Polylactic Acid Improves the Rheological Properties, and Promotes the Degradation of Sodium Carboxymethyl Cellulose-Modified Alkali-Activated Cement
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Measurements
3. Results and Discussion
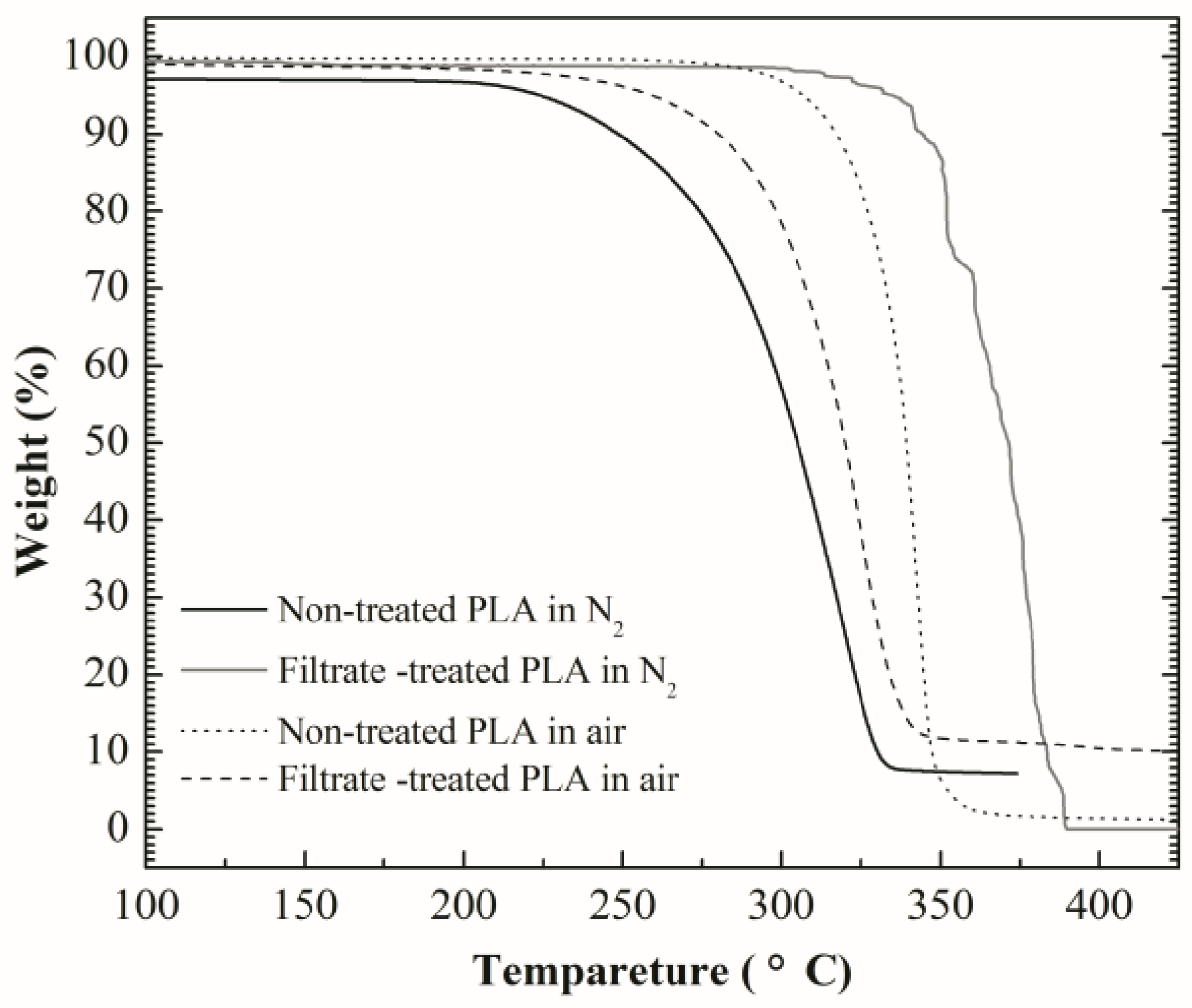
3.1. Thermogravimetric Analysis (TGA)
