1. Introduction
It is not clear what level of energy consumption would allow a future 100% renewable energy (RE) mix. Some studies suggest that a RE mix would allow the onset of a new growth cycle based on green technologies until a final stationary state were reached with an input of energy larger or similar to the present one [
1,
2]. However, they also conclude that if the investment in electrification of the economy is not enough, the future stationary RE production could be under the present level. This scenario is close to the scenario of “energy decline” defended by [
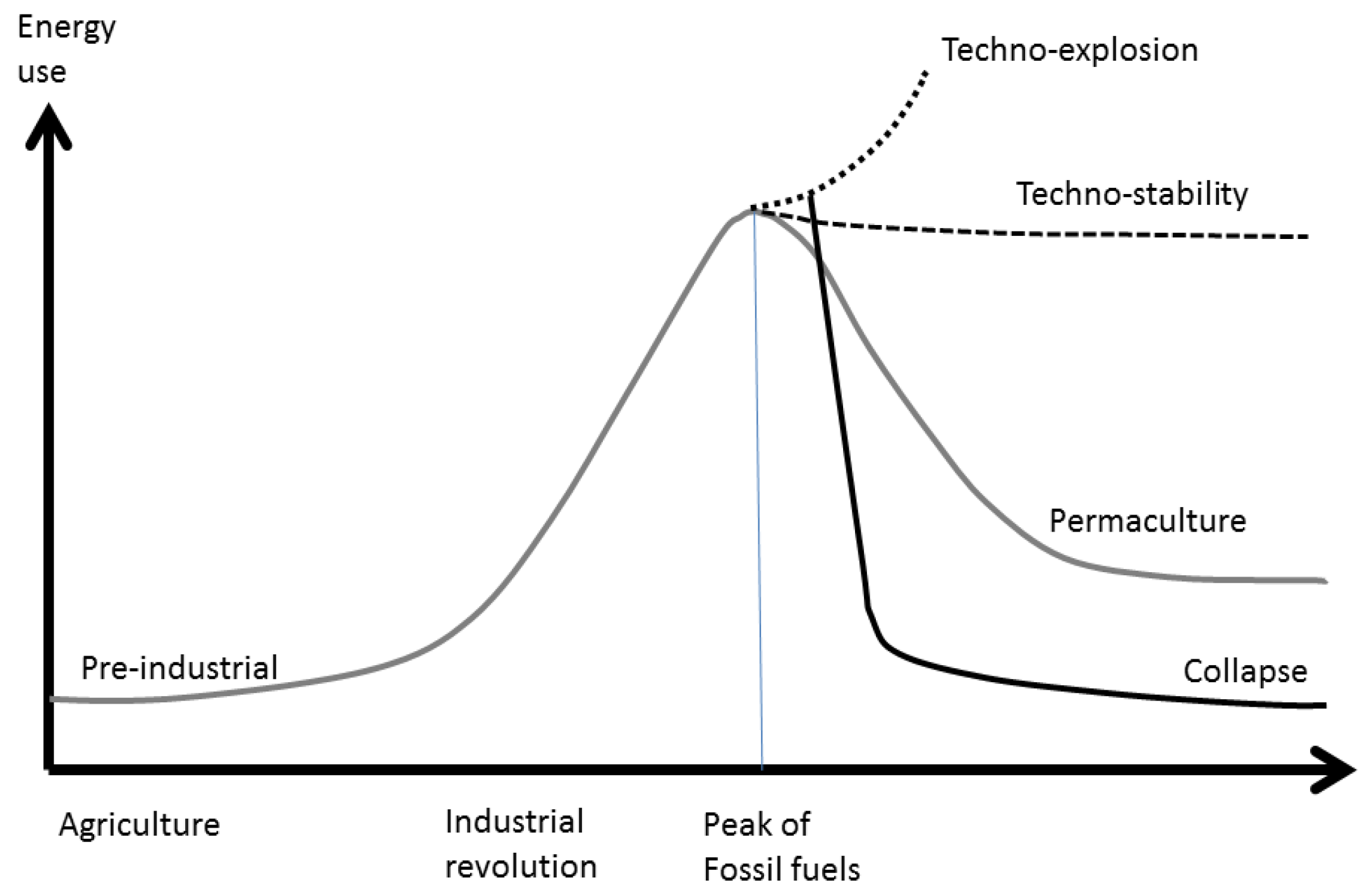
3]. Holmgren, like other members of the Permaculture Movement, assumes that the peak of fossil fuels will involve a reduction of energy input to the economy, because the substitution of renewables for fossil fuels will not be sufficient to maintain the enormous diversity of economic activities that were fueled by oil, coal and gas. He suggests that a lower energy density of RE will, over time, force a ruralisation of settlements and the economy, with less consumption of energy and resources, a progressive decline in human populations, and abandonment of high technology. He also outlines four potential ways in which our global society could respond and adapt to the fossil fuel peak and climate change (
Figure 1): (i) techno-explosion; (ii) techno-stability; (iii) controlled energy descent; or (iv) collapse.
