Development and Characterization of Non-Conventional Micro-Porous Layers for PEM Fuel Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation
| Sample | CB/W | CB/T | CB/IPA | Polymer/CB |
|---|---|---|---|---|
| GDM-PTFE | 0.13 | 5.6 | – | 0.12 PTFE |
| GDM-PFPE-12 | – | – | 7.72 | 0.12 P56 |
| GDM-PFPE-6 | – | – | 7.72 | 0.06 P56 |
2.2. Characterization
3. Results and Discussion
3.1. Physical Characterization
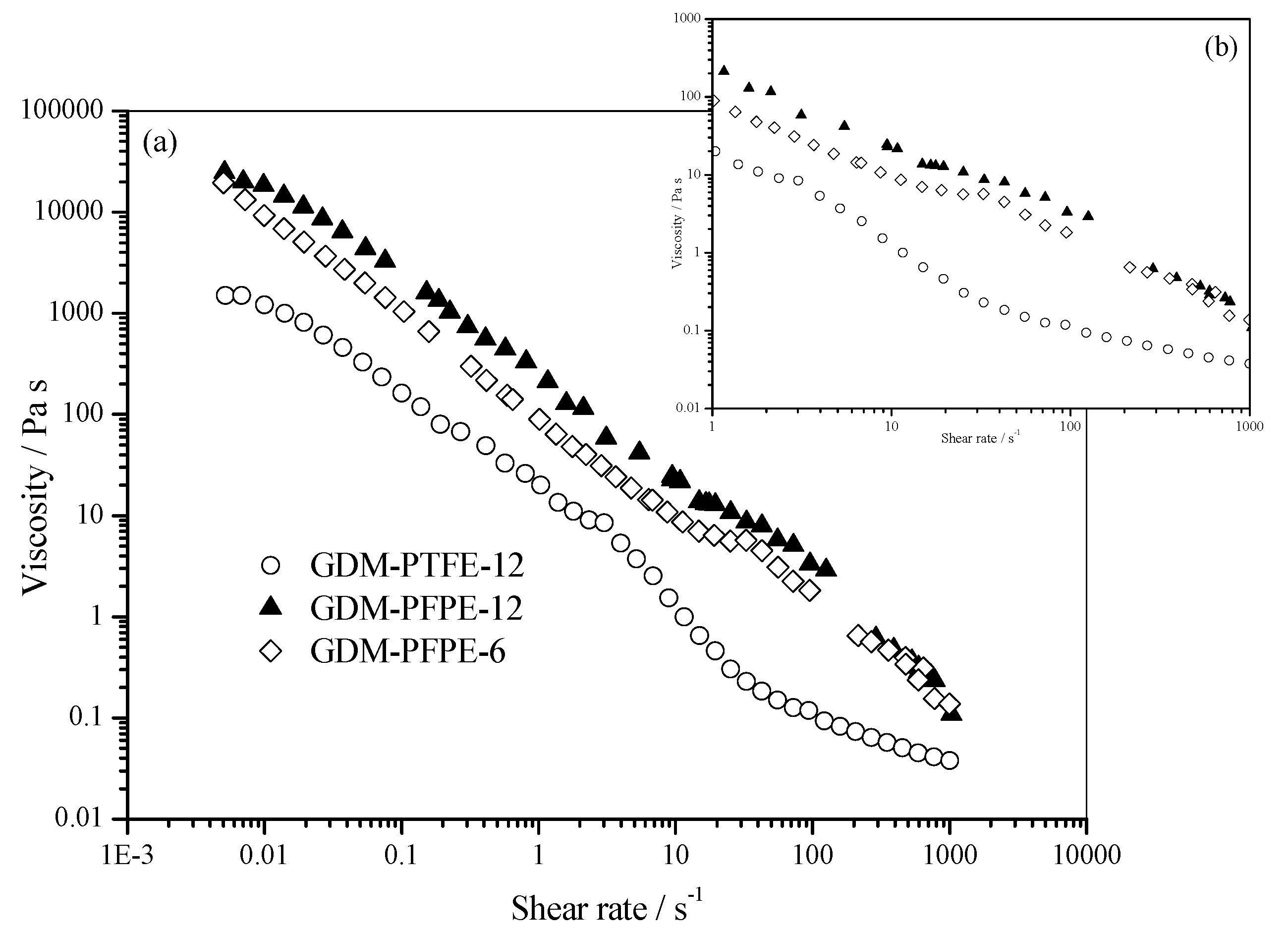
| Sample | BCT [°] | ACT-A [°] | ACT-C [°] |
|---|---|---|---|
| GDM-PTFE | 145 ± 4 | 146 ± 2 | 146 ± 2 |
| GDM-PFPE-12 | 151 ± 6 | 149 ± 5 | 151 ± 2 |
| GDM-PFPE-6 | 146 ± 5 | 154 ± 3 | 145 ± 2 |


3.2. Electrochemical Characterization
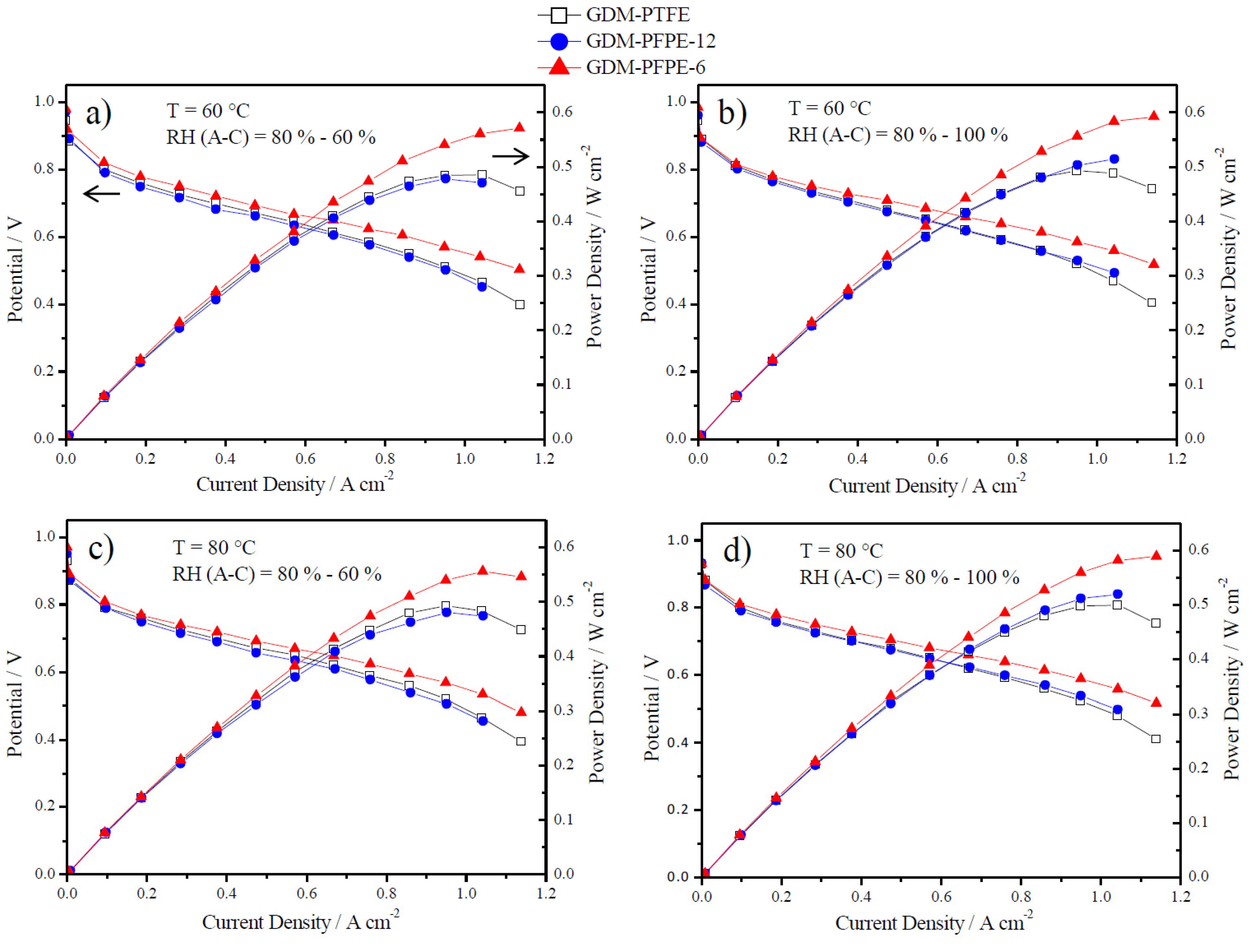
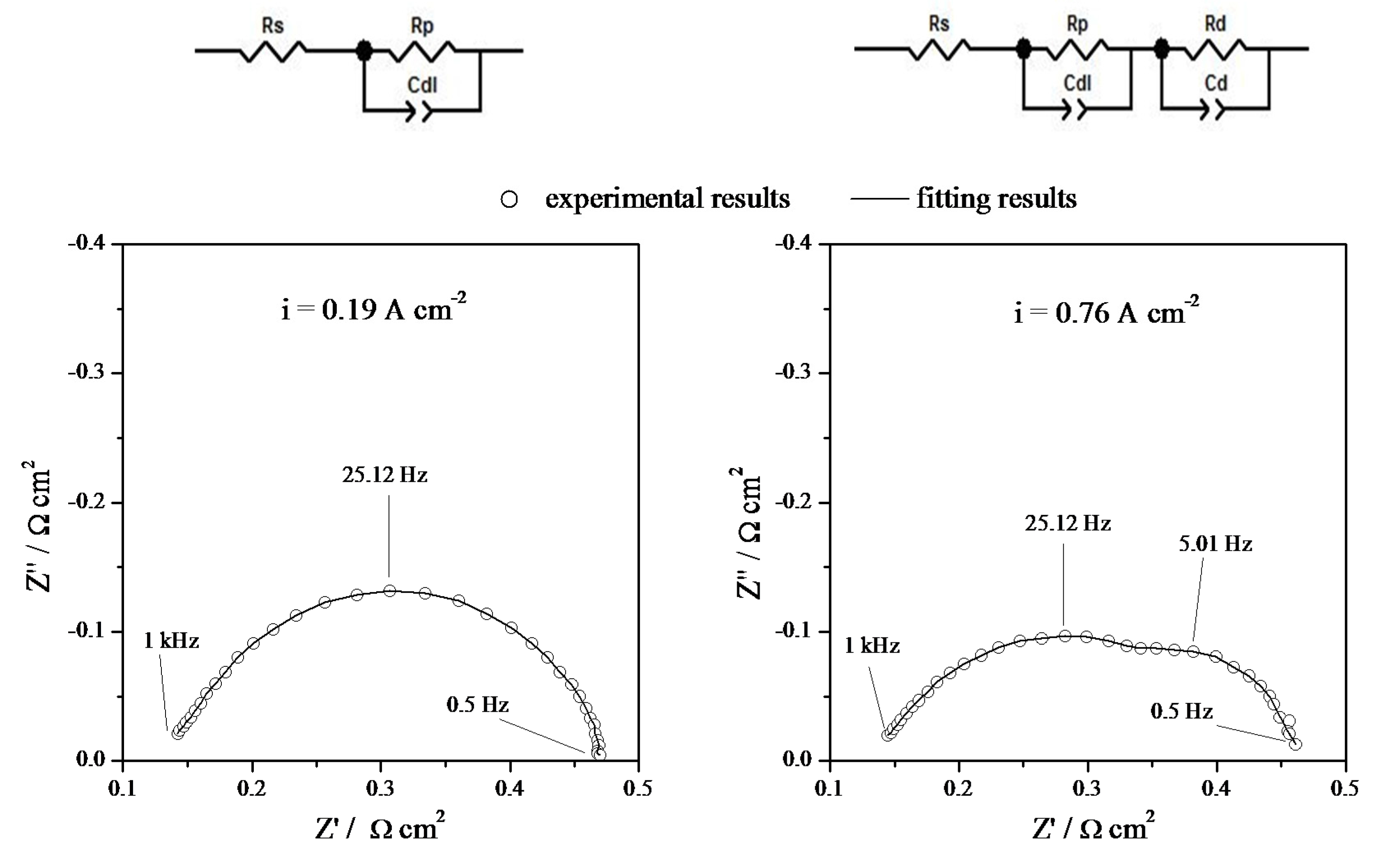
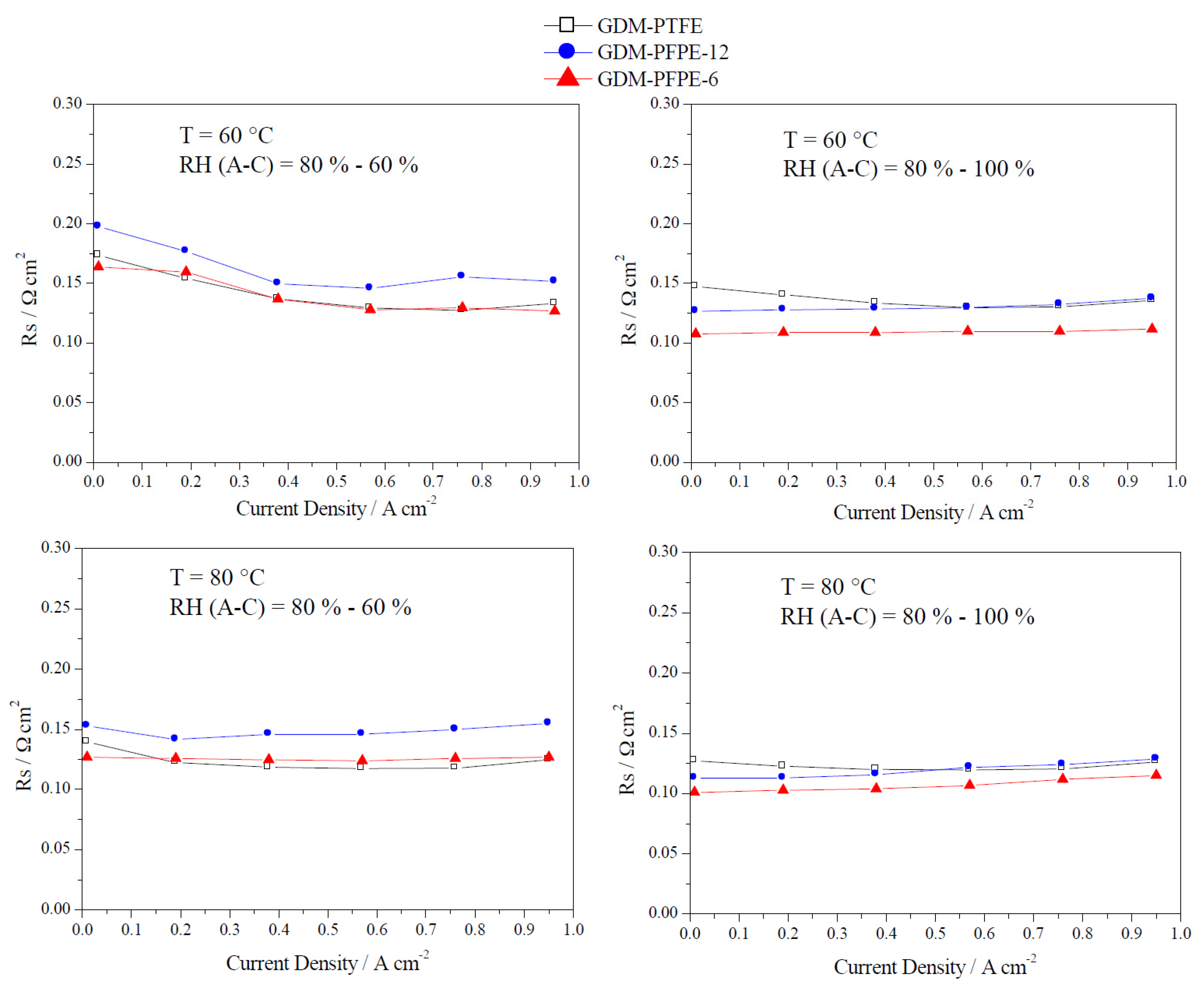
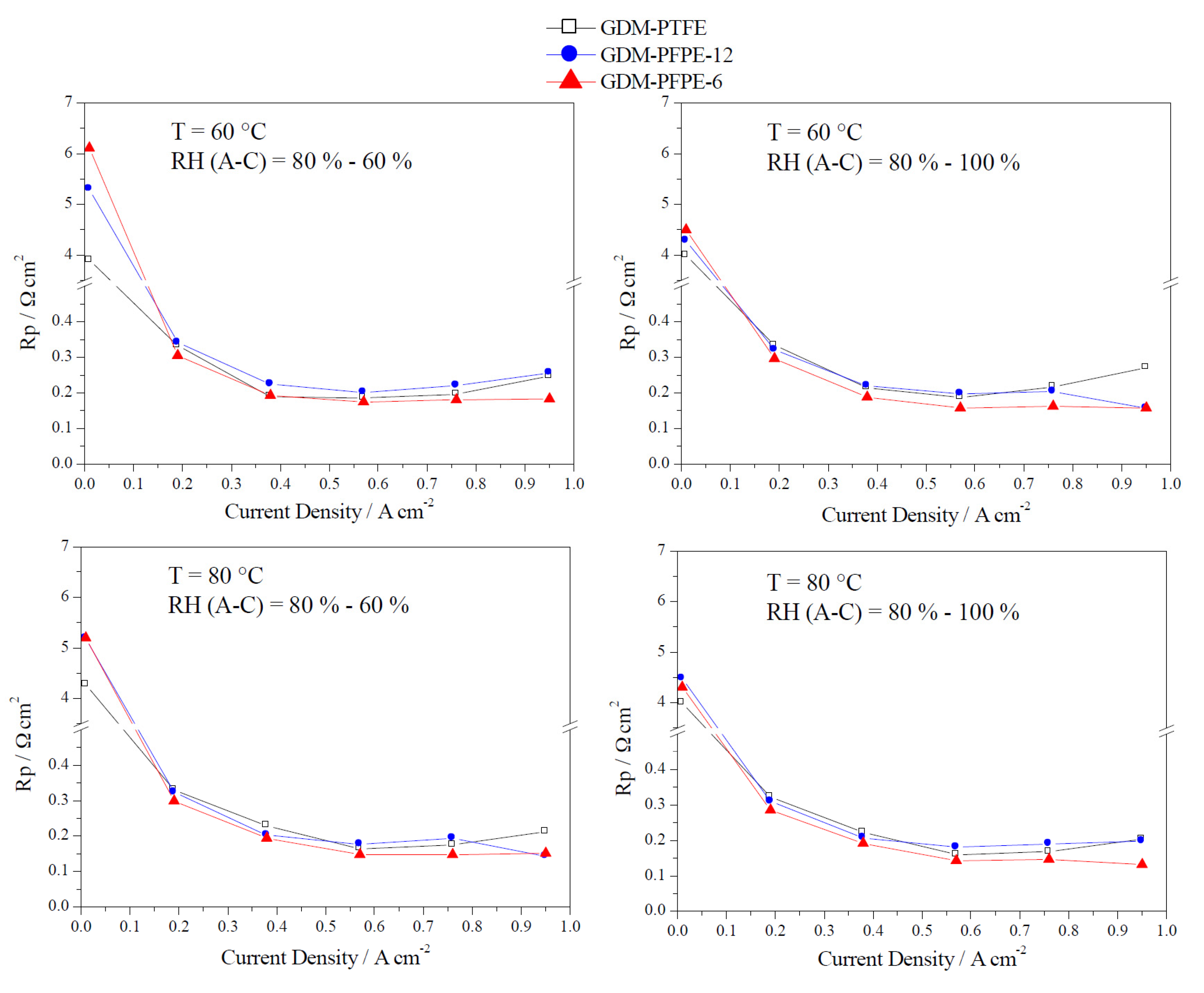
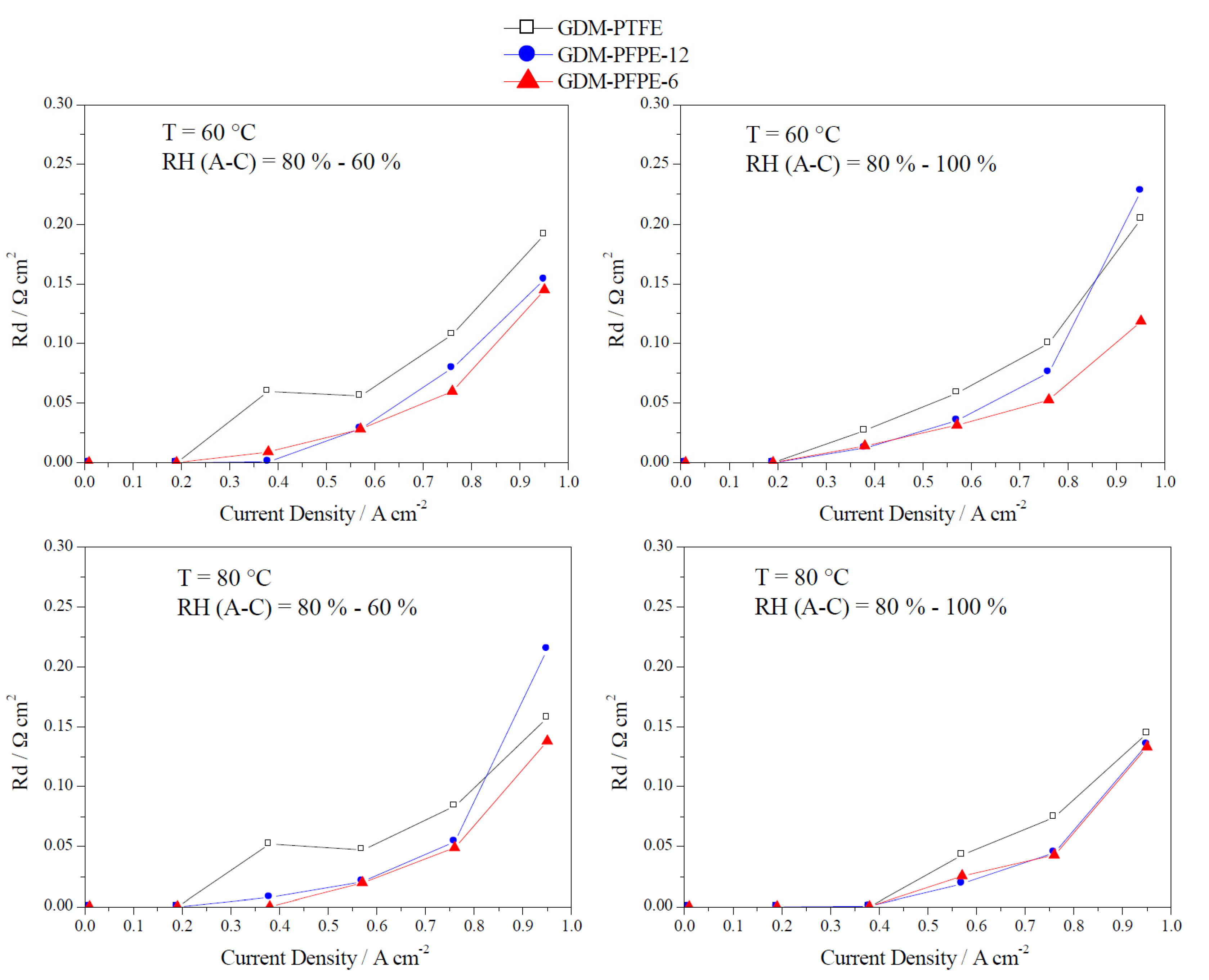
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Nagl, S.; Fursch, M.; Paulus, M.; Richter, J.; Truby, J.; Lindenberger, D. Energy policy scenarios to reach challenging climate protection targets in the German electricity sector until 2050. Util. Policy 2011, 19, 185–192. [Google Scholar] [CrossRef]
