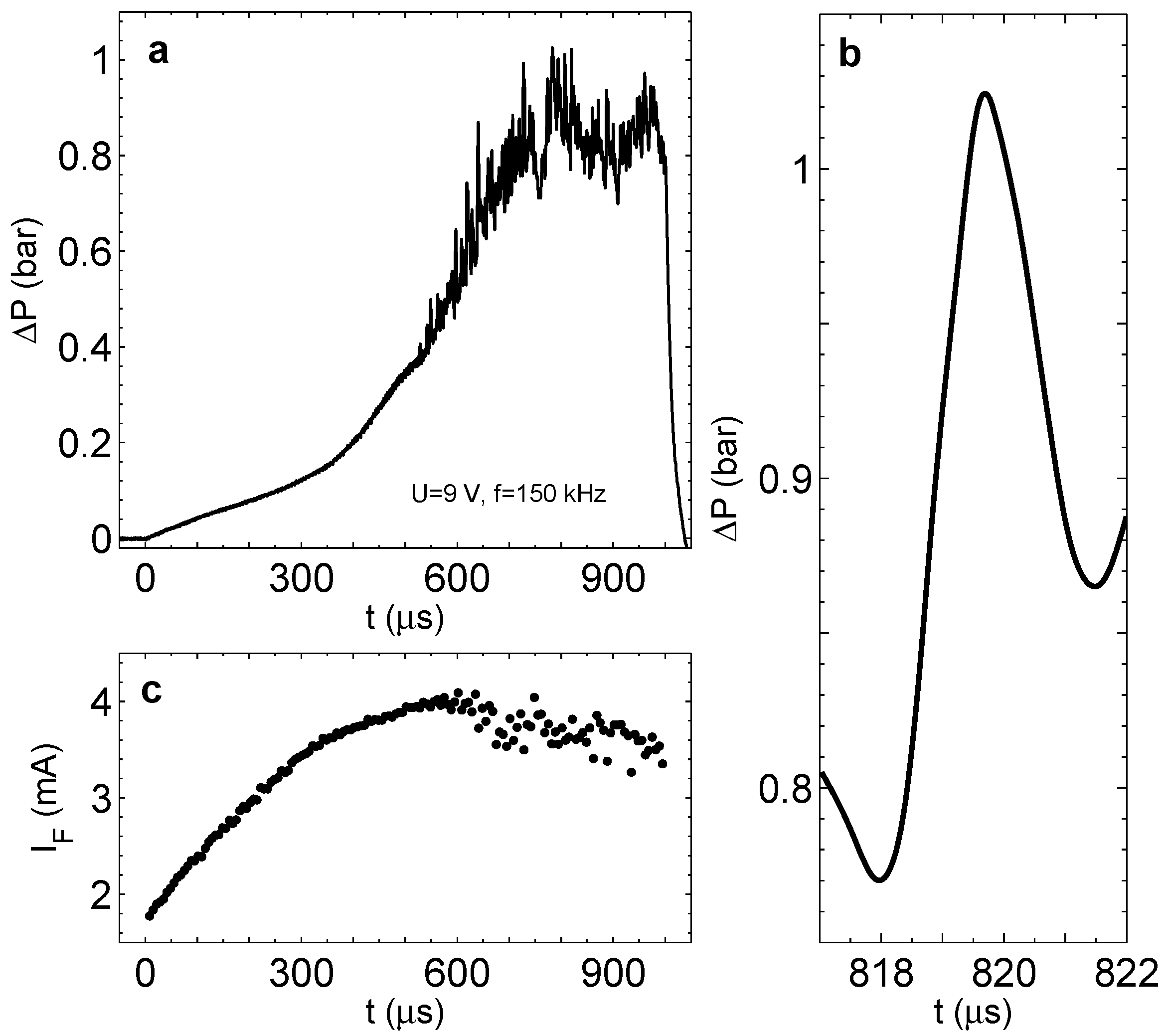
If the process is run for a long time, the pressure in the chamber saturates similarly to that presented in
Figure 6c; however, instead of regular oscillations, we observe significant fluctuations of pressure in the steady state. It can be seen in
Figure 10a, where overpressure is shown for
V and
kHz. A typical pressure jump for these driving pulses is shown in Panel (b). It has a magnitude of
bar and a width of around
s. It is also interesting to see the behavior of the Faraday current shown in Panel (c). When the pressure starts to jump, the current does not grow anymore and develops a fluctuating component. This is in agreement with the short-lived microbubbles appearing in the chamber. These microbubbles have a size comparable to the distance between the electrodes. The appearance and disappearance of even one bubble of this size in the chamber has to influence on the current, resulting in its fluctuations. Some reduction of the current magnitude is explained by better heat transfer in the chamber due to the flickering microbubbles. We conclude that the pressure jumps and the current fluctuations are induced by the short-lived bubbles observed optically.
