Effects of Design/Operating Parameters and Physical Properties on Slag Thickness and Heat Transfer during Coal Gasification
Abstract
:1. Introduction
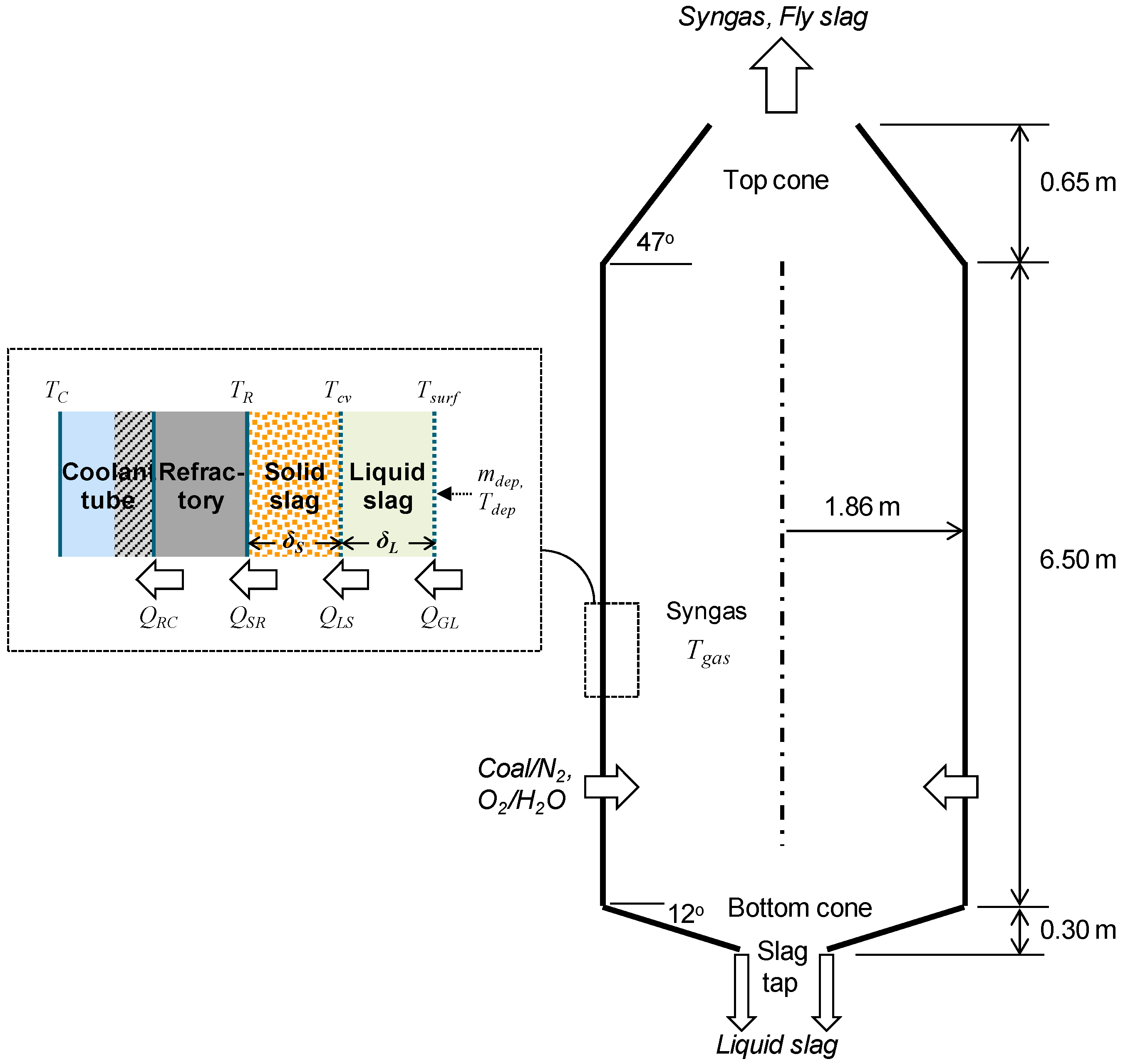
2. Numerical Methods and Test Parameters
| Test Parameters | Reference Value | Accompanying Changes |
|---|---|---|
| Gas temp. (Tgas) | 1800 K | Tdep (=Tgas − 50) changed correspondingly |
| Ash deposition rate (mdep) | 5 kg/s | |
| Ash deposition temp. (Tdep) | 1750 K | |