- Connolly, D.; Lund, H.; Mathiesen, B.V.; Werner, S.; Moller, B.; Persson, U.; Boermans, T.; Trier, D.; Ostergaard, P.A.; Nielsen, S. Heat roadmap europe: Combining district heating with heat savings to decarbonise the EU energy system. Energy Policy 2014, 65, 475–489. [Google Scholar] [CrossRef]
- Wee, J.-H. Applications of proton exchange membrane fuel cell systems. Renew. Sustain. Energy Rev. 2007, 11, 1720–1738. [Google Scholar] [CrossRef]
- Costamagna, P.; Srinivasan, S. Quantum jumps in the PEMFC science and technology from the 1960s to the year 2000 Part I. Fundamental scientific aspects. J. Power Sources 2001, 102, 242–252. [Google Scholar] [CrossRef]
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sources 2003, 114, 32–53. [Google Scholar] [CrossRef]
- Chalk, S.G.; Miller, J.F.; Wagner, F.W. Challenges for fuel cells in transport applications. J. Power Sources 2000, 86, 40–51. [Google Scholar] [CrossRef]
- Ji, M.B.; Wei, Z.D. A Review of Water Management in Polymer Electrolyte Membrane Fuel Cells. Energies 2009, 2, 1057–1106. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Morgan, J.M.; Datta, R. Understanding the gas diffusion layer in proton exchange membrane fuel cells. I. How its structural characteristics affect diffusion and performance. J. Power Sources 2014, 251, 269–278. