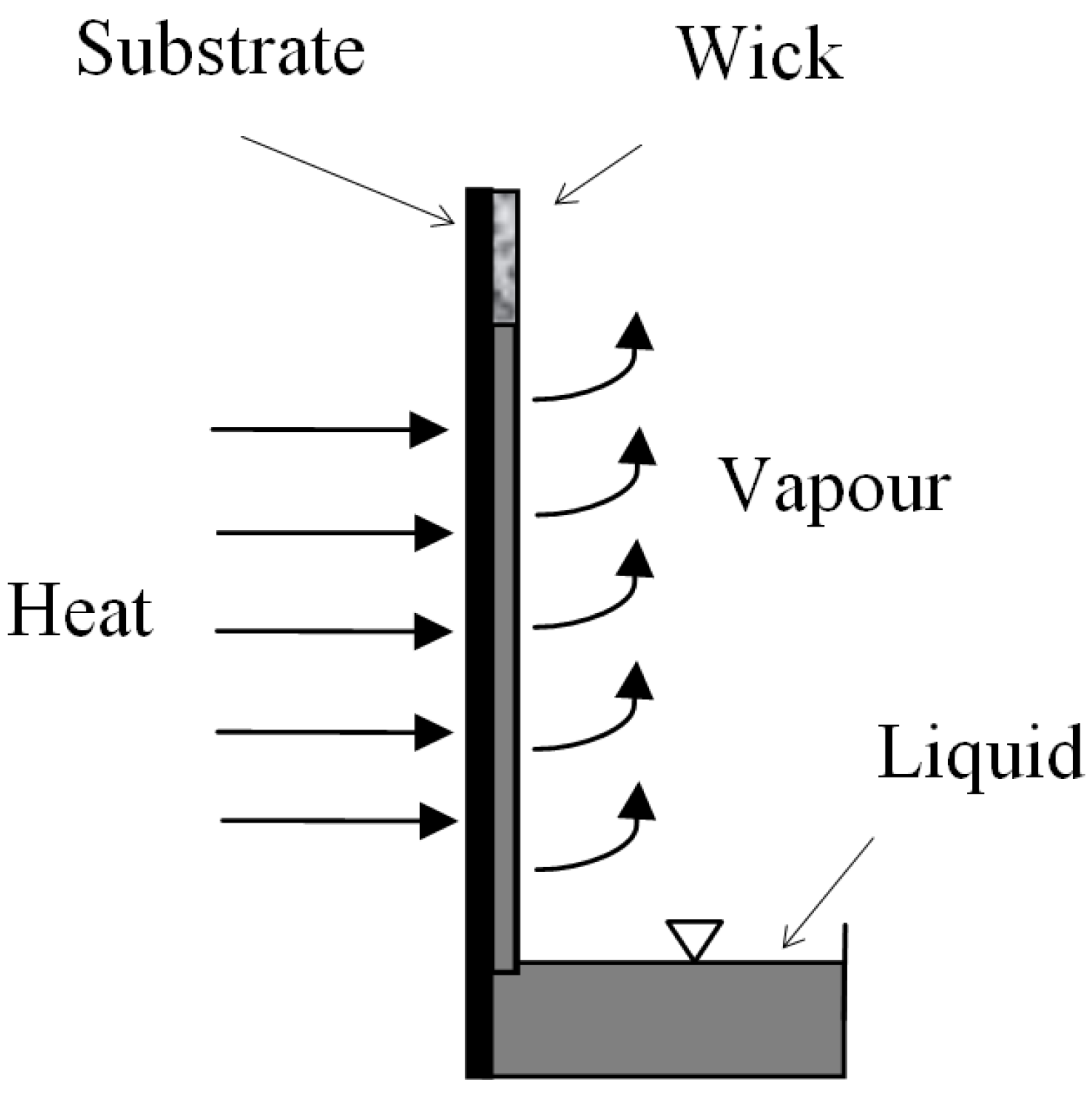
Sintered Nickel Powder Wicks for Flat Vertical Heat Pipes
Abstract
:1. Introduction
- Sintering time.
- Sintering temperature.
- Sintering atmosphere.
- Type and amount of pore former (spacing agent).
- Pressure from weights applied during sintering (forming pressure).
- Method of fabrication of the wick.
- Selection of burn-off and sintering temperature programs from thermogravimetric analysis.
- Determination of the wick characteristics.
- Prediction of the wick maximum heat transfer capacity with potassium.
- Criterion for pore former selection.
2. Method of Fabrication of the Wick
3. Selection of Burn-off and Sintering Temperature Programs from Thermogravimetric Analysis
- Thermogravimetric analysis of pure Inco 255 nickel powder in air was carried out to study the oxidation.