| Bottom cone height | 0.30 m | With the fixed slag tap radius, the wall angle changed from 12° to 13.2° (+10%) and 10.8° (−10%) |
| Viscosity of liquid slag (μ) | Equation (6) | Tcv changed from 1548 K to 1557 K (+10%) and 1538 K (−10%) |
| Thermal conductivity of slag (k) | Equation (7) | |
| Emissivity of liquid slag (ε) | 0.83 |
Below Tglass: k = 1.040+ 2.025 × 10−4T − 0.246/T2 (W/m∙K)
3. Results and Discussion
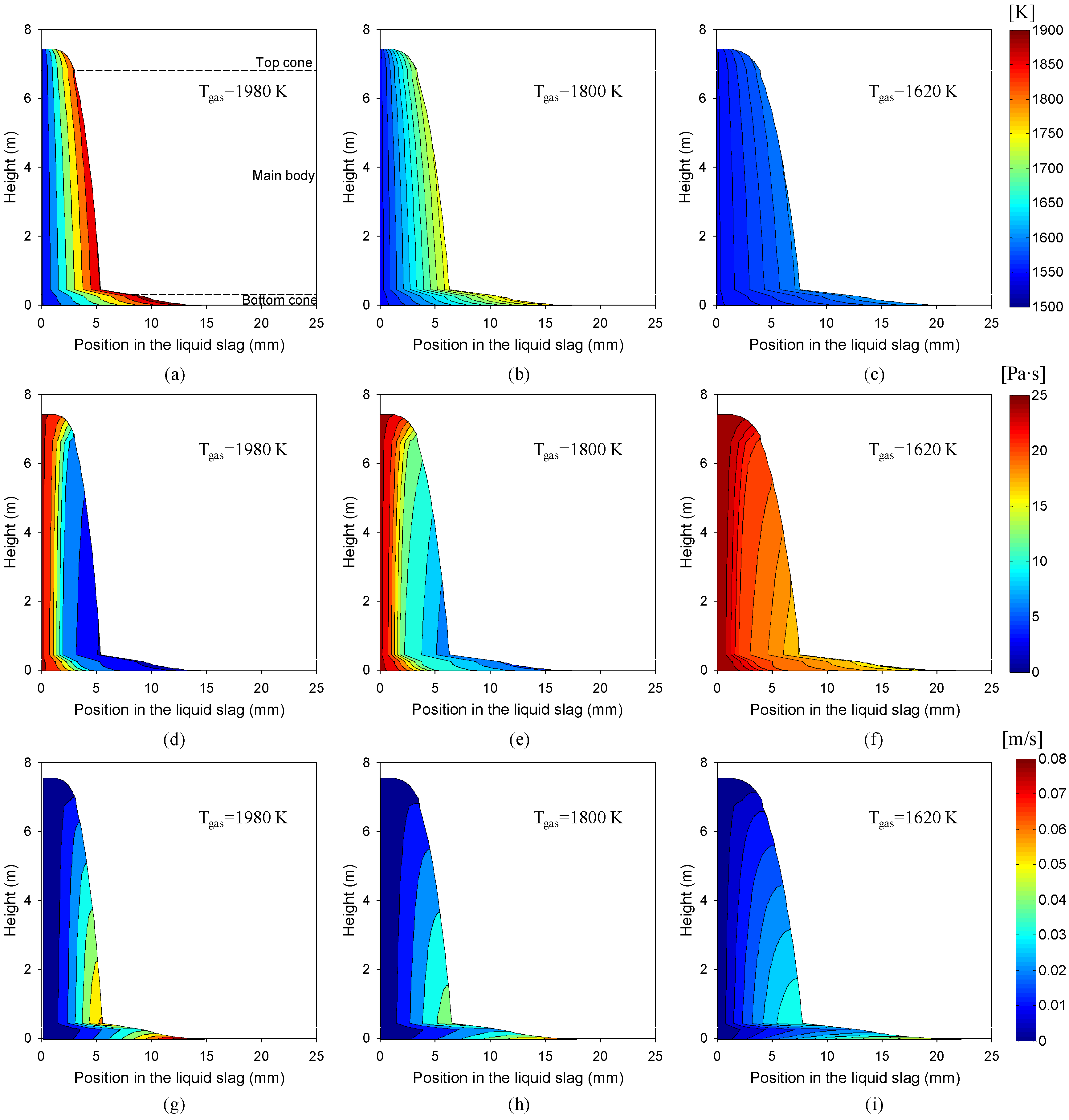