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.W.; Popov, B.N. A review of gas diffusion layer in PEM fuel cells: Materials and designs. Int. J. Hydrogen Energy 2012, 37, 5850–5865. [Google Scholar] [CrossRef]
- Wilkinson, D.P.; Zhang, J.; Hui, R.; Fergus, J.; Li, X. Proton Exchange Membrane Fuel Cells: Matersials Properties and Performance; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Barbir, F. PEM Fuel Cells: Theory and Practice, 2nd ed.; Elsevier: London, UK, 2013. [Google Scholar]
- Wang, Y.; Wang, C.Y.; Chen, K.S. Elucidating differences between carbon paper and carbon cloth in polymer electrolyte fuel cells. Electrochim. Acta 2007, 52, 3965–3975. [Google Scholar] [CrossRef]
- Frey, T.; Linardi, M. Effects of membrane electrode assembly preparation on the polymer electrolyte membrane fuel cell performance. Electrochim. Acta 2004, 50, 99–105. [Google Scholar] [CrossRef]
- Qi, Z.G.; Kaufman, A. Improvement of water management by a microporous sublayer for PEM fuel cells. J. Power Sources 2002, 109, 38–46. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.-W.; Popov, B.N. Effect of PTFE content in microporous layer on water management in PEM fuel cells. J. Power Sources 2008, 177, 457–463. [Google Scholar] [CrossRef]
- Weber, A.Z.; Newman, J. Effects of microporous layers in polymer electrolyte fuel cells. J. Electrochem. Soc. 2005, 152, A677–A688. [Google Scholar] [CrossRef]
- Park, S.; Popov, B.N. Effect of hydrophobicity and pore geometry in cathode GDL on PEM fuel cell performance. Electrochim. Acta 2009, 54, 3473–3479. [Google Scholar] [CrossRef]
- Lee, E.; Yang, K.; Jung, H.; Kim, T.; Han, S.; Lee, J.; Seo, Y.; Park, J. Preparation of Gas Diffusion Layer for Fuel Cell. U.S. Patent. 2009/0011308 A1, 2009. [Google Scholar]
- Latorrata, S.; Stampino, P.G.; Amici, E.; Pelosato, R.; Cristiani, C.; Dotelli, G. Effect of rheology controller agent addition to Micro-Porous Layers on PEMFC performances. Solid State Ionics 2012, 216, 73–77. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Song, C.; Wang, H.; Zhang, J. Electrochemical Impedance Spectroscopy in PEM Fuel Cells: Fundamentals and Applications; Springer: London, UK, 2010; pp. 1–420. [Google Scholar]