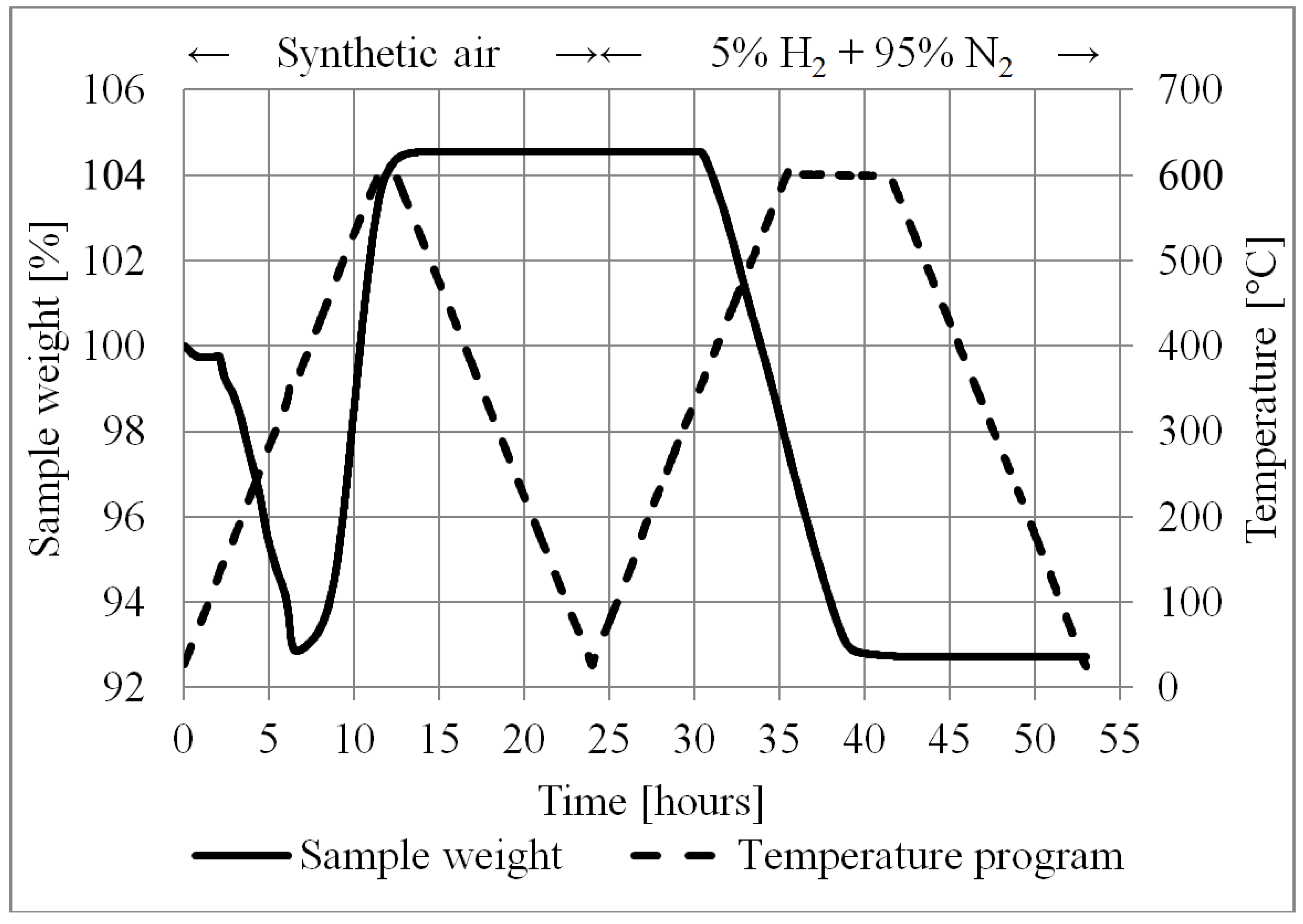
- Thermogravimetric analysis was carried out for the tape cast tape in order to determine the programs for burn-off and reduction. In this test a 900 µL crucible was filled with approximately 40 layers of tape. The burn-off TGA was carried out using synthetic air atmosphere, and the reduction in a H2 (5%) and N2 (95%) atmosphere.
- A thermogravimetric analysis with pure graphite powder in air was performed. Pore former was not used in the fabrication of the final wick presented in this study, but some work was performed to analyse the suitability of the graphite powder as pore former. Graphite was selected because it leaves very little residues after burn-off. The graphite powder (from Sigma-Aldrich) had particle sizes smaller than 20 µm.
- A thermogravimetric analysis was also performed for the burn-off of a tape casted tape consisting of approximately equal amounts of Inco 255 nickel powder and graphite powder, plus the tape casting additives.