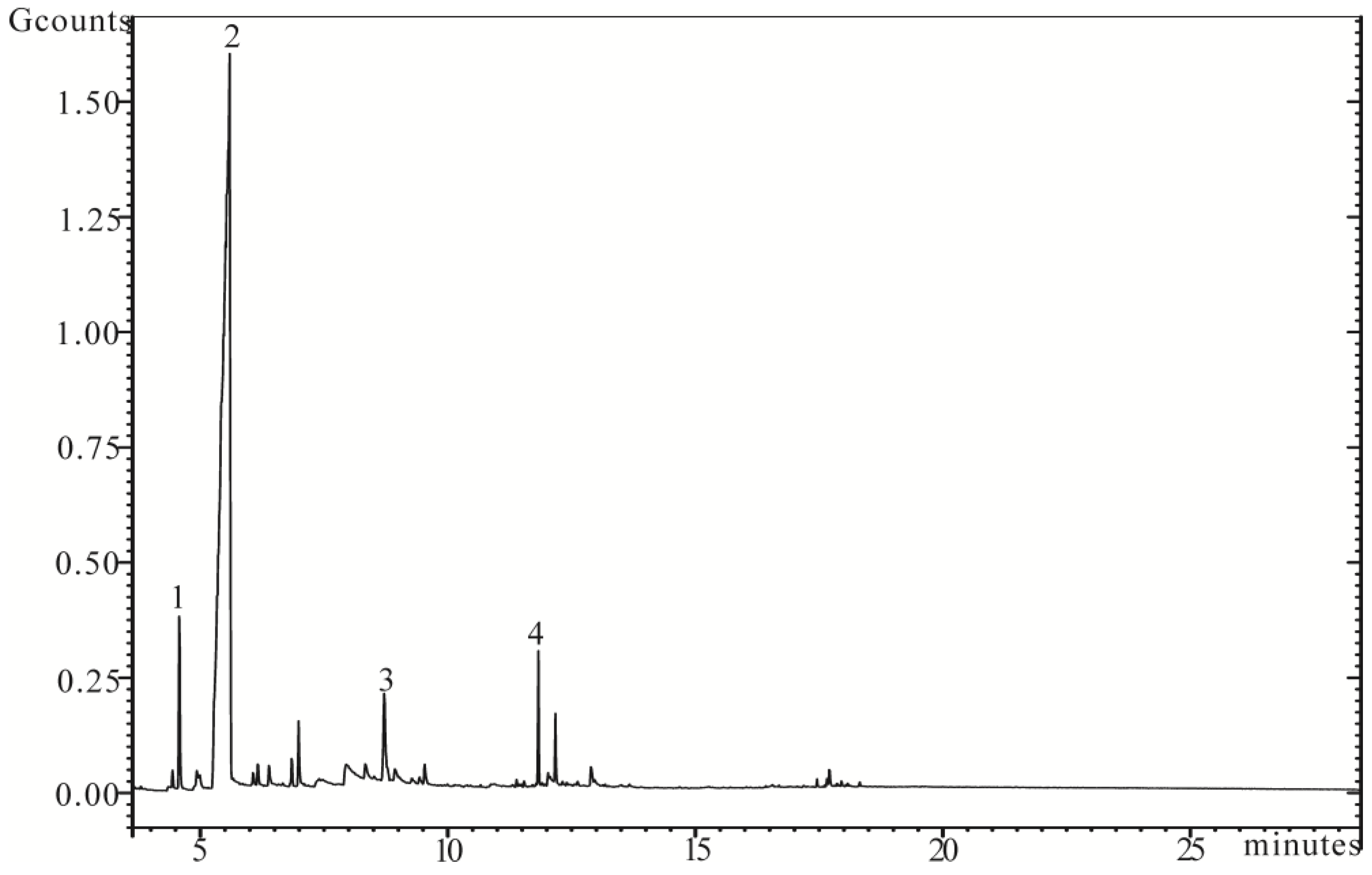
3.2. Pyrolysis-Gas Chromatography/Mass Spectroscopy (Py-GC/MS)
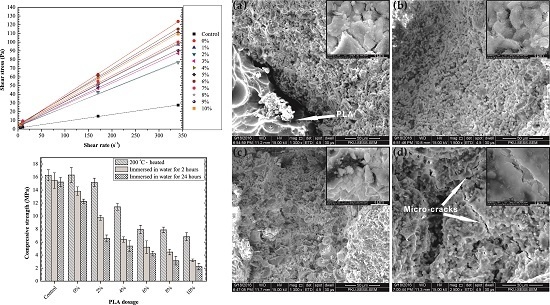
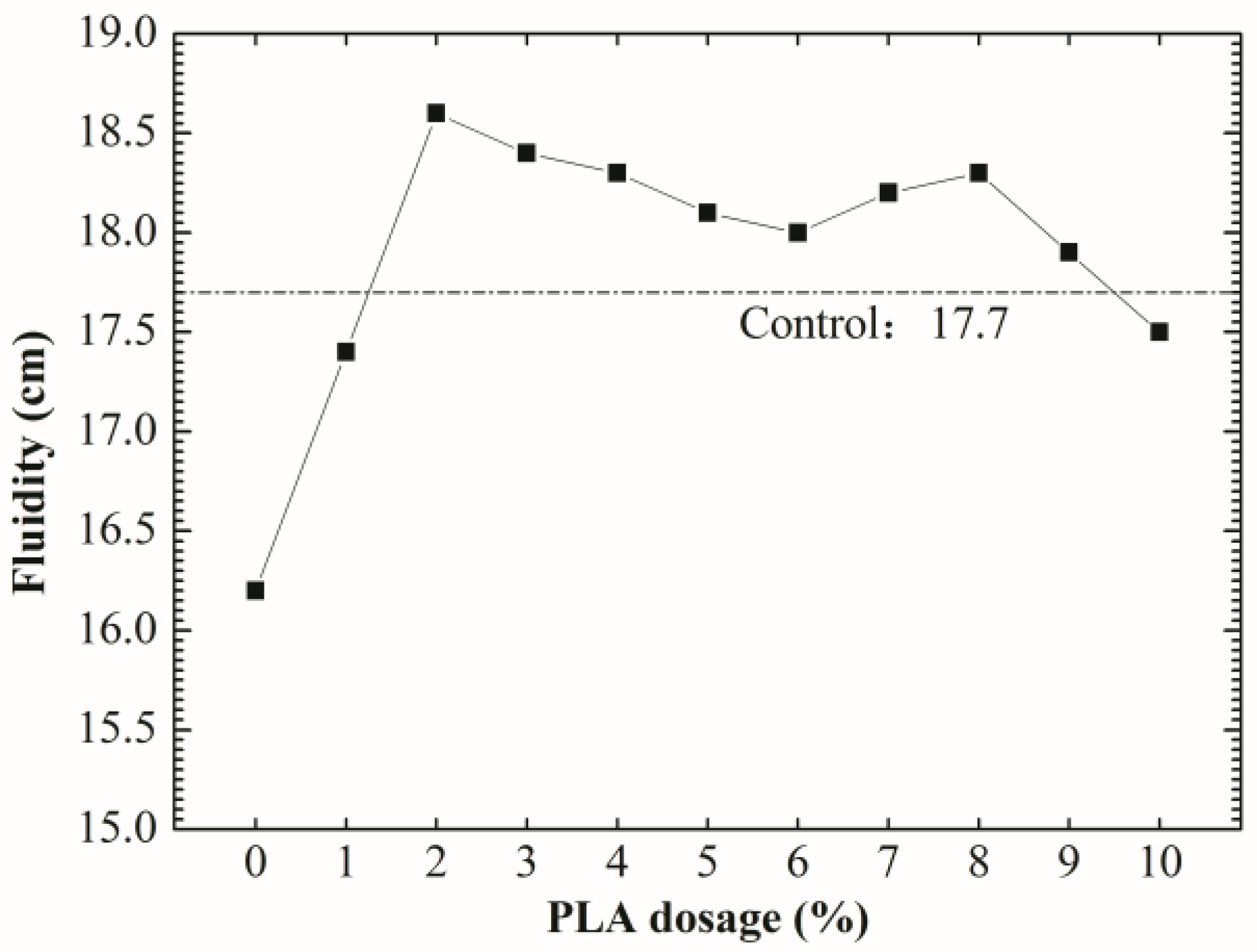
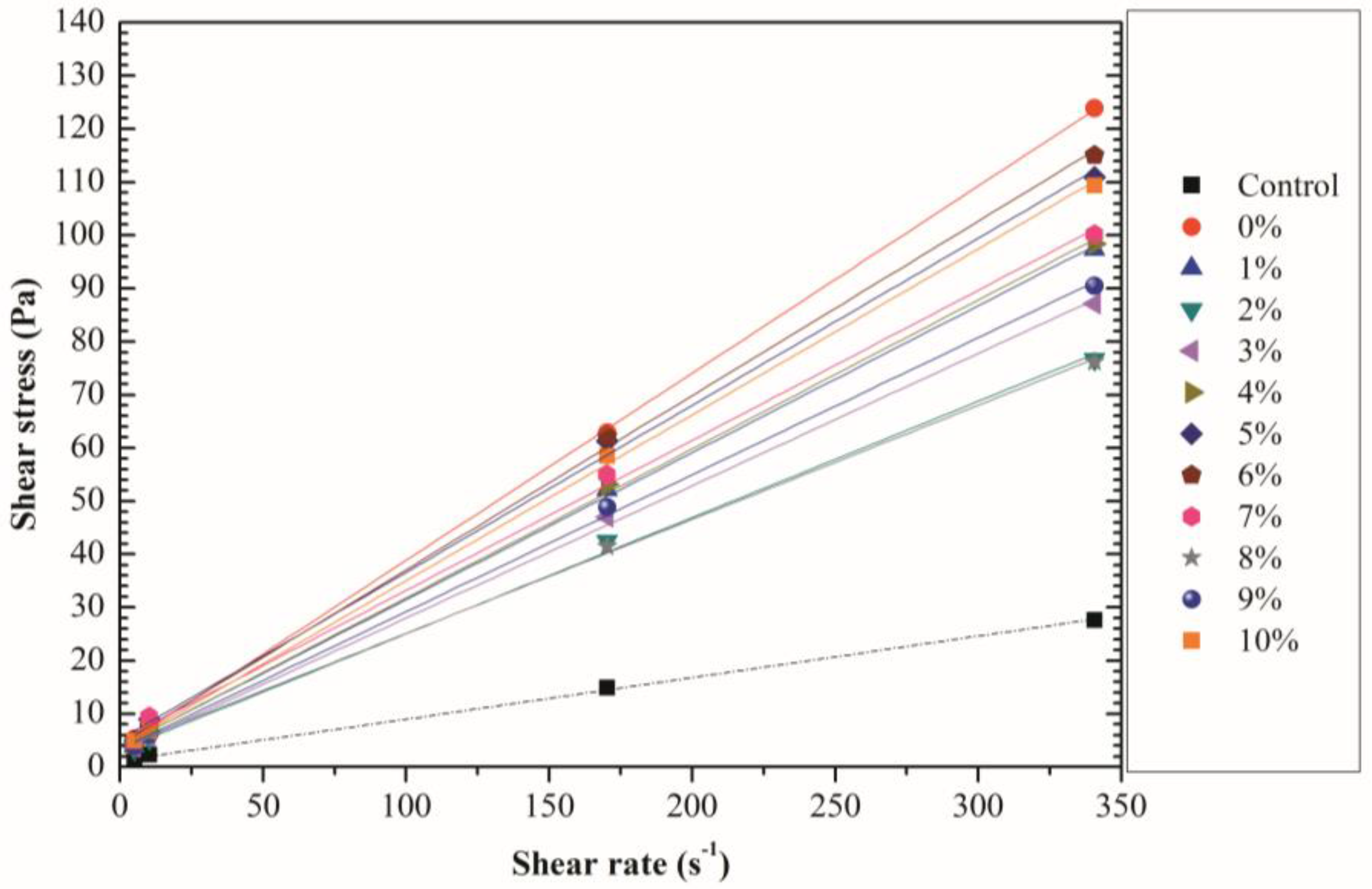
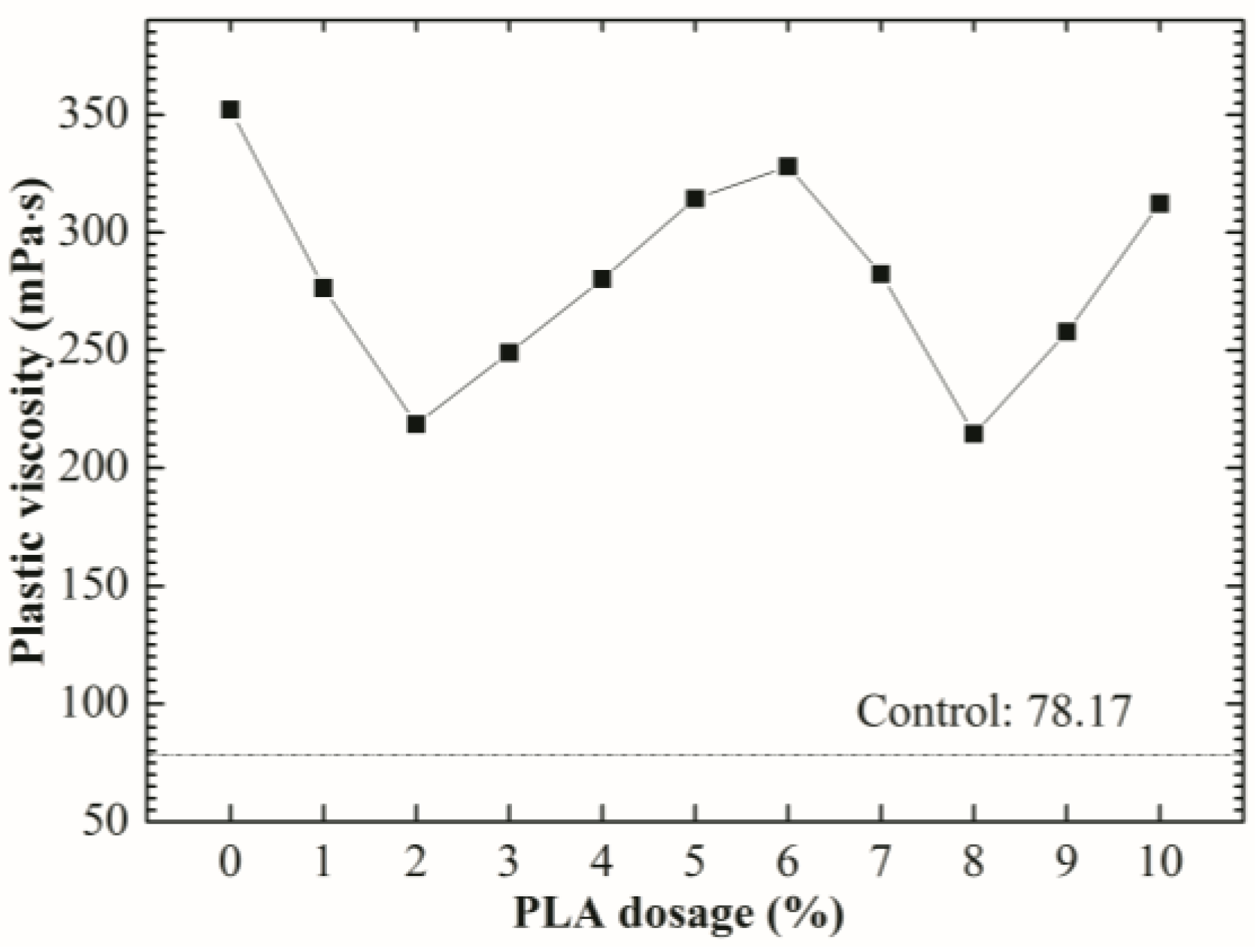
3.3. Rheological Properties