- Wagner, N.; Gülzow, E. Change of electrochemical impedance spectra (EIS) with time during CO-poisoning of the Pt-anode in a membrane fuel cell. J. Power Sources 2004, 127, 341–347. [Google Scholar] [CrossRef]
- Asghari, S.; Mokmeli, A.; Samavati, M. Study of PEM fuel cell performance by electrochemical impedance spectroscopy. Int. J. Hydrogen Energy 2010, 35, 9283–9290. [Google Scholar] [CrossRef]
- Wagner, N. Characterization of membrane electrode assemblies in polymer electrolyte fuel cells using a.c. impedance spectroscopy. J. Appl. Electrochem. 2002, 32, 859–863. [Google Scholar] [CrossRef]
- Ciureanu, M.; Roberge, R. Electrochemical impedance study of PEM fuel cells. Experimental diagnostics and modeling of air cathodes. J. Phys. Chem. B 2001, 105, 3531–3539. [Google Scholar] [CrossRef]
- Ramasamy, R.P.; Kumbur, E.C.; Mench, M.M.; Liu, W.; Moore, D.; Murthy, M. Investigation of macro- and micro-porous layer interaction in polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2008, 33, 3351–3367. [Google Scholar] [CrossRef]
- Dhirde, A.M.; Dale, N.V.; Salehfar, H.; Mann, M.D.; Han, T.-H. Equivalent Electric Circuit Modeling and Performance Analysis of a PEM Fuel Cell Stack Using Impedance Spectroscopy. IEEE Trans. Energy Convers. 2010, 25, 778–786. [Google Scholar] [CrossRef]