4. Determination of the Wick Characteristics
4.1. Capillarity and Flow Characteristics
4.2. Wick Porosity
4.3. Rate-of-Rise

5. Results and Discussion
5.1. Thermogravimetric Analysis and Temperature Programs
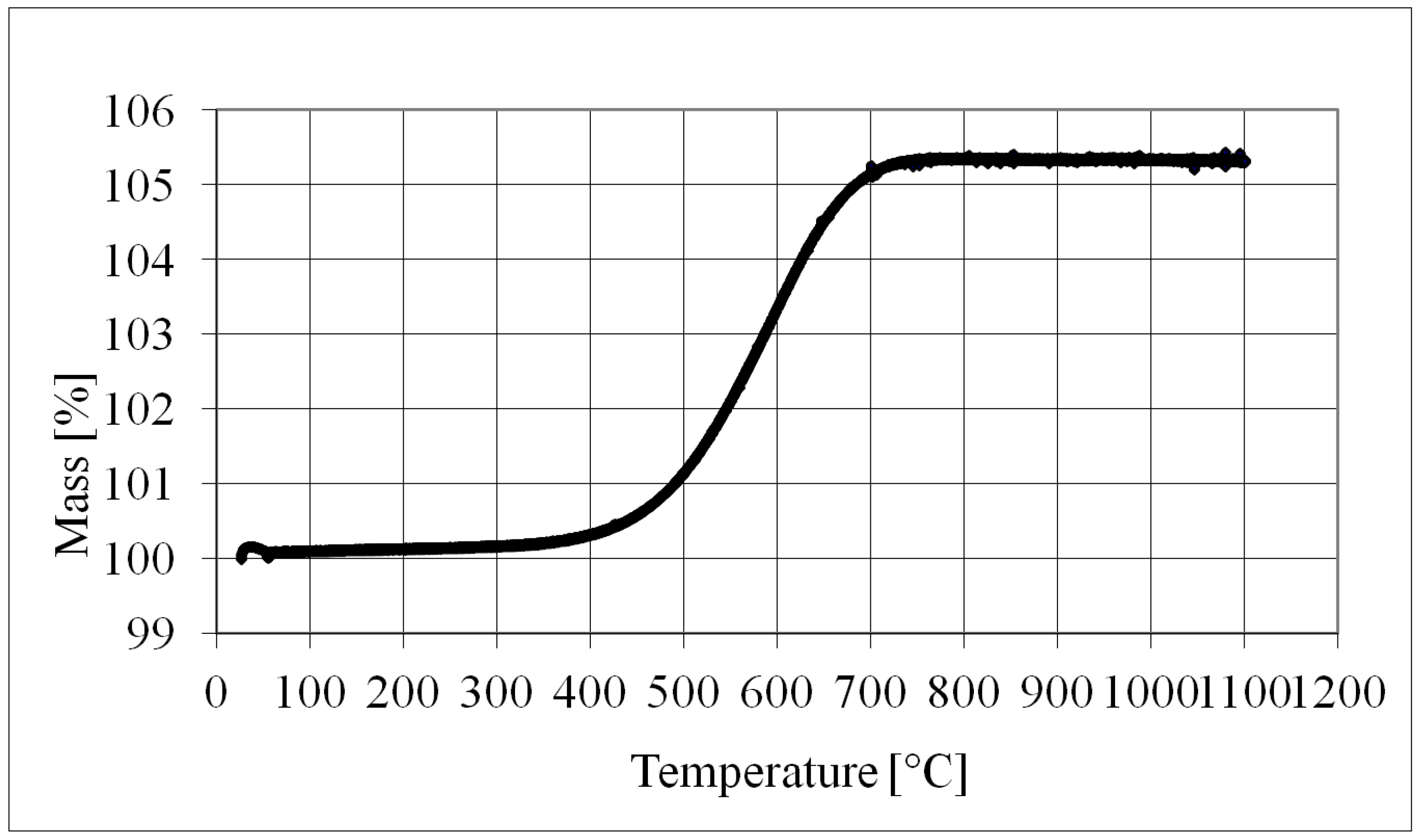
5.1.1. Nickel Oxide Formation
5.1.2. Burn-off and Reduction
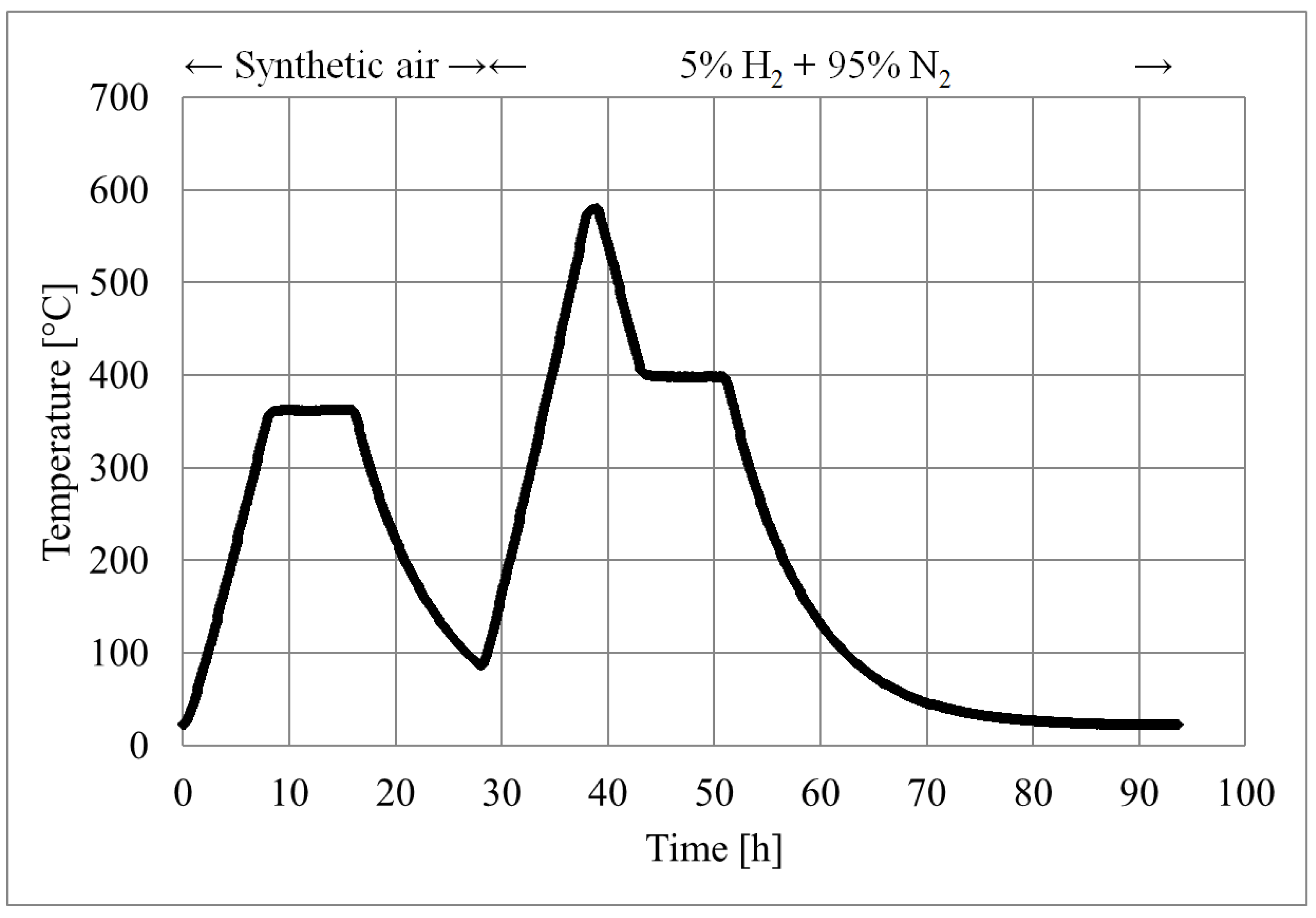
5.1.3. The Temperature Program
- Sufficient time and temperature for the burn-off.