The techno-explosion scenario (i) would be related to the discovery of new energy sources that would allow a rising consumption of energy despite the fossil fuel decline; techno-stability (ii) would involve a deployment of RE sufficient to sustain stationary consumption of resources, population and economic activity, with new electric industrial processes able to maintain if not improve the quality of services currently available.
Figure 1.
Four scenarios of energy consumption: techno-explosion, techno-stability, controlled energy descent (“permaculture”), and collapse, based on Holmgren [
3].
Figure 1.
Four scenarios of energy consumption: techno-explosion, techno-stability, controlled energy descent (“permaculture”), and collapse, based on Holmgren [
3].
The energy descent scenario (iii) would involve reduction of energy consumption and economic activity, adoption of “low technology” systems and permaculture farm techniques, and a final stationary energy consumption something over the pre-industrial level; finally, the collapse scenario (iv) would be an uncontrolled breakdown of economic and social systems close to the Duncan [
4] projection. It would involve a major “die-off” of human population and a loss of the knowledge and infrastructure necessary for industrial civilization.
We consider implausible the techno-explosion scenario because new technologies and energy systems take about 50 years to diffuse throughout the economy [
5]. Thus, the new energy systems that will replace fossil fuels in fifty years are most probably those that are currently being tested,
i.e., renewables. However, RE deployment is very dependent upon a set of materials with limited reserves, especially copper [
1,
6]. Therefore, it is highly improbable that a 100% RE economy may continue the customary exponential growth of energy supply, as is discussed in
Section 2.
We assume that our society has enough means and prudence to avoid the collapse scenario at any price. As was discussed by [
6], RE technologies are presently available which allow the supply of about 12 TW of electricity globally without shrinking present reserves of copper, lithium and nickel. It is improbable that, having a global energy source available, knowledge and technology will not be developed to use it efficiently. We do not believe in the cornucopian myth according to which there would always be enough energy resources if only the stock of knowledge increased quickly enough, but, in line with an argument used by Greer [
7] we do believe that, provided a new energy source has been found, human ingenuity will create technologies able to exploit it, especially if the alternative is collapse.
The two remaining scenarios (ii and iii) are both plausible and we think that the evolution of our economy will be closer to (ii) or to (iii) depending on the degree of substitutability of electricity for fossil fuels in the present economy sectors, and the extent to which irreplaceable processes are crucial for the working of the rest of the economy.
In this study we take as a reference a future stationary economy based on a 100% renewable mix, and we evaluate which present economic processes should be replaced by others based on electricity and renewable feedstocks, the concrete form that the new processes should adopt in terms of materials and energy flows, and the degree of substitutability of the main economic sectors. The objective is envisage if a post-carbon society would be compatible with an industrial economy similar to the present one, and what would be the main changes that the different economic sectors would suffer in such future society.
Some studies have quantified the monetary [
8] and energetic costs ([
9], submitted) that infrastructures would require in a 100% RE economy. Here we assume a scenario where this huge investment has already taken place, and we focus on the main features of the resulting post-carbon sustainable economy.
The manuscript is organized as follows:
Section 2 discusses the level of power that a 100% RE mix would be able to supply.
Section 3,
Section 4,
Section 5 and
Section 6 discuss the substitutability of the current fuel-based economic processes by electricity in a future stationary post-carbon society. The economic sectors analyzed are, respectively: agriculture, forestry and fisheries (
Section 3), transportation (
Section 4), commercial and residential sectors (
Section 5), and industry (
Section 6). Special care is taken in analyzing the main material and energy inputs of the industrial sector and their future substitutes, and the technical details of this discussion are shown in the
Appendix.