- Sullivan, T.M.; Middleman, S. Film thickness in blade coating of viscous and viscoelastic liquids. J. Non-Newton. Fluid Mech. 1986, 21, 13–38. [Google Scholar] [CrossRef]
- Kong, C.S.; Kim, D.Y.; Lee, H.K.; Shul, Y.G.; Lee, T.H. Influence of pore-size distribution of diffusion layer on mass-transport problems of proton exchange membrane fuel cells. J. Power Sources 2002, 108, 185–191. [Google Scholar] [CrossRef]
- Lee, H.K.; Park, J.H.; Kim, D.Y.; Lee, T.H. A study on the characteristics of the diffusion layer thickness and porosity of the PEMFC. J. Power Sources 2004, 131, 200–206. [Google Scholar] [CrossRef]
- Hao, L.; Cheng, P. Lattice Boltzmann simulations of anisotropic permeabilities in carbon paper gas diffusion layers. J. Power Sources 2009, 186, 104–114. [Google Scholar] [CrossRef]
- Chun, J.H.; Jo, D.H.; Kim, S.G.; Park, S.H.; Lee, C.H.; Lee, E.S.; Jyoung, J.Y.; Kim, S.H. Development of a porosity-graded micro porous layer using thermal expandable graphite for proton exchange membrane fuel cells. Renew. Energy 2013, 58, 28–33. [Google Scholar] [CrossRef]