- Sufficient time and temperature for proper sintering and acceptable mechanical properties.
- Sufficient time and temperature for the reduction of the nickel oxide.
5.1.4. Pure Graphite Powder
5.1.5. Tape Containing Pore Former
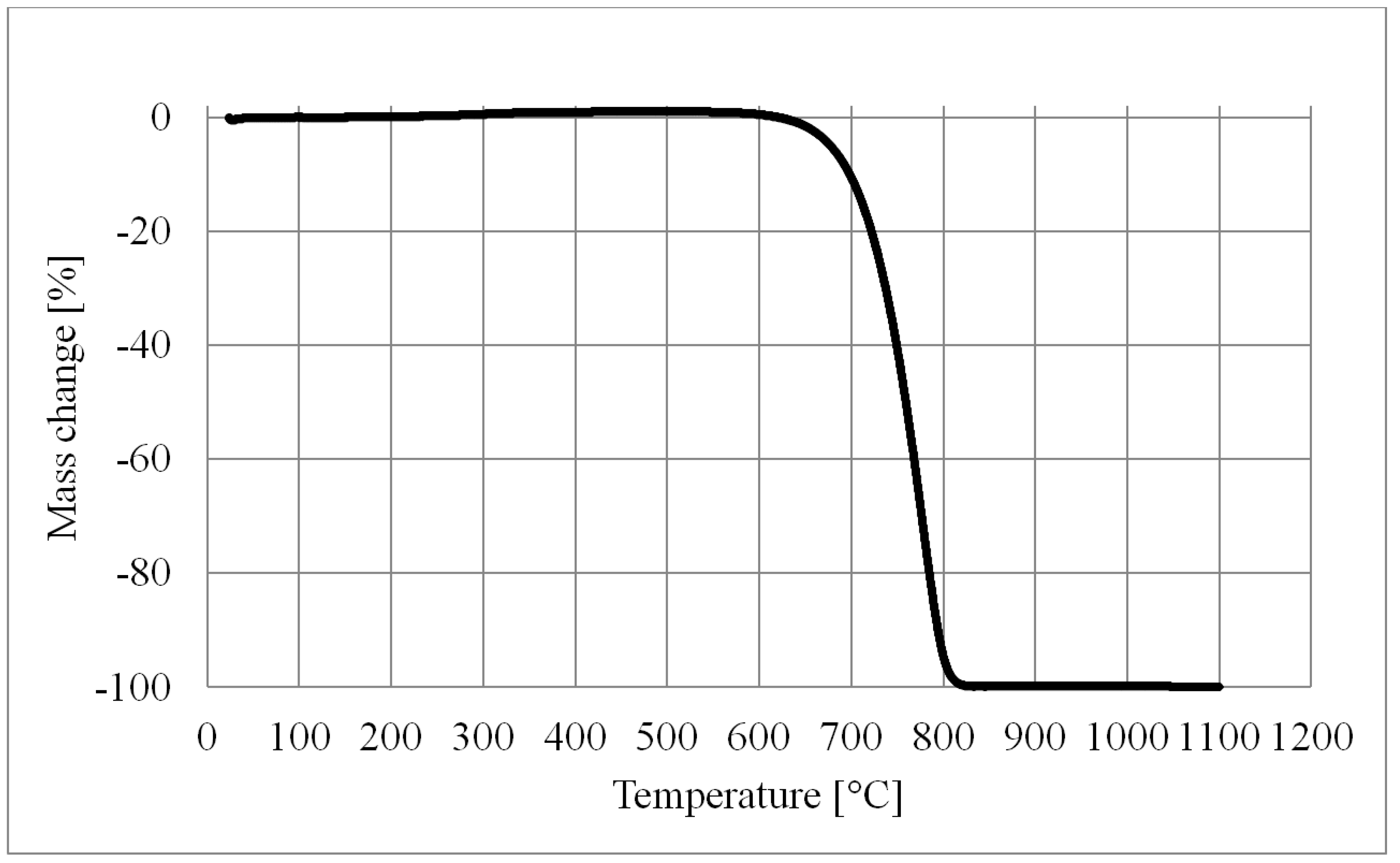

5.2. The Contact Angle
5.3. Wick Porosity
5.4. Rate-of-Rise

5.5. Prediction of the Wick Maximum Heat Transfer Capacity with Potassium
Static Pressure Limitation

5.6. Sensitivity Analysis