Figure 10.
(a) Overpressure in the chamber with well-developed pressure fluctuations. The process is driven at V and kHz during s. (b) A zoomed peak from (a) represents a typical pressure jump. (c) The Faraday current corresponding to the run in (a) (one point per period).
Figure 10.
(a) Overpressure in the chamber with well-developed pressure fluctuations. The process is driven at V and kHz during s. (b) A zoomed peak from (a) represents a typical pressure jump. (c) The Faraday current corresponding to the run in (a) (one point per period).
4.3. Formation Mechanism
The described observations of the short-lived microbubbles can be explained with the following sequence of events (a detailed description is presented in [
23]). When the electrolysis is driven by the alternating polarity pulses, three types of nanobubbles are formed nearby the electrode surface, as shown in
Figure 2. The bubbles containing the stoichiometric mixture of the gases disappear in the spontaneous reaction contributing to the temperature increase. The nanobubbles containing hydrogen or oxygen are collected in the chamber, resulting in the observed monotonous pressure increase in the chamber. With time, the concentration of nanobubbles containing H
or O
becomes so large that the bubbles are nearly touching. This can happen only in between the electrodes, and the precise place depends on the fluctuations in the concentration of bubbles. In a place with a high concentration, the bubbles merge, forming a microbubble containing the stoichiometric mixture of gases. It is interesting to note that a similar process of fast coalescence of small vapor bubbles was observed in liquids that were superheated in a very short time [
26,
33,
34,
35].
The volume of microbubbles emerging in the chamber is much smaller than the volume of the chamber. Nevertheless, such bubbles are able to produce large pressure jumps in the whole chamber. This means that the appearance and disappearance of the short-lived microbubble is a highly energetic event. Only combustion of gases inside of the microbubble has an appropriate energy scale. The energy produced by the reaction is:
where
kJ/mol is the enthalpy of water formation and
is the number of molecules in the microbubble. The bubbles, which are observed at
V and
kHz, have this number in the range
(0.5–1.5)
, so that the combustion energy is
1.5–4 nJ.
This energy is spent on the increase of pressure in the chamber, on the evaporation of water molecules from the bubble walls, on the temperature increase in the bubble, on the increase of the elastic energy of the membrane and on the kinetic energy of the liquid. The last two channels are related to each other and are not very important [
23]. Significant temperature increase in the microbubble is not possible, since the characteristic time
for the heat transfer to liquid is very short. For a radius of
m, this time is just
ns, as was estimated earlier. This means that the reaction in microbubbles is also a surface-dominated process as the reaction in nanobubbles. The absence of high temperature spots in the chamber is confirmed by the observation of the process with a low-light Andor iXon +855 EMCCD camera.
This discussion shows that there are only two important channels to which the combustion energy may go. The pressure increase in the chamber (in
Figure 10a, it is
bar) corresponds to the energy
nJ. On the other hand, the reaction between H
and O
has to produce energetic molecules and radicals, which are able to vaporize water molecules from the wall. Because the heat of vaporization is rather large (
kJ/mol), this channel is able to absorb the excessive energy produced by the combustion.
5. Discussion
We provided experimental evidence that the combustion reaction between hydrogen and oxygen can proceed in microscopic and nanoscopic volumes. The reaction is ignited spontaneously at temperatures below 100
C and at slightly elevated pressure. According to the standard combustion theory [
27,
28], the reaction at this low temperature should not proceed, because radicals are efficiently terminated on the surface of microbubbles and because the reaction cannot sustain itself due to the fast heat escape from the microscopic volume.
The strongest argument in favor of the reaction in micro- and nano-bubbles is the amount of gas that is produced by the Faraday current. Without the reaction, this gas would fill a few volumes of the chamber. It is easy to check that the Faraday current really produces the gases by breaking the stoichiometric ratio, for example by applying single polarity pulses or changing the duty cycle of the alternating polarity pulses. We do not know the mechanism of the reaction in a small volume, but it is clear that the bubble surface has to play a significant role. The catalytic activity of the electrodes can be excluded, since we observed similar properties of the process for very different metals. In microsystems, we used Pt, Au, W and Pd, but we tried also Cu, Al, Ta, Ti and Fe for larger systems (in the millimeter range).