- Latorrata, S.; Balzarotti, R.; Stampino, P.G.; Cristiani, C.; Dotelli, G.; Guilizzoni, M. Design of properties and performances of innovative gas diffusion media for polymer electrolyte membrane fuel cells. Prog. Org. Coat. 2015, 78, 517–525. [Google Scholar] [CrossRef]
- Hiramitsu, Y.; Sato, H.; Honi, M. Prevention of the water flooding by micronizing the pore structure of gas diffusion layer for polymer electrolyte fuel cell. J. Power Sources 2010, 195, 5543–5549. [Google Scholar] [CrossRef]
- Jordan, L.R.; Shukla, A.K.; Behrsing, T.; Avery, N.R.; Muddle, B.C.; Forsyth, M. Diffusion layer parameters influencing optimal fuel cell performance. J. Power Sources 2000, 86, 250–254. [Google Scholar] [CrossRef]
- Paganin, V.A.; Ticianelli, E.A.; Gonzalez, E.R. Development and electrochemical studies of gas diffusion electrodes for polymer electrolyte fuel cells. J. Appl. Electrochem. 1996, 26, 297–304. [Google Scholar] [CrossRef]
- Nam, J.H.; Lee, K.J.; Hwang, G.S.; Kim, C.J.; Kaviany, M. Microporous layer for water morphology control in PEMFC. Int. J. Heat Mass Tran. 2009, 52, 2779–2791. [Google Scholar] [CrossRef]
- Tseng, C.J.; Lo, S.K. Effects of microstructure characteristics of gas diffusion layer and microporous layer on the performance of PEMFC. Energy Convers. Manag. 2010, 51, 677–684. [Google Scholar] [CrossRef]