The Influence of Parameter Variations Above and Below Pcap/Psat = 1
| Parameter | Change | Change in heat transfer capacity at 850 °C (vapour static pressure limited) | Change in heat transfer capacity at 1050 °C (capillary pressure limited) |
|---|---|---|---|
| Temperature | +1.2 K | −28% | −0.2% |
| Porosity | +1% | −40% | −0.9% |
| Evaporation rate | +1% | −28% | −0.05% |
| Cross sectional area | +1% | −47% | −6% |
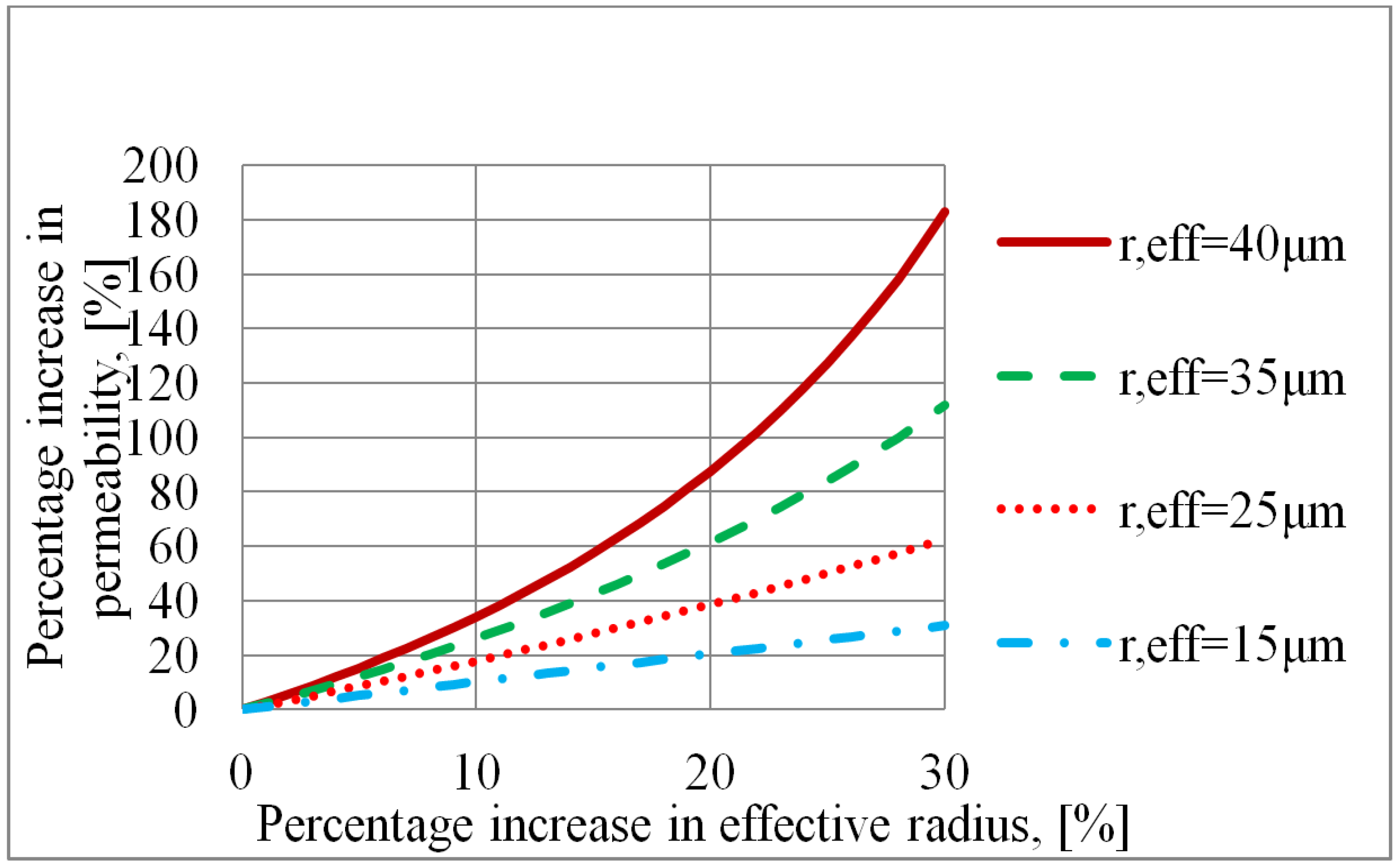