Section 7 studies the inputs of biomass, charcoal and biogas that such an RE mix would require and the limits that these materials would impose on the economy. Finally,
Section 8 summarizes the main conclusions resulting from the analysis.
2. Energy Supply in a 100% RE Economy
In previous works [
1,
6] we have argued that the fossil fuels peak could take place around 2030. In that context, a feasible way to avoid the risk of energy decline and to combat climate change is to build a worldwide 100% renewable energy mix. However, a globally electrified economy cannot grow much above 12 year-average electric terawatts (TWe) without putting pressure on the limits of copper reserves. A way to achieve RE up to 12 TWe would involve deployment of floating turbines over 10% of the continental shelves to depths of 225 m, land turbines over 5% of non-frozen continental areas, and installation of concentrating solar power farms over 5% of the areas of high insolation (deserts). New photovoltaic (PV) silicon panels do not use silver metallization or other scarce materials and could contribute up to 1 TW of decentralized residential power. Hydroelectricity has a potential of 1 TW but a fraction of this would have to be sacrificed for energy storage purposes. Hydro, concentrating solar power, wave energy and grid integration at continental scales may be sufficient to fit supply to demand while avoiding intermittency [
6,
10].
In 2005 1.47 TW of primary biomass was used for energy purposes, resulting in 1 TW of final energy after discounting losses in transport and processing [
11]. This consumption has not changed significantly over the last few decades and we can assume that it will remain the same in a future post-carbon society. Therefore, the total renewable power (including biomass) consumed by end users may be 13 TW, and the contribution of biomass to the renewable mix would be 8%.
An RE mix such as the one proposed above would have an Energy Return on Energy Investment (EROEI) of about 15, 35% lower than the estimated EROEI of the present energy mix. That should be sufficient to sustain an industrialized economy in the second half of this century provided substitution of electricity for fossil fuels is feasible and intelligently made [
10].
Could technological progress enable this cap of about 12 TW to be surpassed, and hence make indefinite exponential growth of energy production possible? The incorporation of fusion energy to the electric grid is expected at some date close to the end of this century [
12]; however a fusion-based energy system has the same dependence that renewables have on copper-based devices, and thus the electrified economy that it makes possible is as copper-consuming as the one based on renewables. An energy production mix based on renewables and fusion could be scaled exponentially only if a major substitution of copper for aluminum (which is abundant), graphene, and high temperature superconductors (HTS) were successfully implemented in electricity generators, engines and wires. Most of the consumption of copper in a post-carbon economy will come from windings in generators and electric motors [
1]. Squirrel-cage motors frequently use aluminum instead of copper for the conductive bars, however they are built for low and medium power (a few kW) and are outside the range required by a power generator (MW). Thus, the feasibility of this substitution is very uncertain [
2] and, also, even if new aluminum-based generators and motors were developed in the future, technological innovations take 40–50 years to expand throughout the economy [
5]. Thus, the scenario of a ceiling of 12 TW for a future 100% RE mix at the second half of this century seems more realistic and prudent than assuming major breakthroughs in the expected technological development.
World primary energy production was 496 EJ in 2005, which is equivalent to 15.7 TW of mean annual power. Primary energy includes the energy embodied in energy repositories such as oil (167.4 EJ), natural gas (99 EJ), coal (122.2 EJ), nuclear power (28.5 EJ), biomass (46.3 EJ), hydropower (30.1 EJ) and new renewable energies captured by solar panels, wind turbines,
etc. (2.3 EJ). Due to conversion and distribution losses the final energy that fueled the global economy was 330 EJ, or equivalently, 10,460 GW of fuels and electricity. The expected error value is a few percent due to uncertainties in the statistical processing of the original data. The share of this power consumed by end-use sector in 2005 was: 27.7% transportation, 26.8% industrial, 9.5% commercial, 24.6% residential, 9.2% feedstocks, and 2.3% agriculture, forestry and fisheries ([
11], Table 1.2 and Figure 1.5).
In what follows we analyze whether 12 TW of electricity and 1 TW of biomass (final) power will be able to fuel a future post-carbon economy that can sustain similar services to those of the 2005 economy, and what changes should be introduced into the main processes to maintain, as far as possible, the current economic services.