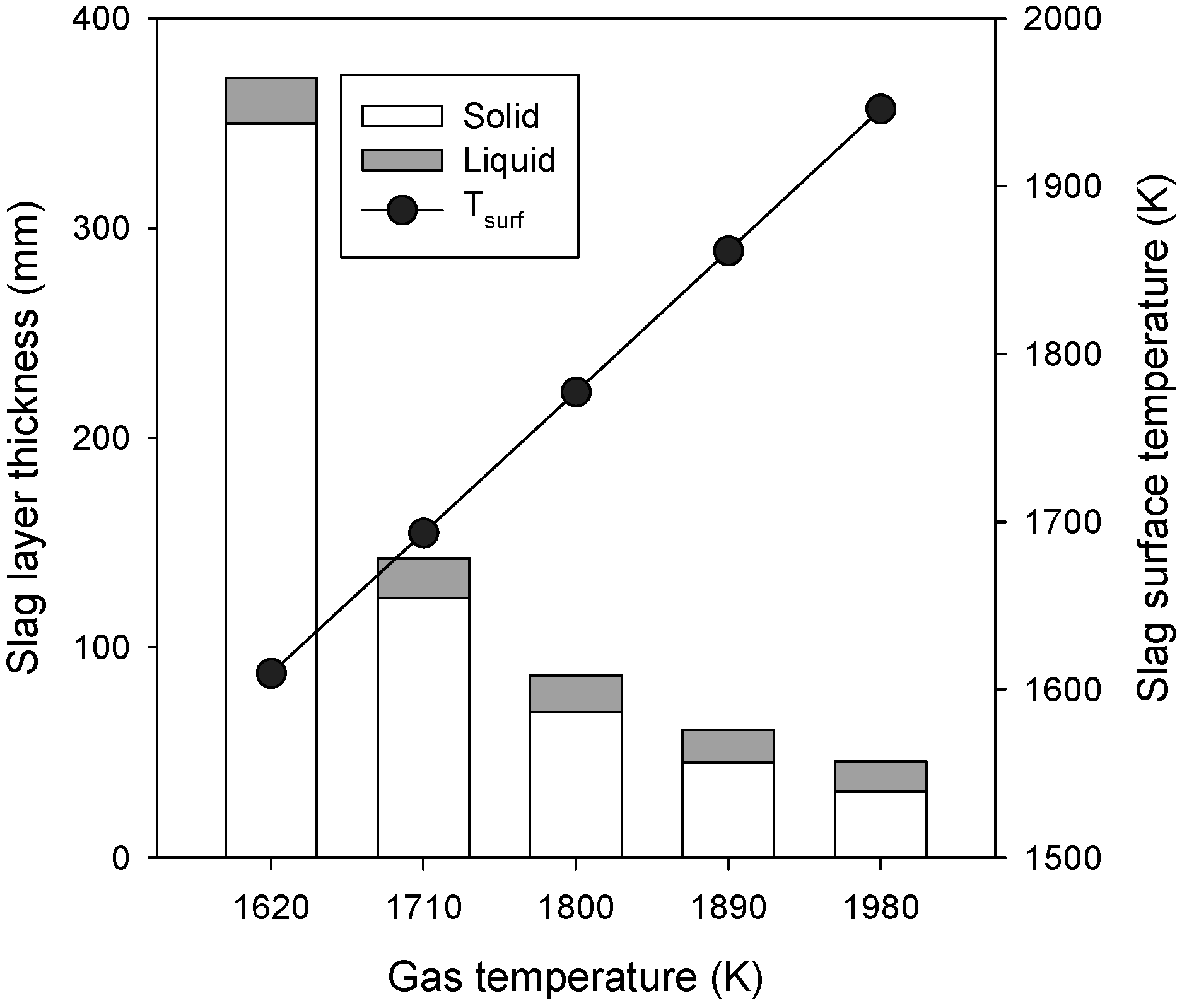
3.1. Effects of Gas Temperature
| Varied Parameters | QGL (%) | QLS (%) | δL (%) | δS (%) | Tsurf (K) | TR (K) | |
|---|---|---|---|---|---|---|---|
| Gas temperature (Tgas) | +10% | 106.4 | 107.1 | −17.4 | −54.8 | 168.6 | 50.1 |
| −10% | −74.3 | −78.2 | 24.0 | 405.5 | −167.5 | −35.7 | |
| Ash deposition rate (mdep) | +10% | −2.6 | −2.0 | 3.2 | 3.1 | 0.5 | −1.3 |
| −10% | 2.9 | 2.3 | −3.4 | −3.4 | −0.5 | 1.5 | |