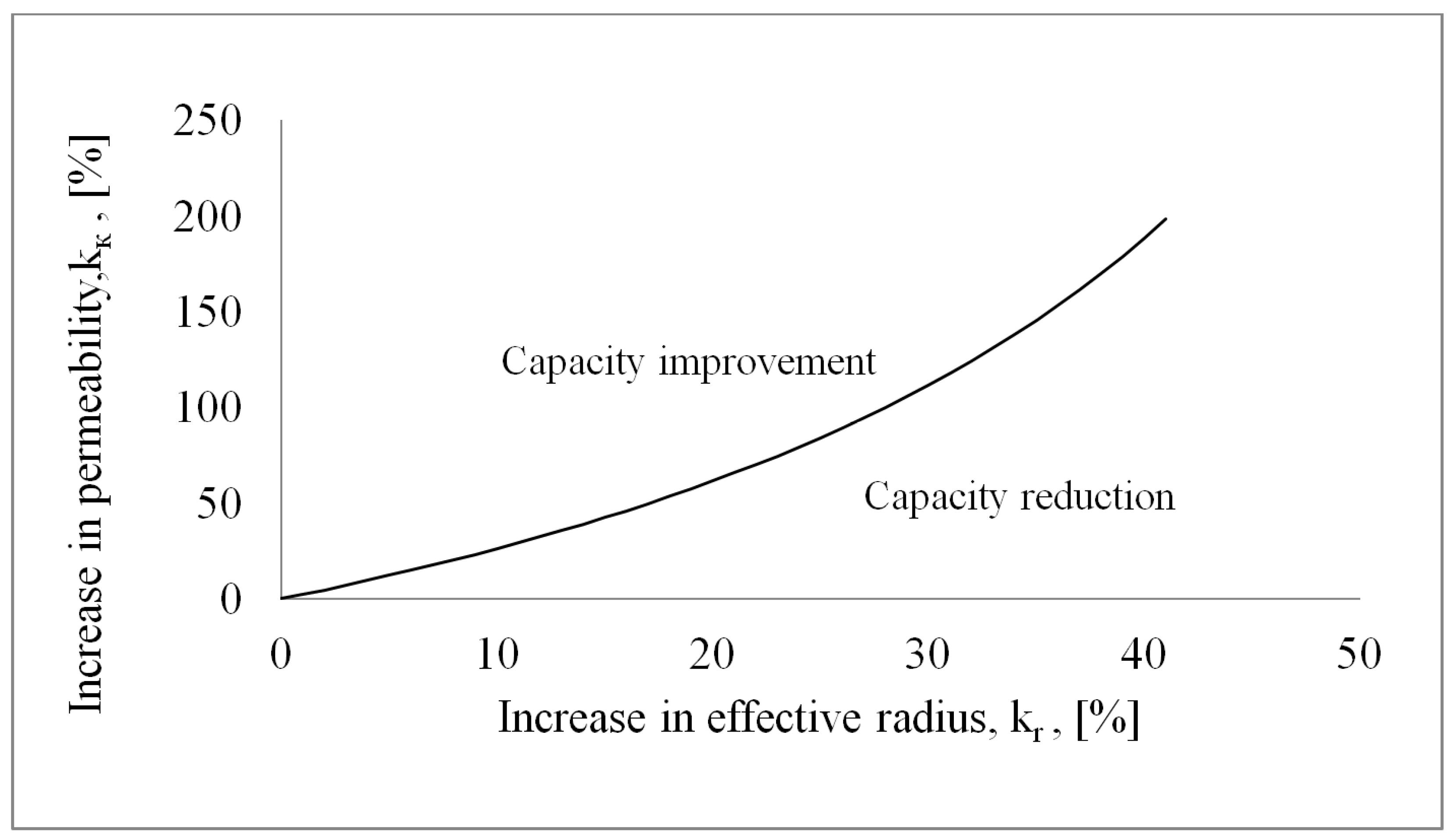
5.7. Criterion for Pore Former Selection
6. Conclusions
- The capillary pressure inside a heat pipe can never exceed the vapour static pressure of the working fluid. This will limit the heat transfer capacity and the useful operating temperature range for heat pipes with wicks with small effective pore radii operated with working fluids with low vapour static pressures, for instance alkali metals.