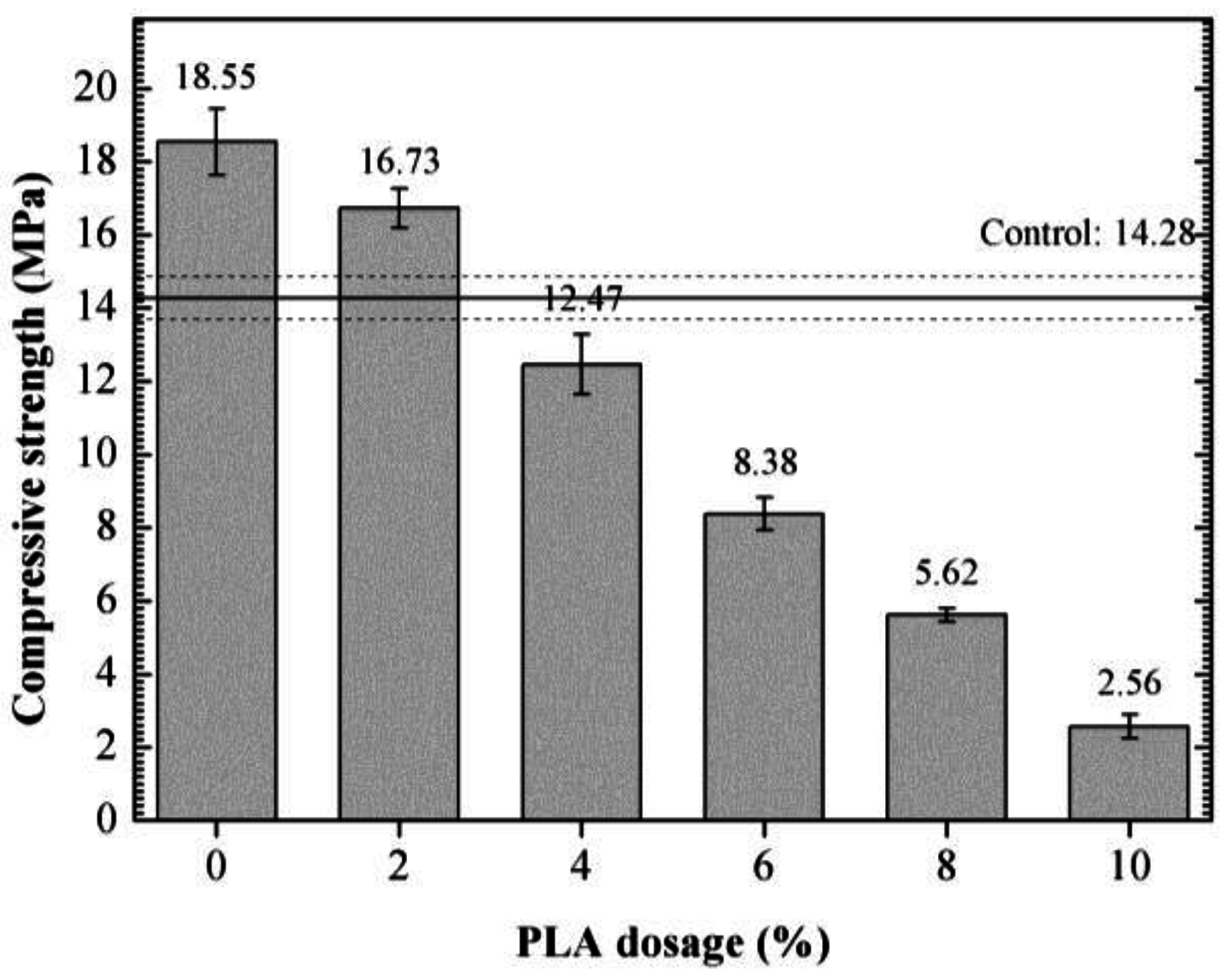
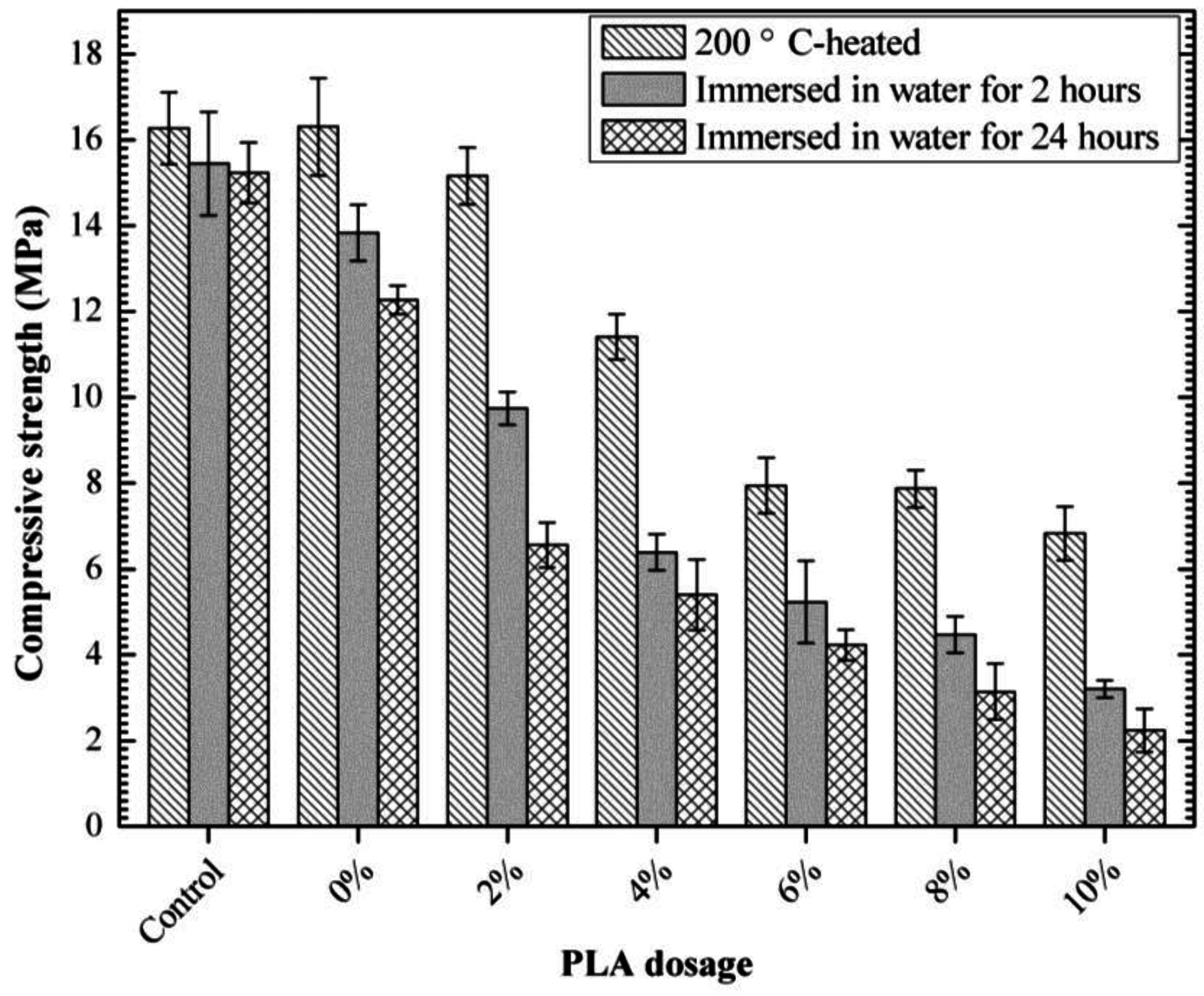
3.4. Compressive Strength of 85 °C-Cured Specimens
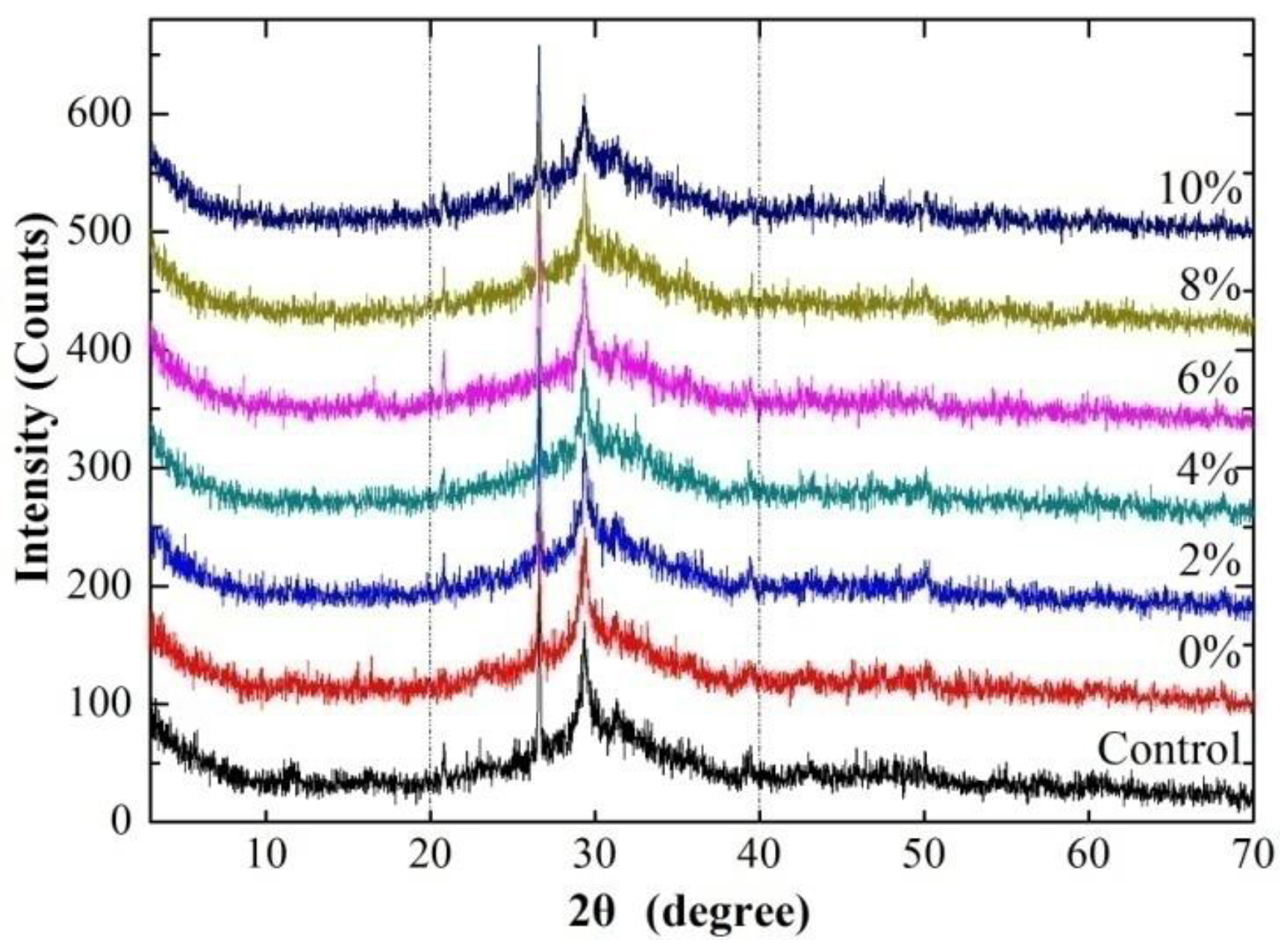
3.5. Powder X-ray Diffraction (XRD)
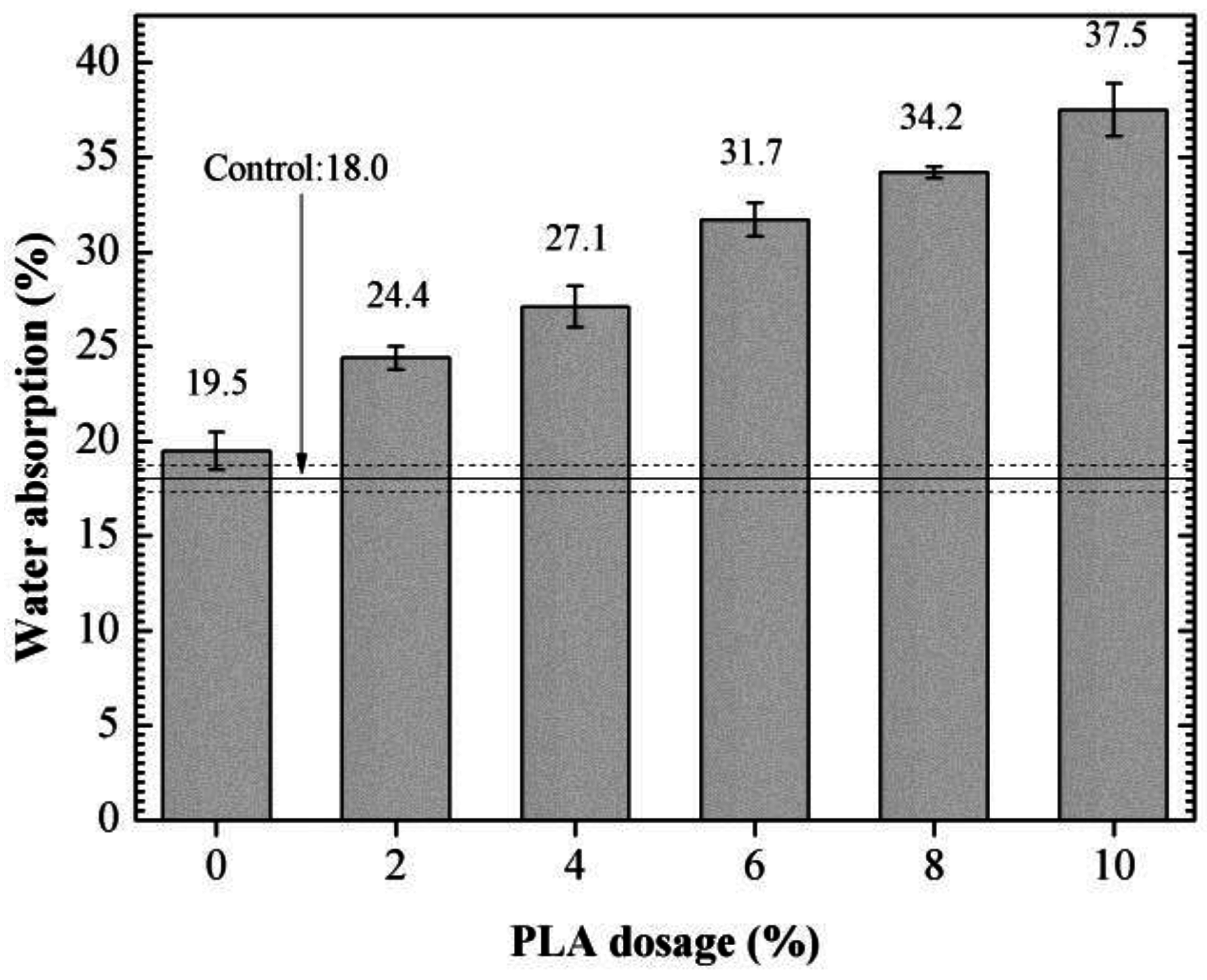
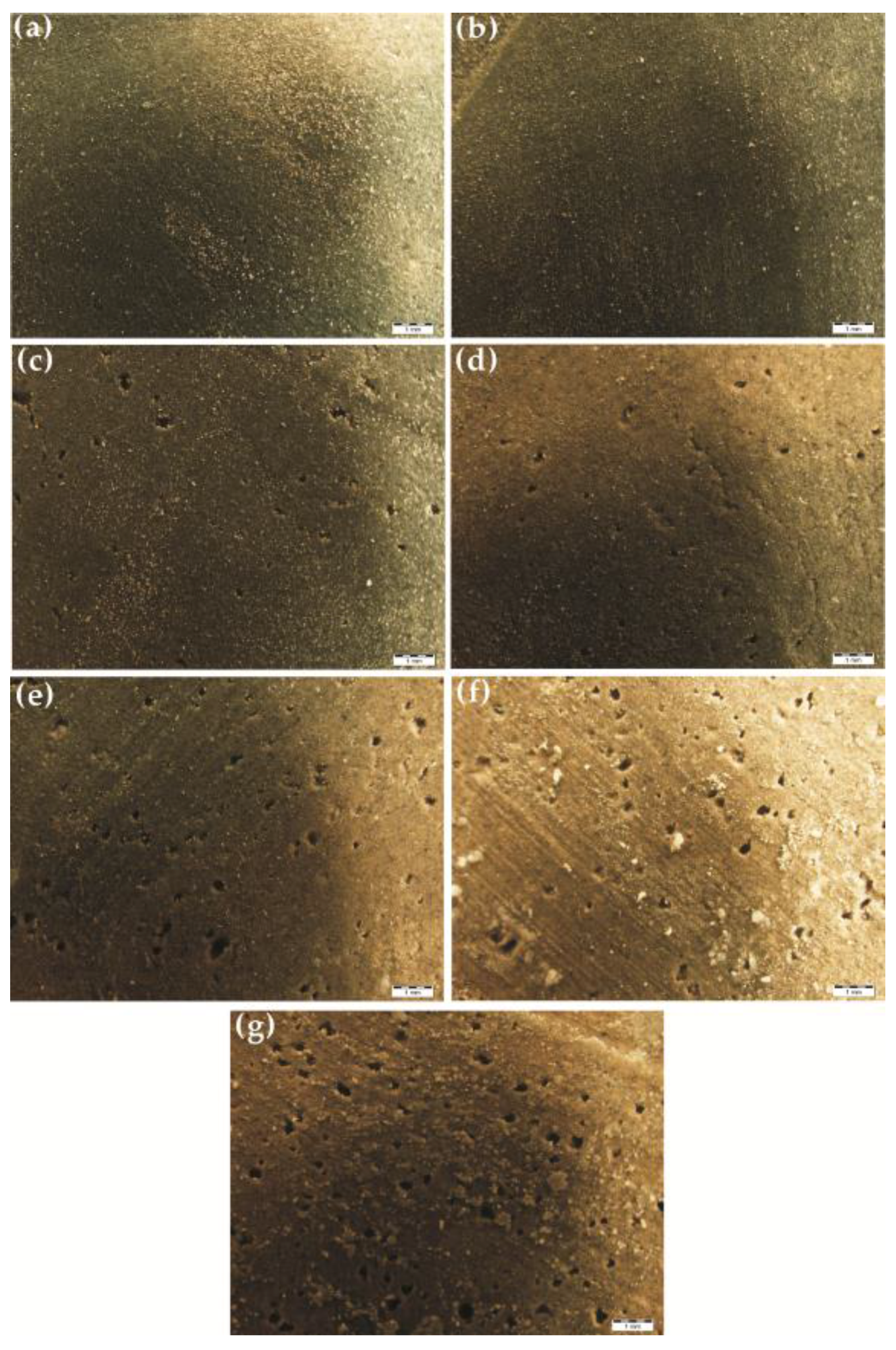