3. Agriculture, Forestry and Fisheries
Energy input to agriculture was 0.24 TW in 2005 (
Table 5) (from [
11], Table 1.2). We will assume that 8% of world biomass input of energy into the economy is also representative of the biomass consumption of agriculture. The share of fossil fuels in agriculture is highly variable between countries. In the USA 60% of energy input was fuel (diesel and gasoline), 5% gas and liquid petroleum, and 35% electricity in 2013 [
13]. We will take this share as representative of all developed countries. However, western agriculture is an extreme case of high use of machinery and irrigation and low use of labor, while developing countries are closer to the opposite extreme. Direct energy inputs of a typical rainfed arable crop farm are probably close to this second extreme and, according to Sims ([
14], see Figure 2.19) consist of 20% liquid fuels (diesel), 0% electricity and 80% feedstocks. We will take this latter share as representative of the fossil fuels used for agriculture in developing countries. According to Pimentel [
15] roughly the same energy is used in developed and developing countries in agricultural production.
We assume that oil is used mainly for transportation, which will be electrified in the future. Tillage sometimes requires high power tractors that, in the future, could be powered by fuel cells. However, for other generic farm work, if a grid connection point is installed on the farm, a fleet of smaller electric tractors would have no problem doing the open field work, since the battery recharge could be as frequent as needed, and some electric tractors would be working while others are recharging. For small farmers, the minimal traction equipment required would consist of one tractor and two rechargeable batteries. Of course, in such a future system, full connection of farms to the electric grid will become necessary. If that connection was not available or the farmer could not afford to own a tractor, tillage would have to be based on human labor, as is the case currently with small farmers in developing countries. We will assume that electric battery vehicles will replace only 23% of the total oil consuming machines of the sector, and 77% of it will be replaced with fuel cells tractors. The former figure corresponds to the “miscellaneous” category in the diesel input of a typical arable farm (see Figure 2.20 in [
14]).
We assume that gas is used for heating, which will be replaced by electric resistance heating that is 97% efficient [
16]; and that biomass will be used in the future with the same efficiency, about 22% for heat production in rural stoves [
17]. We assume that gas is used mainly for water and space heating with the typical efficiency of a condensing boiler (about 90%) [
18]). Oil is assumed to be used for transport and tilling purposes with the efficiency of diesel motors (40%) (
Table 1).
Given the above efficiencies, the 2005 power used in this sector (240 GW) will become 188 GW in a post-carbon economy. The expression used to make the calculation is the following:
where p
r is the mean annual power demanded by a renewable agricultural sector; p
f is the demand of the sector in 2005; and e
di, e
ba, e
fc, e
st, e
cb, e
er are the efficiencies of diesel engines, battery motors, fuel cell motors, rural stoves, condensing boilers, and electric resistance, respectively. Similar expressions are used for each economic sector hereafter.
Appendix A7 of the
Appendix analyzes the feasibility of producing ammonia and other agricultural feedstocks in a post-carbon society. While it seems feasible to produce ammonia renewably from biogas and hydrogen, renewable sources of phosphorous and potassium are not available to the present-day agriculture and this is a major problem that must be solved in a future post-carbon economy.
In the long term (from a few decades to a century) the world will run out of potassium and phosphorus, so the only available way to sustain productive agriculture will be to resort to organic farming [
19]. A move to fully organic farming will require good knowledge of soil ecosystems, and must be fine-tuned to the local climate. Also, it could reduce the output of grains by 20%–30% in the short term. However, in the long term, organic soils hold up in quality and even improve, and they resist erosion better than standard farmed soils. Therefore, in a few decades organic soils may achieve productivities close to those of artificially fertilized soils. Some models indicate that organic agriculture could produce enough food for the current world population: 2640–4380 kcal/person/day [
20,
21,
22]. In addition, in the majority of cases studied, organic systems are more profitable than non-organic ones due to lower input costs [
23]. Use of plant, animal and human wastes for production of compost and natural fertilizers will allow recycling of phosphorous and other nutrients. This is the only available solution to the future decline of phosphorous mining that has been predicted for 2040–2050 [
24,
25].
Organic farming as a solution to the decline of fertilizer availability is, however, not compatible with an always rising population, since available arable land
per capita has decreased from 0.5 to 0.24 ha between 1961 and 2005 [
26]. There is not much room for additional decrease of arable land
per capita, because the supply of the principle cereals is saturating at their biological limits: 7–8 tonnes/ha for wheat and 7 tonnes/ha for rice, despite the increasing input of fertilizers [
27]. Depletion of groundwater basins must also be curbed, which requires putting a stop to population growth. About one third of the Earth's largest groundwater basins are being rapidly depleted by human consumption, which means that significant segments of the population are consuming groundwater without knowing when it might run out [
28].