| Ash deposition temp. (Tdep) | +10% | −16.7 | 6.1 | −1.2 | −5.8 | 10.5 | 2.7 |
| −10% | 16.6 | −6.1 | 1.2 | 6.6 | −10.6 | −2.7 | |
| Bottom cone height | +10% | 2.3 | 3.4 | −2.9 | −2.7 | −0.5 | 1.1 |
| −10% | −2.4 | −3.6 | 3.3 | 3.2 | 0.6 | −1.3 | |
| Liquid slag viscosity (μ) | +10% | −4.1 | −4.3 | 1.2 | 6.0 | 1.0 | −2.0 |
| −10% | 4.6 | 4.7 | −1.3 | −6.1 | −1.1 | 2.3 | |
| Slag conductivity (k) | +10% | 7.1 | 6.9 | 0.3 | 1.3 | −2.0 | 3.7 |
| −10% | −7.5 | −7.3 | −0.3 | −1.3 | 2.0 | −3.7 | |
| Liquid slag emissivity (ε) | +10% | 2.8 | 2.4 | −0.3 | −1.7 | 2.0 | 0.8 |
| −10% | −3.2 | −2.8 | 0.4 | 2.1 | −2.4 | −0.9 | |
| Values in reference case | 179.4 kW | 182.6 kW | 17.4 mm | 69.3 mm | 1777.2 K | 567.1 K | |
3.2. Effects of Ash Deposition Rate
3.3. Effects of Ash Deposition Temperature
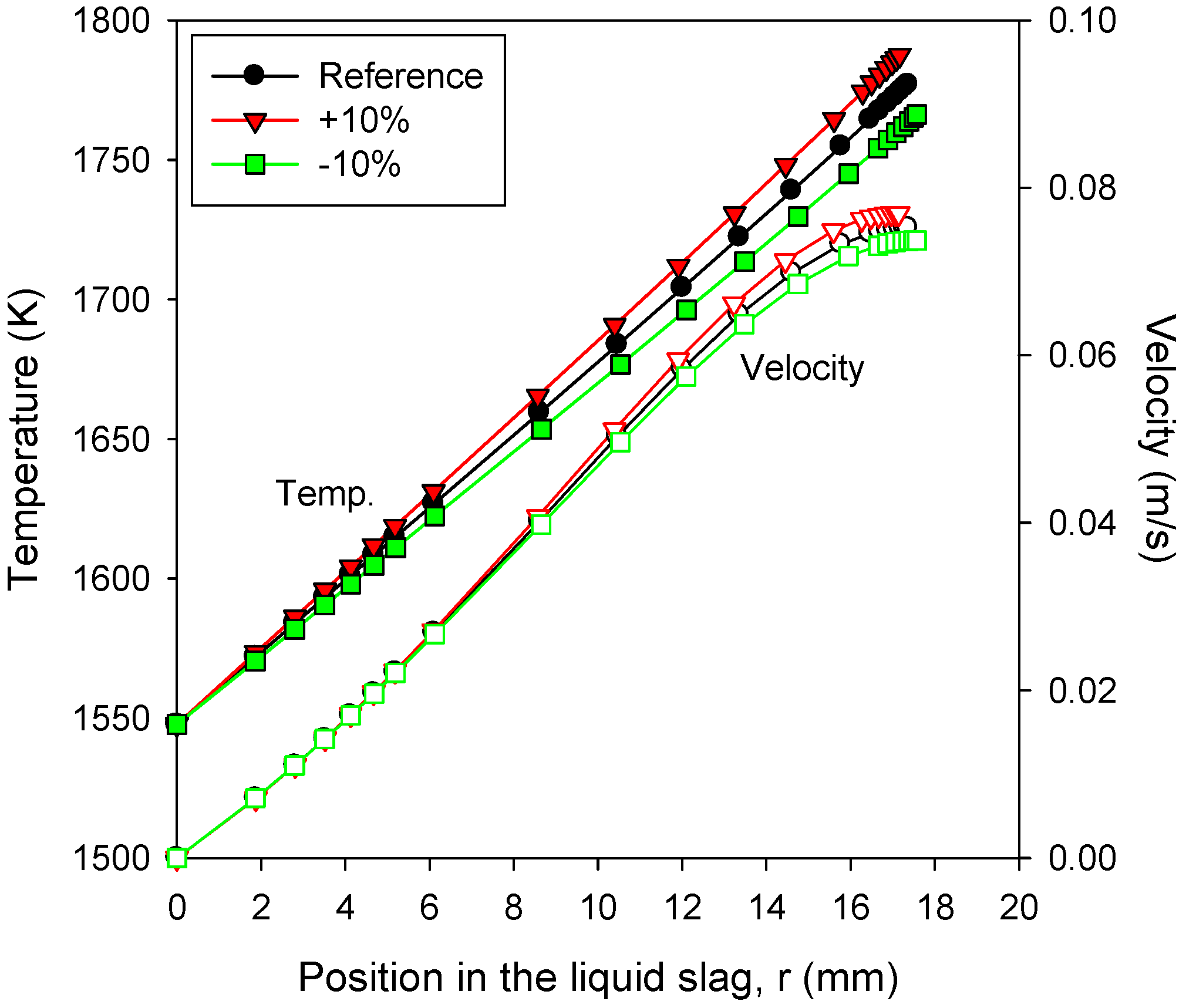
3.4. Effects of Bottom Cone Design
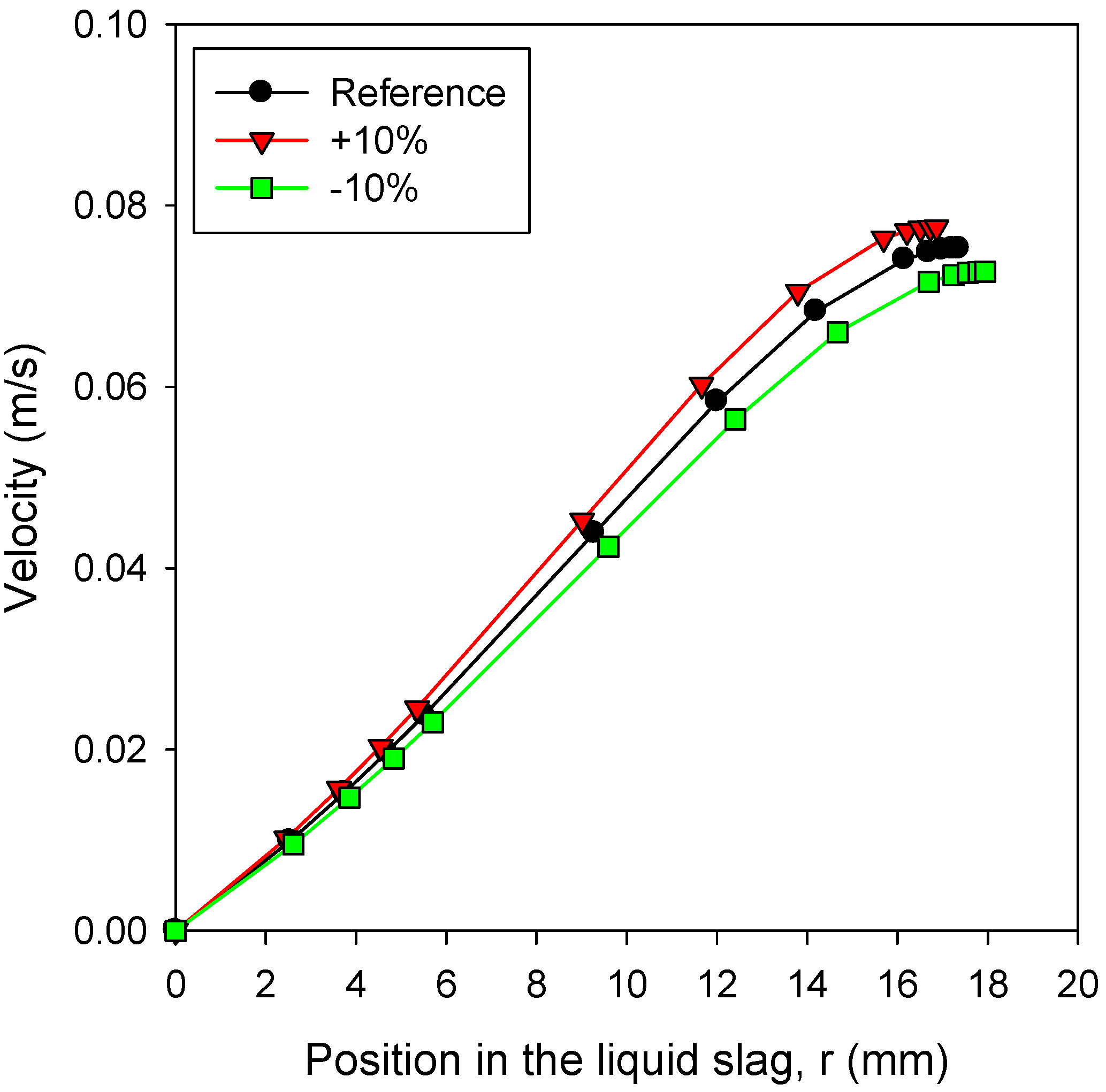
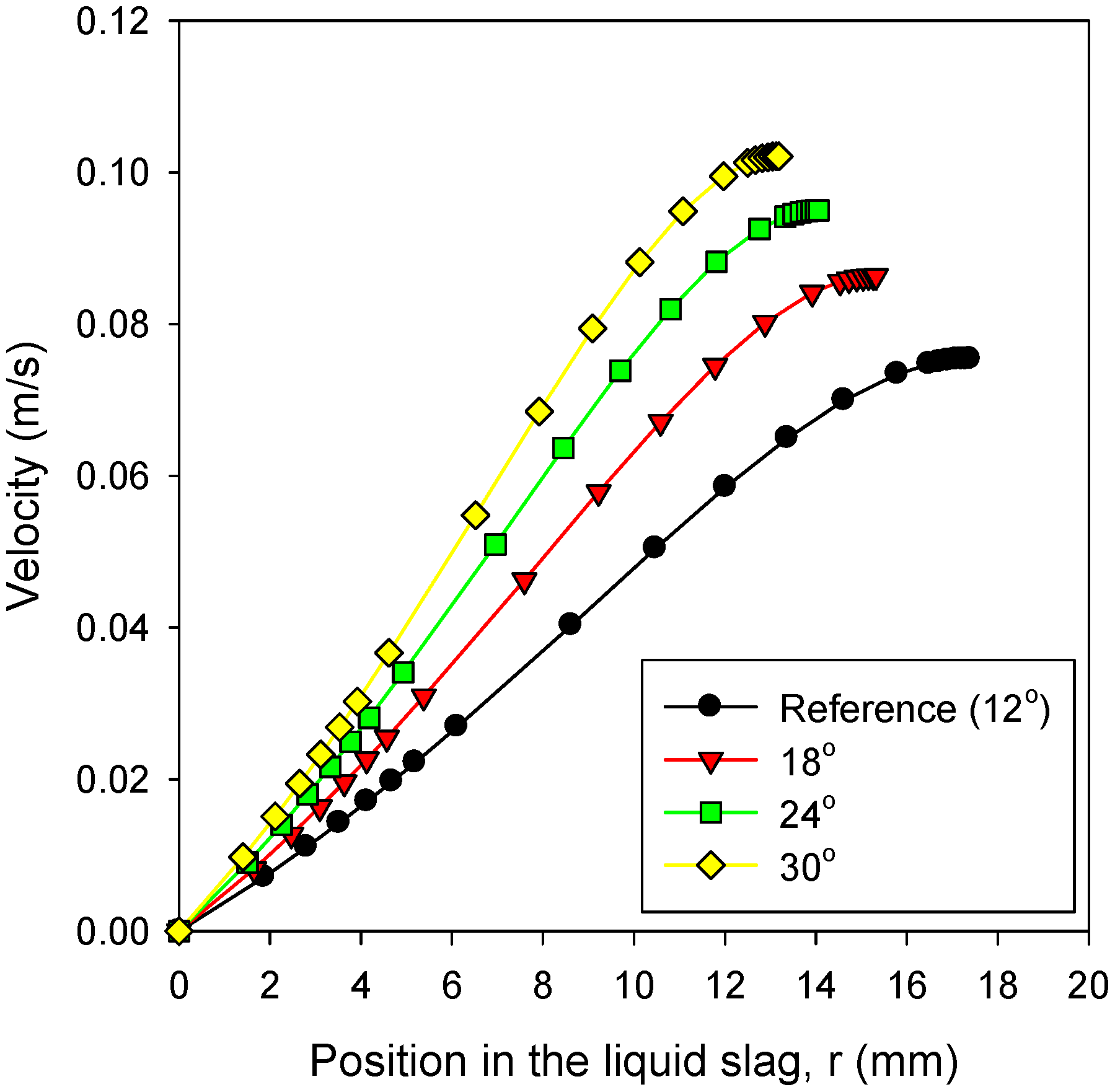