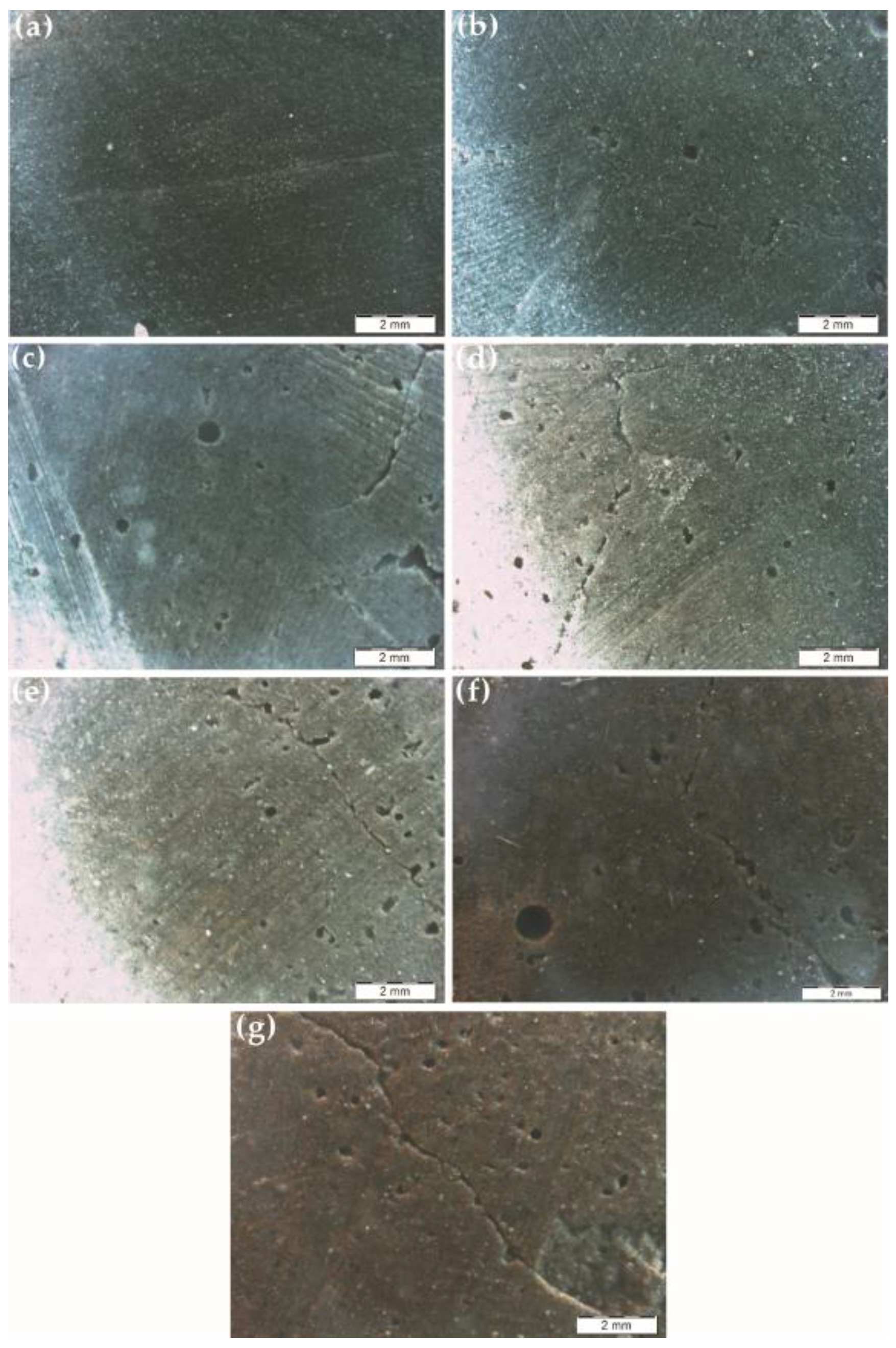
3.6. Water Absorption
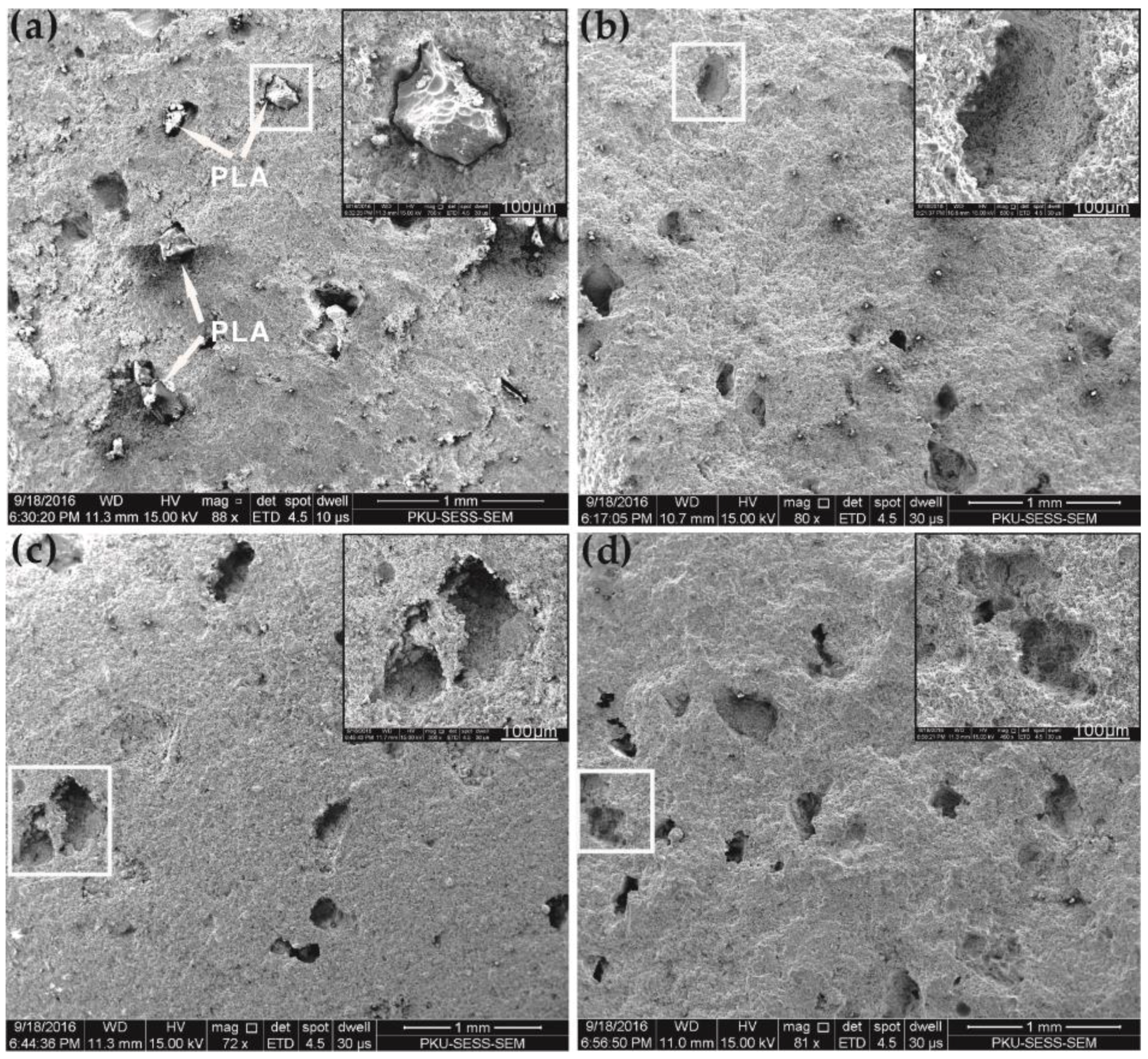
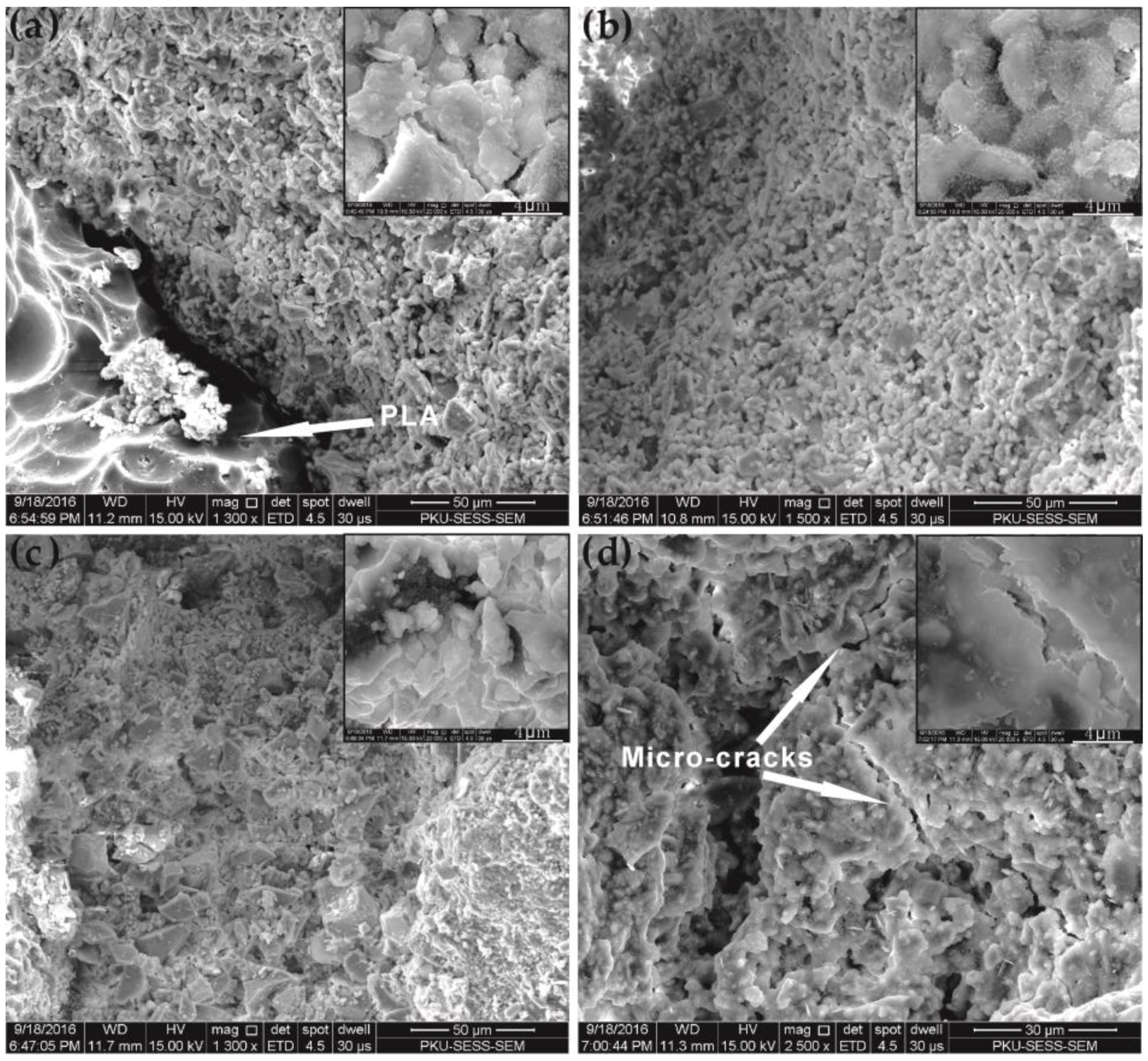
3.7. Self-Degradation
3.8. Self-Degradation Mechanism
4. Conclusions
- (1)
- The thermal decomposition temperature of PLA declined after treatment with filtrate of SDTSM. The main pyrolysis products of PLA are γ-butyrolactone, o-xylene, hexyl alcohol and 3-methylpent-1-en-3-ol.
- (2)
- The optimum PLA dosage is 2%. The addition of PLA increased the fluidity and reduced the viscosity of SDTSM, thus improving its rheological and pumping properties. 2% PLA enhanced the level of self-degradation, and ensured the SDTSM obtain a relatively larger 85 °C compressive strength to resume drilling.
- (3)
- PLA or CMC have a small effect on the types of hydrated products of the 85 °C-cured alkali activated slag/Class C fly ash cement, however, the hydrated products decrease as the dosage of PLA increases.