Renewable exploitation of forests will also be crucial in a post-carbon economy, not only for reasons of climate and biodiversity, but also because of their importance in renewable production of wood biomass and charcoal (see
Section 7).
Regarding fisheries, fishing fleets consisted of about 4 million vessels in 2002, 1/3 decked and 2/3 undecked, with 65% of undecked boats not using mechanical propulsion systems [
29]. This is equivalent to 1.32 million vessels with large motors and 0.23 million vessels with light motors; 0.005 and 4 × 10
−4 times the number of large and light motors being used for the transportation sector, respectively. In principle, all these combustion engines could be replaced by fuel cell motors in a future post-carbon economy, as discussed in
Section 4.
4. Transportation
Hydrogen has certainly been proposed as an energy source that is similar to oil and natural gas, and that could be used for transportation. However, present electrolytic systems require around 60 kWh to produce 1 kg of hydrogen [
30], which implies an energy efficiency of 65% if we take HHV of hydrogen as output. This implies that hydrogen produced and consumed on-site has 1.53 times more electricity embedded than its own HHV content. If losses along the hydrogen conversion chain,
i.e., containing, liquefaction, transport and handling are also taken into account, the result is that the production of hydrogen for consumption by a jet turbine or fuel cell requires 1.8 times its HHV energy content in the form of electricity [
31] or, equivalently, 2.1 times its LHV content that is the metric that is being used in
Table 4.
In addition, electrical motors are more efficient than fuel cell motors (
Table 1) and, for both reasons, a fuel cell vehicle requires 3.6 times more integrated electricity consumption than an electric vehicle [
31]. Also, the hydrogen produced is five times more expensive than the direct use of renewable electricity [
32]. Thus, the direct use of electricity by motors is a cheaper and more efficient way to produce movement, and is the most promising option in future ground transportation [
1]. The exception would be aircraft and other forms of transport that are not able to receive energy from the electric grid, as well as vehicles with specific requirements for both autonomy and power, such as ambulances, fire engines and police cars.
Efficient land transport should ideally be based on electric trains for freight and passengers between cities, and electric vehicles (EV) for short-distance transport between cities and villages [
1,
33]. However, we will assume, pessimistically, that future land transport will instead be based on the electrification of the present vehicle fleet.
About 600 million small vehicles, 205 million commercial (heavy) vehicles and 215 million motorcycles were circulating in 2005 if we linearly extrapolate the trend observed by [
34] for the period 2000–2003. The number of commercial vehicles that will use fuel cells is very dependent of the future weight given to trains for long distance freight. We will assume that its number will be only 10% of the number of commercial vehicles, because with this percentage 99% of Pt reserves (and 22% of Pt and Palladium reserves) would have to be used in the fuel cell electrodes. Palladium is more abundant than Pt and it has been reported to be a possible substitute of Pt for fuel cells, although not with identical performance [
35]. Lithium-ion batteries have the largest energy density and, for this reason, are the most used in current electric cars. Taking typical battery capacities and power for these three classes of vehicles (
Table 1) and the density of metal used in their respective motors (
Table 2), the quantity of lithium (Li) that such a fleet would require would be 7.8 Mt. Alternatively, nickel Na-NiCl
2 (Zebra) batteries are technically feasible. If these batteries were used to renew the world fleet, 65 Mt of nickel would be used [
1]. These figures amount to 58% and 80% of present reserves of Li and Ni, respectively [
36]. If 50% of Li batteries and 50% of Ni batteries were used, 29% and 40% of the present reserves of Li and Ni would be used, respectively. Electrification of vehicles will be necessary, but given that reserves cannot be indefinitely expanded [
2], the number of vehicles that a future post-carbon society could sustain is roughly the number we have currently. A larger number would endanger the availability of Li and Ni for other economic demands.
Table 1.
Classes of electric vehicles (column 1), global number in 2005 (column 2), typical peak power of its battery (column 3) and battery capacity (column 5). The parameters used are discussed in [
1].
Table 1.
Classes of electric vehicles (column 1), global number in 2005 (column 2), typical peak power of its battery (column 3) and battery capacity (column 5). The parameters used are discussed in [1].
| Kind of EV | Number in 2011 (millions) | Power (KW) | Battery Capacity (KWh) |
|---|
| Light | 600 | 60 | 22.4 |
| Heavy | 205 | 179 | 67 |
| Motorcycle | 215 | 3.6 | 1.2 |
Table 2.