- Markoetter, H.; Haussmann, J.; Alink, R.; Toetzke, C.; Arlt, T.; Klages, M.; Riesemeier, H.; Scholta, J.; Gerteisen, D.; Banhart, J.; et al. Influence of cracks in the microporous layer on the water distribution in a PEM fuel cell investigated by synchrotron radiography. Electrochem. Commun. 2013, 34, 22–24. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzarotti, R.; Latorrata, S.; Stampino, P.G.; Cristiani, C.; Dotelli, G. Development and Characterization of Non-Conventional Micro-Porous Layers for PEM Fuel Cells. Energies 2015, 8, 7070-7083. https://doi.org/10.3390/en8077070
Balzarotti R, Latorrata S, Stampino PG, Cristiani C, Dotelli G. Development and Characterization of Non-Conventional Micro-Porous Layers for PEM Fuel Cells. Energies. 2015; 8(7):7070-7083. https://doi.org/10.3390/en8077070
Chicago/Turabian StyleBalzarotti, Riccardo, Saverio Latorrata, Paola Gallo Stampino, Cinzia Cristiani, and Giovanni Dotelli. 2015. "Development and Characterization of Non-Conventional Micro-Porous Layers for PEM Fuel Cells" Energies 8, no. 7: 7070-7083. https://doi.org/10.3390/en8077070