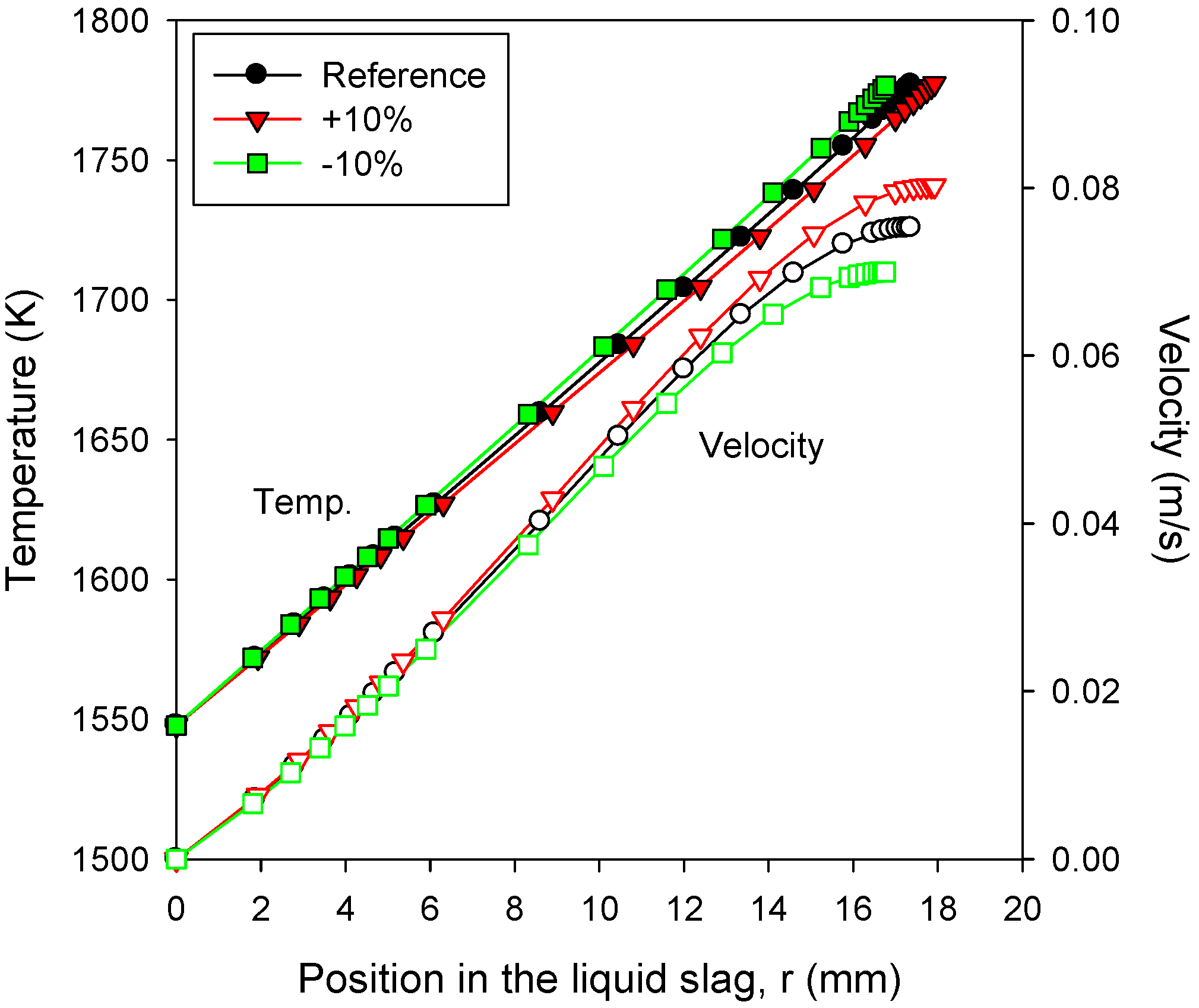
3.5. Effects of Slag Properties: Viscosity
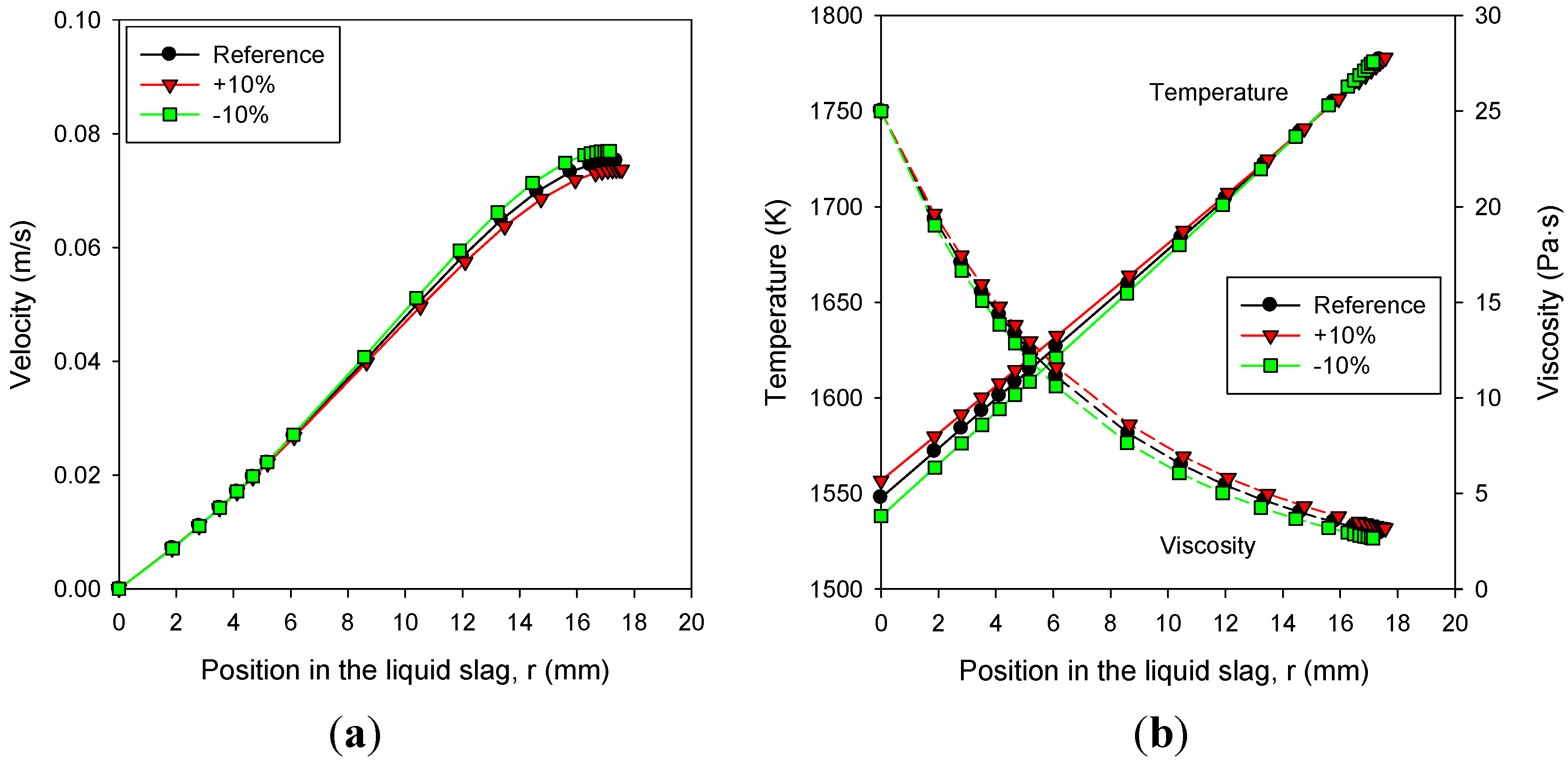
3.6. Effects of Slag Properties: Thermal Conductivity and Emissivity
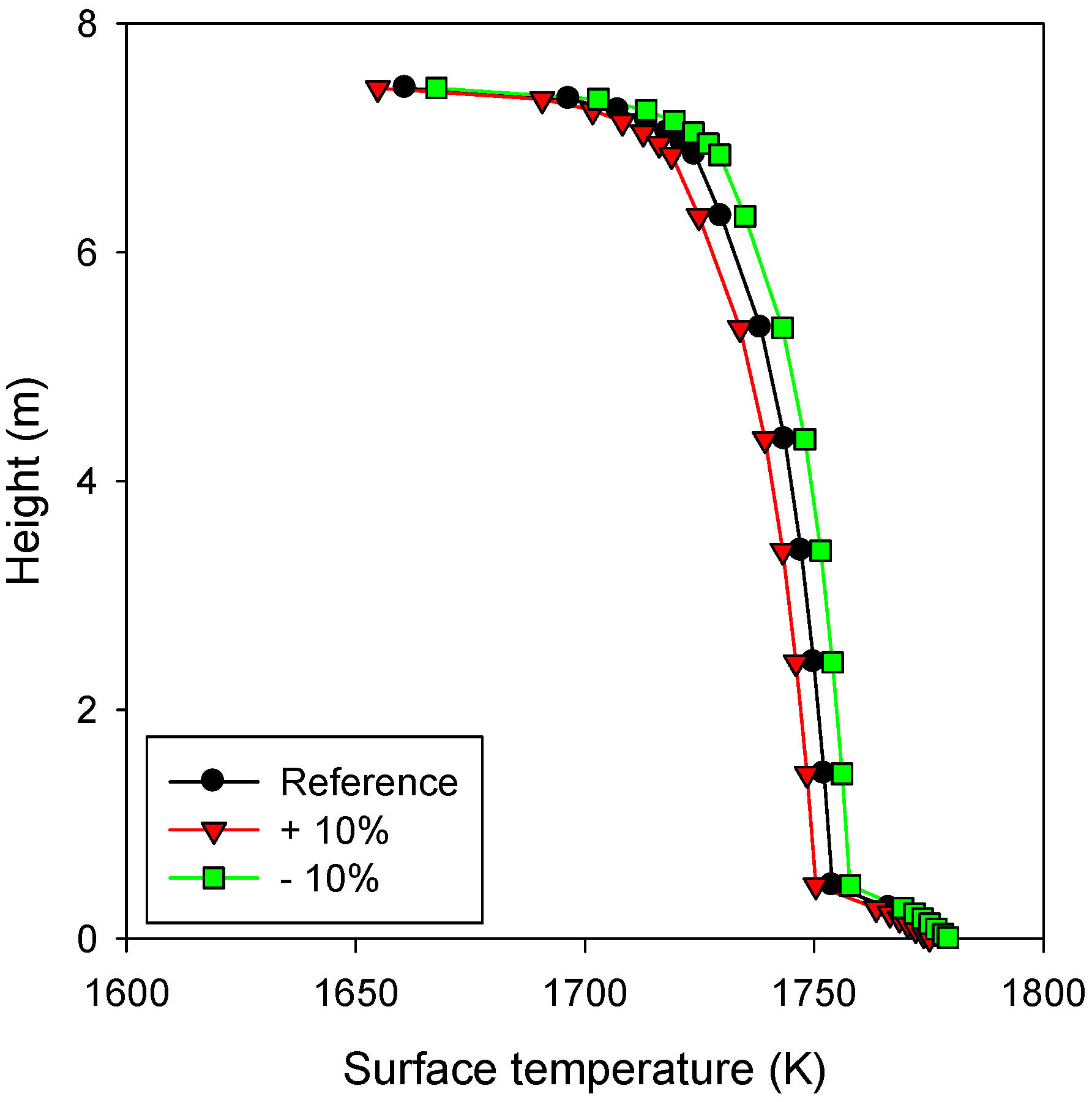
4. Conclusions
- The velocity profile of the liquid slag was less sensitive to the variations in the parameters, and therefore, the change in the thickness of the liquid slag was typically smaller than that of the solid slag.