- (4)
- The existence of the large amount of pores generated by melting and thermal decomposition is the major reason for PLA enhancement of the self-degradation level of SDTSM. This works in two ways: first, the large amount of pores leads to a looser microstructure which results in lower strength; second, these pores make the water impregnate the cement more easily, and this made the dissolution progress faster to generate heat and destroy the microstructure of the cement, ultimately, facilitating the self-degradation.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olasolo, P.; Juarez, M.C.; Morales, M.P.; D’Amico, S.; Liarte, I.A. Enhanced geothermal systems (EGS): A review. Renew. Sustain. Energy Rev. 2016, 56, 133–144. [Google Scholar] [CrossRef]
- Preisig, G.; Eberhardt, E.; Gischig, V.; Roche, V.; Van der Baan, M.; Valley, B.; Kaiser, P.K.; Duff, D.; Lowther, R. Development of connected permeability in massive crystalline rocks through hydraulic fracture propagation and shearing accompanying fluid injection. Geofluids 2015, 15, 321–337. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Li, Z.; Yu, Z.; Xu, T.; Lan, C. Electricity generation and heating potential from enhanced geothermal system in Songliao Basin, China: Different reservoir stimulation strategies for tight rock and naturally fractured formations. Energy 2015, 93, 1860–1885. [Google Scholar] [CrossRef]
- Sugama, T.; Pyatina, T. Effect of sodium carboxymethyl celluloses on water-catalyzed self-degradation of 200 °C-heated alkali-activated cement. Cem. Concr. Compos. 2015, 55, 281–289. [Google Scholar] [CrossRef]
- Sugama, T.; Butcher, T.; Brothers, L. Temporary Cementitious Sealing Materials. In Proceedings of the Geothermal Resources Council 2011 Annual Meeting, San Diego, CA, USA, 23–26 October 2011.
- Li, F.Y. Experiments Research on Self-Degradable Slag/Fly Ash Cement for EGS Geothermal Wells. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2015. [Google Scholar]
- Gao, X.; Xu, Q.L.; Brouwers, H.J.H. Characterization of alkali activated slag-fly ash blends containing nano-silica. Constr. Build. Mater. 2015, 98, 397–406. [Google Scholar] [CrossRef]
- Puertas, F.; Fernández-Jiménez, A. Mineralogical and microstructural characterisation of alkali-activated fly ash/slag pastes. Cem. Concr. Compos. 2003, 25, 287–292. [Google Scholar] [CrossRef]
- Kastner, U.; Hoffmann, H.; Donges, R.; Hilbig, J. Structure and solution properties of sodium carboxymethyl cellulose. Colloids Surf. 1997, 123, 307–328. [Google Scholar] [CrossRef]
- Akther, S.; Hwang, J.; Lee, H. Sedimentation characteristics of two commercial bentonites in aqueous suspensions. Clay Miner. 2008, 43, 449–457. [Google Scholar] [CrossRef]
- Vipulanandan, C.; Mohammed, A. Smart cement rheological and piezoresistive behavior for oil well applications. J. Petrol. Sci. Eng. 2015, 135, 50–58. [Google Scholar] [CrossRef]
- Feng, S.Q.; Zhang, N.W.; Ren, J. Thermal Degradation and Stability of Poly Lactic Acid. Plastics 2011, 40, 59–62. [Google Scholar]
- Habraken, W.J.E.M.; Liao, H.B.; Zhang, Z.; Wolke, J.G.C.; Grijpma, D.W.; Mikos, A.G.; Feijen, J.; Jansen, J.A. In vivo degradation of calcium phosphate cement incorporated into biodegradable microspheres. Acta Biomater. 2010, 6, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yang, F.; Wolke, J.G.C.; Li, Y.; Jansen, J.A. Incorporation of biodegradable electrospun fibers into calcium phosphate cement for bone regeneration. Acta Biomater. 2010, 6, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Krueger, R.; Seitz, J.; Ewald, A.; Bach, F.; Groll, J. Strong and tough magnesium wire reinforced phosphate cement composites for load-bearing bone replacement. J. Mech. Behav. Biomed. Mater. 2013, 20, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, S.; Hayes, D.G.; Wadsworth, L.C.; Dunlap, R.N.; DeBruyn, J.M.; Lee, J.; Wszelaki, A.L. Soil Degradation of Polylactic Acid/Polyhydroxyalkanoate-Based Nonwoven Mulches. J. Polym. Environ. 2015, 23, 302–315. [Google Scholar] [CrossRef]
- Ingrao, C.; Tricase, C.; Cholewa-Wojcik, A.; Kawecka, A.; Rana, R.; Siracusa, V. Polylactic acid trays for fresh-food packaging: A Carbon Footprint assessment. Sci. Total Environ. 2015, 537, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Halliburton Energy Services Inc. Re-Fracturing a Fracture Stimulated Subterranean Formation. Patent WO2,015,105,488 (A1), 9 January 2014. [Google Scholar]
- Liang, F.; Reddy, B.R.; Nguyen, P.D. Tailoring Thermal, Mechanical, and Physical Properties of Degradable Polymers for Downhole Applications. In Proceedings of the 2014 AADE Fluids Technical Conference and Exhibition, Houston, TX, USA, 15–16 April 2014.
- Halliburton Energy Services Inc. Wellbore Servicing Materials and Methods of Making and Using Same. Patent US8714249B1, 6 May 2014. [Google Scholar]
- General Administration of Quality Supervision; Inspection and Quarantine of the People’s Republic of China; Standardization Administration of the People’s Republic of China. Method for Testing Uniformity of Concrete Admixture, 2nd ed.Standards Press of China: Beijing, China, 2012; pp. 15–16.
- American Petroleum Institute. Recommended Practice for Testing Well Cements, 2nd ed.; API Publishing Services: Washington, DC, USA, 2013; pp. 59–65. [Google Scholar]
- General Administration of Quality Supervision; Inspection and Quarantine of the People’s Republic of China. Standardization Administration of the People’s Republic of China, Oil Well Cement, 3rd ed.Standards Press of China: Beijing, China, 2005; pp. 13–16.
- National Energy Administration. Performance Requirements for Oil Well Cement Slurries, 2nd ed.Petroleum Industry Press: Beijing, China, 2010; pp. 1–3.
- Hakamy, A.; Shaikh, F.U.A.; Low, I.M. Characteristics of nanoclay and calcined nanoclay-cement nanocomposites. Comp. B Eng. 2015, 78, 174–184. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E. Influence of the type of buffer solution on thermal and structural properties of polylactide-based composites. Polym. Degrad. Stab. 2016, 129, 87–95. [Google Scholar] [CrossRef]
- Stloukal, P.; Kalendova, A.; Mattausch, H.; Laske, S.; Holzer, C.; Koutny, M. The influence of a hydrolysis-inhibiting additive on the degradation and biodegradation of PLA and its nanocomposites. Polym. Test. 2015, 41, 124–132. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, C.N.; Song, J.; Qiu, J.H. Analysis of the Factors Influencing Degradability and Degradation Mechanism of Polylactic Acid. Pack Eng. 2008, 29, 16–19. [Google Scholar]
- Zhang, X.; Espiritu, M.; Bilyk, A.; Kurniawan, L. Morphological behavior of poly (lactic acid) during hydrolytic degradation. Polym. Degrad. Stab. 2008, 93, 1964–1970. [Google Scholar] [CrossRef]
- Cam, D.; Marucci, M. Influence of residual monomers and metals on poly (l-lactide) thermal stability. Polymer 1997, 38, 1879–1884. [Google Scholar] [CrossRef]
- Zhang, S.G. Handbook of the Practical Fine Chemicals, 2nd ed.; Chemical Industry Press: Beijing, China, 1996; p. 513. [Google Scholar]
- Zhang, Z.L.; Sui, T.B.; Chen, F.Y.; Wen, S.J.; Shi, H.X.; Wang, J.; Fan, L. Determined therheological properties of HBC cement paste slurry using rotational viscometer. Liaoning Build. Mater. 2005, 1, 18–20. [Google Scholar]