- Determination of the effective pore radius and the permeability from a rate-of-rise experiment alone can lead to great uncertainties about the actual values. Many different pairs of (reff, к) give good curve fits, and in such case they can all be used for approximate wick capacity predictions for (Pcap/Psat < 1). For wick capacity predictions for (Pcap/Psat > 1) the permeability has to be known with higher accuracy.
- In the vapour static pressure limited region the use of a pore former which increases the wick permeability will always improve the wick performance.
- In the capillary pressure limited region, a pore former evaluation plot may be made in order to analyse the effects of using a pore former. If the effective radius and permeability increase with an equal percentage the overall effect on the wick heat transfer capacity is negative. A pore former which increases the permeability more than the effective pore radius would be a better choice than a powderous pore former. One such candidate can be carbon fibers.
- The oxidation of Inco 255 nickel powder accellerates at about 350 °C.
- The reduction of nickel oxide in 5%H2 + 95%N2 accellerates at about 350 °C.
- In order to burn off a graphite powder pore former a temperature of about 800 °C is required.
Acknowledgments
Author Contributions
Symbols
| Ac | wick cross sectional area, m2 |
| C | constant |
| E | Deviation |
| g | gravity constant, g = 9.81 m/s2 |
| H | height, m |
| hfg | latent heat of evaporation, kJ/kg |
| kк | factor expressing change in permeability |
| kr | factor expressing change in radius |
mass flow rate, kg/s | |
| P | pressure, Pa |
heat flow rate, W | |
| r | radius, m |
| reff | effective radius, m |
| t | time, s |
| V | volume |
velocity, m/s | |
| y | height, m |
Greek symbols
| Г | evaporation rate per unit height of wetted wick, kg/(m·s) |
| ε | porosity |
| θ | solid-liquid-vapour contact angle, degrees |
| к | permeability, m2 |
| µ | dynamic viscosity, kg/(m·s) |
| ρ | density, kg/m3 |
| σ | surface tension, N/m |
Subscripts
| cap | capillary |
| eff | effective (pore radius) |
| f | friction |
| g | gravity |
| l | liquid |
| sat | saturated |
| v | vapour |
Conflicts of Interest
References
- Reay, D.A.; Kew, P.A.; Dunn, P.D. Heat Pipes; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Çengel, Y.A. Heat and Mass Transfer: A Practical Approach; McGraw-Hill: Boston, MA, USA, 2007. [Google Scholar]
- De Schampheleire, S.; De Kerpel, K.; Deruyter, T.; De Jaeger, P.; De Paepe, M. Experimental study of small diameter fibres as wick material for capillary-driven heat pipes. Appl. Therm. Eng. 2015, 78, 258–267. [Google Scholar] [CrossRef]
- Faghri, A. Heat Pipe Science and Technology; Taylor & Francis: Washington, DC, USA, 1995. [Google Scholar]
- Franchi, G.; Huang, X. Development of composite wicks for heat pipe performance enhancement. Heat Transf. Eng. 2008, 29, 873–884. [Google Scholar] [CrossRef]
- Huang, X.; Franchi, G. Design and fabrication of hybrid bi-modal wick structure for heat pipe application. J. Porous Mat. 2008, 15, 635–642. [Google Scholar] [CrossRef]
- Huang, X.; Franchi, G.; Cai, F. Characterization of porous bi-modal ni structures. J. Porous Mat. 2009, 16, 165–173. [Google Scholar] [CrossRef]
- Li, J.; Zou, Y.; Cheng, L.; Singh, R.; Akbarzadeh, A. Effect of fabricating parameters on properties of sintered porous wicks for loop heat pipe. Powder Technol. 2010, 204, 241–248. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.-H.; Kong, Y.C.; Jang, S.P.; Choi, J.H.; Koo, J. Long-term reliability of the thermal performance of a flat-plate heat pipe using a prognostics method. Int. J. Heat Mass Transf. 2015, 82, 369–372. [Google Scholar] [CrossRef]
- Sheehan, J.; Queheillalt, D.T.; Norris, P.M. An evaluation of the wicking characteristics of stochastic open-cell nickel foams. In Proceedings of the 2006 ASME International Mechanical Engineering Congress and Exposition, IMECE2006, Chicago, IL, USA, 5–10 November 2006.