- The gas temperature was found to be highly influential, because of its dominant effect on the radiative heat transfer to the slag layer. The solid slag thickness increased exponentially with an increase in the gas temperature.
- The effect of the variations in the ash deposition rate was diminished by the high-velocity region developed near the liquid slag surface. Increasing the ash deposition rate by 10% caused an approximate 3% increase in the thickness of the slag layers.
- The slag viscosity significantly influenced the solid slag thickness through the corresponding changes in the temperature (Tcv) and its gradient (heat flux) at the interface of the solid and liquid slag layers. Decreasing the slag viscosity by 10% reduced the thickness of the liquid slag by only 1.3%, whereas that of the solid slag was reduced by 6%.
- A higher thermal conductivity of the slag directly increased the heat transfer rate across the slag layer, whereas its effect on the thickness of the slag layers was very small.
- For the bottom cone of the gasifier, steeper angles were favorable to reduce the slag layer thickness.
Acknowledgments
Author Contributions
Nomenclature
| A | area, m2 | g | gravity, 9.81 m/s2 |
| H | enthalpy, J/s | k | thermal conductivity, W/m·K |
| M | momentum, kg∙m/s2 | m | mass flow rate, kg/s |
| Q | heat transfer rate, W | q | heat flux, W/m2 |
| r | radius perpendicular to the wall, m | T | temperature, K |
| V | volume, m3 | v | streamwise velocity, m/s |
| y | length parallel to the wall, m | ||
| Greek | |||
| α | angle from the horizontal plane ° | δ | thickness of a slag layer, m |
| ε | emissivity | μ | viscosity, Pa·s |
| ρ | density, kg/m3 | ||
| Subscript | |||
| cond | conduction | cv | critical viscosity |
| dep | depositing slag | gas | gas |
| GL | from gas to liquid slag | glass | glass transition of slag |
| in | inflow | L | liquid slag |
| LS | from liquid slag to solid slag | out | outflow to the section below |
| react | reactions of residual carbon or the phase transformation | R | refractory |
| S | solid slag | surf | liquid slag surface facing gas |
Conflicts of Interest
References
- Stiegel, G.J.; Maxwell, R.C. Gasification technologies: The path to clean, affordable energy in the 21st century. Fuel Process. Technol. 2001, 71, 79–97. [Google Scholar] [CrossRef]
- Higman, C.; van der Burgt, M. Gasification, 2nd ed.; Elsevier: New York, NY, USA, 2008. [Google Scholar]
- Wang, P.; Massoudi, M. Slag behavior in gasifiers. Part I: Influence of coal properties and gasification conditions. Energies 2013, 6, 784–806. [Google Scholar] [CrossRef]
- Duchesne, M.A.; Hughes, R.W.; Lu, D.Y.; McCalden, D.J.; Anthony, E.J.; Macchi, A. Fate of inorganic matter in entrained-flow slagging gasifiers: Pilot plant testing. Fuel Process. Technol. 2014, 125, 18–33. [Google Scholar] [CrossRef]
- Ni, J.J.; Zhou, Z.J.; Yu, G.S.; Liang, Q.F.; Wang, F.C. Molten slag flow and phase transformation behaviors in a slagging entrained-flow coal gasifier. Ind. Eng. Chem. Res. 2010, 49, 12302–12310. [Google Scholar] [CrossRef]
- Ilyushechkin, A.Y.; Hla, S.S.; Roberts, D.G.; Kinaev, N.N. The effect of solids and phase compositions on viscosity behaviour and TCV of slags from Australian bituminous coals. J. Non-Cryst. Solids 2011, 357, 893–902. [Google Scholar] [CrossRef]
- Song, W.J.; Dong, Y.H.; Wu, Y.Q.; Zhu, Z.B. Prediction of temperature of critical viscosity for coal ash slag. AIChE J. 2011, 57, 2921–2925. [Google Scholar] [CrossRef]
- McLennan, A.R.; Bryant, G.W.; Bailey, C.W.; Stanmore, B.R.; Wall, T.F. An experimental comparison of the ash formed from coals containing pyrite and siderite mineral in oxidizing and reducing conditions. Energy Fuels 2000, 14, 308–315. [Google Scholar] [CrossRef]
- McLennan, A.R.; Bryant, G.W.; Bailey, C.W.; Stanmore, B.R.; Wall, T.F. Index for iron-based slagging for pulverized coal firing in oxidizing and reducing conditions. Energy Fuels 2000, 14, 349–354. [Google Scholar] [CrossRef]
- Duchesne, M.A.; Hall, A.D.; Hughes, R.W.; McCalden, D.J.; Anthony, E.J.; Macchi, A. Fate of inorganic matter in entrained-flow slagging gasifiers: Fuel characterization. Fuel Process. Technol. 2014, 118, 208–217. [Google Scholar] [CrossRef]
- Yun, Y.; Yoo, Y.D.; Chung, S.W. Selection of IGCC candidate coals by pilot-scale gasifier operation. Fuel Process. Technol. 2007, 88, 107–116. [Google Scholar] [CrossRef]
- Song, W.; Tang, L.; Zhu, X.; Wu, Y.; Rong, Y.; Zhu, Z. Fusibility and flow properties of coal ash and slag. Fuel 2009, 88, 297–304. [Google Scholar] [CrossRef]
- Kong, L.; Bai, J.; Bai, Z.; Guo, Z.; Li, W. Improvement of ash flow properties of low-rank coal for entrained flow gasifier. Fuel 2014, 120, 122–129. [Google Scholar] [CrossRef]