- Domone, P.; HsiWen, C. Testing of binders for high performance concrete. Cem. Concr. Res. 1997, 27, 1141–1147. [Google Scholar] [CrossRef]
- Assaad, J.J. Correlating water extraction to viscosity variations of injection grouts. Constr. Build. Mater. 2015, 77, 74–82. [Google Scholar] [CrossRef]
- Celik, F.; Canakci, H. An investigation of rheological properties of cement-based grout mixed with rice husk ash (RHA). Constr. Build. Mater. 2015, 91, 187–194. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, D.; Yang, C.; Liu, Y.Y.; Liu, Y. Effect of graphene oxide on the rheological properties of cement pastes. Constr. Build. Mater. 2015, 96, 20–28. [Google Scholar] [CrossRef]
- Bentz, D.P.; Ferraris, C.F.; Galler, M.A.; Hansen, A.S.; Guynn, J.M. Influence of particle size distributions on yield stress and viscosity of cement-fly ash pastes. Cem. Concr. Res. 2012, 42, 404–409. [Google Scholar] [CrossRef]
- Vance, K.; Kumar, A.; Sant, G.; Neithalath, N. The rheological properties of ternary binders containing Portland cement, limestone, and metakaolin or fly ash. Cem. Concr. Res. 2013, 52, 196–207. [Google Scholar] [CrossRef]
- Abdalqader, A.F.; Jin, F.; Al-Tabbaa, A. Development of greener alkali-activated cement: Utilisation of sodium carbonate for activating slag and fly ash mixtures. J. Clean. Prod. 2016, 113, 66–75. [Google Scholar] [CrossRef]
- Abdalqader, A.F.; Jin, F.; Al-Tabbaa, A. Characterisation of reactive magnesia and sodium carbonate-activated fly ash/slag paste blends. Constr. Build. Mater. 2015, 93, 506–513. [Google Scholar] [CrossRef]
- Shi, C.J.; Krivenko, P.V.; Roy, D. Alkali-Activated Cements and Concretes, 1st ed.; Chemical Industry Press: Beijing, China, 2008; pp. 70–74. [Google Scholar]
- Kumar, S.; Kumar, R. Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Avirneni, D.; Peddinti, P.R.T.; Saride, S. Durability and long term performance of geopolymer stabilized reclaimed asphalt pavement base courses. Constr. Build. Mater. 2016, 121, 198–209. [Google Scholar] [CrossRef]
- Francis, A.A. Crystallization kinetics of magnetic glass-ceramics prepared by the processing of waste materials. Mater. Res. Bull. 2006, 41, 1146–1154. [Google Scholar] [CrossRef]
- Jeon, D.; Jun, Y.; Jeong, Y.; Oh, J.E. Microstructural and strength improvements through the useof Na2CO3 in a cementless Ca(OH)2-activated Class F fly ash system. Cem. Concr. Res. 2015, 67, 215–225. [Google Scholar] [CrossRef]
- Thokchom, S.; Ghosh, P.; Ghosh, S. Effect of water absorption, porosity and sorptivity on durability of geopolymer mortars. J. Eng. Appl. Sci. 2009, 4, 28–34. [Google Scholar]
- Hossain, M.M.; Karim, M.R.; Hossain, M.K.; Islam, M.N.; Zain, M.F.M. Durability of mortar and concrete containing alkali-activated binder with pozzolans: A review. Constr. Build. Mater. 2015, 93, 95–109. [Google Scholar] [CrossRef]
- Habib, A.O.; Aiad, I.; Youssef, T.A.; Abd El-Aziz, A.M. Effect of some chemical admixtures on the physico-chemical and rheological properties of oil well cement pastes. Constr. Build. Mater. 2016, 120, 80–88. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Li, L.F.; Ma, X.; Wang, H. Compositional, microstructural and mechanical properties of ambient condition cured alkali-activated cement. Constr. Build. Mater. 2016, 113, 237–245. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, C.; Zeng, Q.P.; Yang, C.H.; Luo, C.Q.; Yang, K. Deterioration of mortars exposed to sulfate attack under electrical field. Constr. Build. Mater. 2016, 117, 121–128. [Google Scholar] [CrossRef]
- Norambuena-Contreras, J.; Thomas, C.; Borinaga-Treviño, R.; Lombillo, I. Influence of recycled carbon powder waste addition on the physical and mechanical properties of cement pastes. Mater. Struct. 2016, 49, 5147–5159. [Google Scholar] [CrossRef]
- Stora, E.; Bary, B.; He, Q.C.; Deville, E.; Montarnal, P. Modelling and simulations of the chemo-mechanical behaviour of leached cement-based materials Leaching process and induced loss of stiffness. Cem. Concr. Res. 2009, 39, 763–772. [Google Scholar] [CrossRef]
- Heukamp, F.H.; Ulm, F.J.; Germaine, J.T. Mechanical properties of calcium-leached cement pastes: Triaxial stress states and the influence of the pore pressures. Cem. Concr. Res. 2001, 31, 767–774. [Google Scholar] [CrossRef]
- Kuhl, D.; Bangert, F.; Meschke, G. Coupled chemo-mechanical deterioration of cementitious materials—Part II: Numerical methods and simulations. Int. J. Solids Struct. 2004, 41, 41–67. [Google Scholar] [CrossRef]
| Physical Properties | Typical Values |
|---|---|
| Density (g/cm3) | 1.25 ± 0.05 |
| Melting temperature (°C) | 155~165 |
| Glass transition temperature (°C) | 56~60 |
| Tensile strength (MPa) | ≥5 |
| Elongation at break (%) | ≥3.0 |
| Impact strength (kJ/m3, Izod) | 1~3 |
| Number | Retention Time (min) | Compounds | Peak Area % of Total |
|---|---|---|---|
| 1 | 4.577 | o-Xylene | 3.29 |
| 2 | 5.514 | γ-Butyrolactone | 82.91 |
| 3 | 8.717 | Hexyl alcohol | 2.95 |
| 4 | 11.829 | 3-Methylpent-1-en-3-ol | 1.68 |
| PLA Dosage (%) | Shear Rates (s−1) | |||
|---|---|---|---|---|
| 5.11 | 10.21 | 170 | 340 | |
| Control | 1.02 | 2.30 | 14.82 | 27.59 |
| 0 | 5.37 | 7.67 | 62.85 | 123.92 |
| 1 | 3.83 | 7.41 | 52.12 | 97.35 |
| 2 | 3.32 | 5.37 | 42.41 | 76.65 |
| 3 | 3.58 | 5.62 | 47.01 | 87.13 |
| 4 | 4.34 | 6.64 | 53.14 | 98.37 |
| 5 | 4.85 | 8.94 | 61.32 | 110.89 |
| 6 | 5.11 | 7.41 | 62.09 | 114.98 |
| 7 | 3.83 | 9.45 | 54.93 | 100.16 |
| 8 | 4.34 | 5.62 | 41.39 | 76.14 |
| 9 | 3.83 | 6.13 | 48.80 | 90.45 |
| 10 | 4.85 | 6.64 | 58.51 | 109.35 |
| PLA Dosage (%) | RMSE | |||
|---|---|---|---|---|
| Control | 1.14954 | 78.17 | 0.99878 | 0.53179 |
| 0 | 3.62560 | 352.13 | 0.99991 | 0.66444 |
| 1 | 3.80992 | 276.44 | 0.99924 | 1.4894 |
| 2 | 3.17759 | 218.16 | 0.99837 | 1.7229 |
| 3 | 3.07989 | 248.97 | 0.99926 | 1.3228 |
| 4 | 3.76898 | 280.15 | 0.99930 | 1.4440 |
| 5 | 5.15655 | 314.27 | 0.99831 | 2.5178 |
| 6 | 4.25199 | 327.94 | 0.99931 | 1.6738 |
| 7 | 4.94911 | 282.35 | 0.99774 | 2.6160 |
| 8 | 3.64514 | 214.57 | 0.99943 | 1.0007 |
| 9 | 3.38908 | 257.79 | 0.99893 | 1.3432 |
| 10 | 3.75633 | 312.99 | 0.99931 | 1.3058 |
| Pore Diameter (μm) | PLA | Slag | Fly Ash |
|---|---|---|---|
| >550 | 0.0903 | 0 | 0 |
| 550~325 | 25.67676 | 0 | 0 |
| 325~250 | 60.11703 | 0 | 0 |
| 250~120 | 12.70065 | 53.76577 | 0.56842 |
| 120~106 | 1.04332 | 11.69448 | 6.977688 |
| <106 | 0.371942 | 34.53976 | 92.45389 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.; Zheng, X.; Duan, C.; Xia, B. Polylactic Acid Improves the Rheological Properties, and Promotes the Degradation of Sodium Carboxymethyl Cellulose-Modified Alkali-Activated Cement. Energies 2016, 9, 823. https://doi.org/10.3390/en9100823
Tan H, Zheng X, Duan C, Xia B. Polylactic Acid Improves the Rheological Properties, and Promotes the Degradation of Sodium Carboxymethyl Cellulose-Modified Alkali-Activated Cement. Energies. 2016; 9(10):823. https://doi.org/10.3390/en9100823
Chicago/Turabian StyleTan, Huijing, Xiuhua Zheng, Chenyang Duan, and Bairu Xia. 2016. "Polylactic Acid Improves the Rheological Properties, and Promotes the Degradation of Sodium Carboxymethyl Cellulose-Modified Alkali-Activated Cement" Energies 9, no. 10: 823. https://doi.org/10.3390/en9100823