- Shirazy, M.R.S.; Blais, S.; Frechette, L.G. Mechanism of wettability transition in copper metal foams: From superhydrophilic to hydrophobic. Appl. Surf. Sci. 2012, 258, 6416–6424. [Google Scholar] [CrossRef]
- Deng, D.; Liang, D.; Tang, Y.; Peng, J.; Han, X.; Pan, M. Evaluation of capillary performance of sintered porous wicks for loop heat pipe. Exp. Therm. Fluid Sci. 2013, 50, 1–9. [Google Scholar] [CrossRef]
- Holley, B.; Faghri, A. Permeability and effective pore radius measurements for heat pipe and fuel cell applications. Appl. Therm. Eng. 2006, 26, 448–462. [Google Scholar] [CrossRef]
- Tracey, V.A. Sintering data for inco nickel powder type 255. In Technical Note; INCO: New York, NY, USA, 1979. [Google Scholar]
- Mistler, R.E.; Twiname, E.R. Tape Casting Theory and Practice; The Amercan Ceramic Society: Westerville, OH, USA, 2000. [Google Scholar]
- Dense Shaped Refractory Products—Determination of Bulk Density, Apparent Porosity and True Porosity; International Organization for Standardization: Geneva, Switzerland, 1998.
- Adkins, D.R.; Dykhuizen, R.C. Procedures for measuring the properties of heat-pipe wick materials. In Proceedings of the 28th Intersociety Energy Conversion Engineering Conference, Atlanta, GA, USA, 8–13 November 1993; American Chemical Society: Washington, DC, USA, 1993; Volume 2, pp. 911–917. [Google Scholar]
- Shirazy, M.R.S.; Frechette, L.G. Investigation of capillary properties of copper metal foams by the rate of rise method in the presence of evaporation. In Proceedings of the 13th InterSociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems, ITherm 2012, San Diego, CA, USA, 30 May–1 June 2012; IEEE Computer Society: San Diego, CA; USA, 2012; pp. 710–716. [Google Scholar]
- Hiemenz, P.C. Principles of Colloid and Surface Chemistry; Dekker: New York, NY, USA, 1986. [Google Scholar]
- Dwyer, O.E. Boiling Liquid-Metal Heat Transfer, 1st ed.; American Nuclear Society: Hinsdale, IL, USA, 1976. [Google Scholar]
- Barlow, M.; Planting, P.J. Wetting of metal surfaces by liquid alkali metals. Z. Metallk. 1969, 60, 719–722. [Google Scholar]
- Reay, D.A.; Kew, P.A.; McGlen, R.; Dunn, P.D. Heat Pipes: Theory, Design, and Applications; Butterworth-Heinemann, an imprint of Elsevier: Kidlington, Oxford, UK, 2014. [Google Scholar]
- Hansen, G.; Næss, E. Performance of compressed nickel foam wicks for flat vertical heat pipes. Appl. Therm. Eng. 2015, 81, 359–367. [Google Scholar] [CrossRef]
- Nam, Y.; Sharratt, S.; Byon, C.; Sung Jin, K.; Ju, Y.S. Fabrication and characterization of the capillary performance of superhydrophilic cu micropost arrays. J. Microelectromech. Syst. 2010, 19, 581–588. [Google Scholar] [CrossRef]
- Deng, D.; Tang, Y.; Huang, G.; Lu, L.; Yuan, D. Characterization of capillary performance of composite wicks for two-phase heat transfer devices. Int. J. Heat Mass Transf. 2013, 56, 283–293. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, G.; Næss, E.; Kristjansson, K. Sintered Nickel Powder Wicks for Flat Vertical Heat Pipes. Energies 2015, 8, 2337-2357. https://doi.org/10.3390/en8042337
Hansen G, Næss E, Kristjansson K. Sintered Nickel Powder Wicks for Flat Vertical Heat Pipes. Energies. 2015; 8(4):2337-2357. https://doi.org/10.3390/en8042337
Chicago/Turabian StyleHansen, Geir, Erling Næss, and Kolbeinn Kristjansson. 2015. "Sintered Nickel Powder Wicks for Flat Vertical Heat Pipes" Energies 8, no. 4: 2337-2357. https://doi.org/10.3390/en8042337