- Kong, L.; Bai, J.; Li, W.; Bai, Z.; Guo, Z. Effect of lime addition on slag fluidity of coal ash. J. Fuel Chem. Technol. 2011, 39, 407–411. [Google Scholar] [CrossRef]
- Kong, L.; Bai, J.; Bai, Z.; Guo, Z.; Li, W. Effects of CaCO3 on slag flow properties at high temperatures. Fuel 2013, 109, 76–85. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Liang, Q.; Xu, J. Experimental and numerical study on slag deposition and growth at the slag tap hole region of Shell gasifier. Fuel Process. Technol. 2013, 106, 704–711. [Google Scholar] [CrossRef]
- Roberts, D.G.; Harris, D.J.; Tremel, A.; Ilyushechkin, A.Y. Linking laboratory data with pilot scale entrained flow coal gasification performance. Part 2: Pilot scale testing. Fuel Process. Technol. 2012, 94, 26–33. [Google Scholar] [CrossRef]
- Sun, B.; Liu, Y.; Chen, X.; Zhou, Q.; Su, M. Dynamic modeling and simulation of shell gasifier in IGCC. Fuel Process. Technol. 2011, 92, 1418–1425. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Z.; Wu, Y.; Wang, J.; Lu, J.; Li, Z.; Ni, W. Dynamic model for an oxygen staged slagging entrained flow gasifier. Energy Fuels 2011, 25, 3646–3656. [Google Scholar] [CrossRef]
- Yong, S.Z.; Gazzino, M.; Ghoniem, A. Modeling the slag layer in solid fuel gasification and combustion—Formulation and sensitivity analysis. Fuel 2012, 92, 162–170. [Google Scholar] [CrossRef]
- Ye, I.; Ryu, C. Numerical modeling of slag flow and heat transfer on the wall of an entrained coal gasifier. Fuel 2015, 150, 64–74. [Google Scholar] [CrossRef]
- Seggiani, M. Modeling and simulation of time varying slag flow in a Prenflo entrained-flow gasifier. Fuel 1998, 77, 1611–1621. [Google Scholar] [CrossRef]
- Kalmanovitch, D.P.; Frank, M. An effective model of viscosity for ash deposition phenomena. In Proceedings of the Mineral Matter and Ash Deposition from Coal, Santa Barbara, CA, USA, 22–26 February 1998; Bryers, R.W., Vorres, K.S., Eds.; United Engineering Trustees Inc.: New York, NY, USA, 1998. [Google Scholar]
- Mills, K.C.; Rhine, J.M. The measurement and estimation of the physical properties of slag formed during coal gasification: 2. Properties relevant to heat transfer. Fuel 1989, 68, 904–910. [Google Scholar] [CrossRef]
- Mills, K.C.; Rhine, J.M. The measurement and estimation of the physical properties of slag formed during coal gasification: 1. Properties relevant to fluid flow. Fuel 1989, 68, 193–200. [Google Scholar] [CrossRef]
- Liu, S.; Tao, M.; Hao, Y. A numerical model for chemical reaction on slag layer surface and slag layer behavior in entrained-flow gasifier. Therm. Sci. 2013, 17, 1389–1394. [Google Scholar] [CrossRef]
- Watt, J.D.; Fereday, F. The flow properties of slags formed from the ashes of British coals: Part 1. viscosity of homogeneous liquid slags in relation to slag composition. J. Inst. Fuel 1969, 42, 99–103. [Google Scholar]
- Reid, W.T. External Corrosion and Deposits: Boilers and Gas Turbines; American Elsevier Publishing Co.: New York, NY, USA, 1971. [Google Scholar]
- Browning, G.J.; Bryant, G.W.; Hurst, H.J.; Lucas, J.A.; Wall, T.F. An empirical method for the prediction of coal ash slag viscosity. Energy Fuels 2003, 17, 731–737. [Google Scholar] [CrossRef]
- Urbain, G. Viscosity estimation of slags. Steel Res. Int. 1987, 58, 111–116. [Google Scholar]
- Zhang, L.; Jahansahi, S. Review and modeling of viscosity of silicate melts: Part I. Viscosity of binary and ternary silicates containing CaO, MgO, and MnO. Metall. Mater. Trans. B 1998, 29, 177–186. [Google Scholar] [CrossRef]
- Kondratiev, A.; Jak, E. Review of Experimental data and modeling of viscosities of fully liquid slags in the Al2O3-CaO-‘FeO’-SiO2 system. Metall. Mater. Trans. B 2001, 32, 1015–1025. [Google Scholar] [CrossRef]
- Seetharaman, S.; Mukai, K.; Sichen, D. Viscosities of slags—An overview. Steel Res. Int. 2005, 76, 267–278. [Google Scholar]
- Duchesne, M.A.; Bronsch, A.M.; Hughes, R.W.; Masset, P.J. Slag viscosity modeling toolbox. Fuel 2013, 114, 38–43. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, I.; Oh, J.; Ryu, C. Effects of Design/Operating Parameters and Physical Properties on Slag Thickness and Heat Transfer during Coal Gasification. Energies 2015, 8, 3370-3385. https://doi.org/10.3390/en8053370
Ye I, Oh J, Ryu C. Effects of Design/Operating Parameters and Physical Properties on Slag Thickness and Heat Transfer during Coal Gasification. Energies. 2015; 8(5):3370-3385. https://doi.org/10.3390/en8053370
Chicago/Turabian StyleYe, Insoo, Junho Oh, and Changkook Ryu. 2015. "Effects of Design/Operating Parameters and Physical Properties on Slag Thickness and Heat Transfer during Coal Gasification" Energies 8, no. 5: 3370-3385. https://doi.org/10.3390/en8053370