We were able to record a periodic decrease in the gas concentration above the electrodes by measuring the change of the refractive index of liquid with the vibrometer. In the closed chamber, variation of the gas concentration, in phase with the driving pulses, can be observed as oscillations of pressure in the chamber. These oscillations are easily visible in all of our measurements. This periodic decrease of the gas is attributed to the combustion of gases in nanobubbles with stoichiometric gas composition.
An important signature of the combustion reaction is the heat production. This heat is difficult to observe in microsystems with a bulk substrate, because the substrate has a large thermal mass. However, the effect becomes well visible in the microchamber covered with a thin membrane. The thermal mass of the membrane is small, and liquid in the chamber can be heated up to 70 C. There are two sources of heating of the liquid: Joule heating by the current flowing through the electrolyte and heating by the reaction. The Joule heating does not depend on the frequency of driving pulses, while we observe clear dependence of the temperature on the frequency. Therefore, at least part of the heating has to be produced by the reaction.
Reaction in microbubbles containing the stoichiometric mixture of hydrogen and oxygen was observed optically using the stroboscope and vibrometer. These bubbles live just a few microseconds, and their existence is accompanied by a significant energy deposition in the chamber. These microbubbles are formed by coalescence of nanobubbles containing only hydrogen or only oxygen. It has to be noted that stoichiometric microbubbles, which were formed in a different way, live a much longer time. For example, the gas produced in a closed chamber by a single polarity pulse first is located above different electrodes (H and O are separated), but at a later time, the gases are mixed. The bubbles formed after mixing do not disappear in a few microseconds, but exist much longer.
It is worth commenting on the relaxation time of pressure in the chamber. When the driving pulses are switched off, the pressure drops down much faster than one could expect on the bases of gas diffusion. While there is no pressure fluctuations the pressure drops down after switching off the pulses in s or so. This can be explained by the local coalescence of H and O nanobubbles followed by the reaction in the formed bubble. When the short-lived microbubbles appear in the chamber, the relaxation time for the pressure is reduced further. This can be as short as s. This is because in this regime, the nanobubbles are packed more densely, so that the coalescence time is reduced.
High pressure in the chamber and its fast relaxation was proposed for use as a new physical principle for fast and strong actuators [
22]. In this device, the pressure is produced by the alternating polarity pulses during a few hundreds of microseconds. When the pulses are switched off, the pressure is reduced six orders of magnitude more quickly than in the fastest electrochemical actuator [
15].
6. Conclusions
In this paper, we collected the latest information that is known about the combustion reaction in nano- and micro-scopic volumes. According to the standard combustion theory, this reaction cannot proceed due to fast heat escape from small volumes. Nevertheless, there are many facts showing that the reaction happens. The mechanism of the reaction is still unclear, but it is obvious that the surface of the volume has to play a principal role.
The process was investigated in nanobubbles produced electrochemically. A special procedure was used to form nanobubbles with a stoichiometric mixture of gases. Although the direct observation of the reaction in nanobubbles is difficult due to short lifetimes (s) and small sizes (nm), a number of observed effects strongly indicated the reaction. The most important effects are the following. (i) There is an obvious disagreement between the gas production predicted on the basis of the Faraday current and a very small gas volume that is actually observed in bubbles. The missed gas reappears in the system as soon as the stoichiometric composition of gases above a specific electrode is broken. (ii) Gas concentration measured above the electrodes demonstrated oscillations in phase with the driving pulses, while without the reaction, it has to increase monotonously with time. (iii) Heat produced by the reaction was separated from the background Joule heating as a frequency-dependent effect. The temperature increase in the microchamber was at least partially due to the reaction.
The reaction was also observed in microbubbles, which were formed by the coalescence of nanobubbles containing only H and only O gases. It is interesting to note that the reaction in microbubbles, which were formed differently, for example, by coalescence of microbubbles, is not going or at least going much slower. Combustion in a microbubble happens for a few microseconds and is accompanied by a large pressure jump in the chamber. The short-lived microbubbles start to appear when the concentration of nanobubbles is so large that they start to merge.
Observation of the reaction in small volumes lifts the fundamental restriction on the volume of the reaction chamber where combustion can proceed. It is too early to speculate on the possible application of the effect for microcombustors because the mechanism of the reaction is still unknown. For this reason, in this paper, we did not discuss applications. Nevertheless, it is worth mentioning that the reaction between hydrogen and oxygen in nanobubbles was successfully used to fabricate fast and strong microactuators [
22].