Values used for the estimation of metals required by the transport system. Density refers to the mass of metal used per unit of power or per unit of energy stored in engine, battery or fuel-cell. The parameters used were discussed in [
1].
Table 2.
Values used for the estimation of metals required by the transport system. Density refers to the mass of metal used per unit of power or per unit of energy stored in engine, battery or fuel-cell. The parameters used were discussed in [1].
| Metal | Density | Reserves (106 t) |
|---|
| Copper | 0.73 kg/kW | 680 |
| Lithium | 0.3 kg/kWh | 13.5 |
| Nickel | 2.5 kg/kWh | 81 |
| Platinum | 0.004 kg/kW | 0.015 (0.07) a |
Marine shipping consists of about 87,500 ships, not including naval and fishing boats [
37]. Naval ships are only a few thousand globally [
38], therefore we can take 100,000 as an upper estimate of the number of non-fishing ships. Assuming that this kind of vessel uses four heavy engines, the total number of marine engines would be 1.72 × 10
6 heavy and 0.23 × 10
6 light engines. We will use 60 kW as a typical power for a light engine, and 179 kW as the power of a heavy engine [
1].
To complete the number of light and heavy motors that would probably require fuel cells, we need to add the world number of heavy farm tractors, the number of ambulances, and the number of police cars and fire vehicles. Farm tractors were estimated to number about 28,570,900 in 2005 [
39]. We will assume that 10% of these will be high power tractors that will need fuel cells. The number of ambulances per capita was about 1 per thirty thousand people in Turkey [
40] and 1 per 4350 people in Australia ([
41] Table 9A.39), figures that we will consider to be representative of an average developing country and a mean developed country, respectively. We will assume that the number of police cars is the same as the number of ambulances. Fire engines will be considered to be far fewer in number. Under these assumptions, and considering that developing countries included 81% of world population in 2005, we obtain a total number of 1,200,000 light engines and 4,577,000 heavy engines, which must be considered as orders of magnitude, and not precise estimates. Assuming that 0.2 kg of Pt or its substitute, palladium, is needed for a fuel cell of 50 kW [
42], the mass of Pt or Pa necessary for such a fleet of fuel cell engines is 18,250 t. This is 28% of present reserves of platinum and palladium (66,000 t) ([
36], platinum). Thus, fuel cell deployment could be sufficient in principle to meet the current requirements of special vehicles and 10% of commercial vehicles, even though not very scalable, except if palladium and new materials were developed to fully substitute for platinum in the fuel cell catalyzers.
Assuming that fuel cell vehicles will mainly be ambulance, police and 10% of commercial vehicles, and taking into account the energy required for producing electrolytic hydrogen, and the efficiencies given in
Table 3, the power required for road transport would be 893 GW.
We assume that half of world train transport has already been electrified and that the other half uses diesel locomotives. Assuming that most of the energy consumption of train transport is for locomotive traction, and using the motor efficiencies shown in
Table 1, we estimate that the power required for 100% electrified railroad transport is 52 GW. The energy demand of the transport system could be substantially reduced if current ground transportation based on cars were replaced with a substantial increase in rail transport. As an example, a typical intercity train transports eight times more seated passengers per MW than a car (204 people/MW
vs. 25 people/MW) [
10]. Therefore, if well organized, it has the potential to reduce the energy consumption of future road transport by a factor of eight.
Regarding marine transport, we assume that most of the energy consumed by the sector goes to vessels and mechanical motors. Under this assumption, we estimate that to produce the service obtained in 2005 with 285 GW, a post-carbon economy would require 830 GW. This factor of three increase in energy demand derives from the need to produce hydrogen for fueling the marine fuel cell engines. However, 10%–35% of this energy could be saved if wind systems were used in tandem with the motors of the vessel, such as the SkySails kite system [
43]. In kite propulsion systems, high-altitude wind energy is directly converted into traction without intermediate conversion to electricity, and with the low cost and maintenance that is typical of low-technology systems.
About 89% of the transport system could possibly be electrified in a post-carbon society, since world air transport uses only 11% of the energy demanded by the transport system ([
11], Table 1.2). To sustain the same volume of air traffic as in 2005, air transport would need 3% (11% of 27%) of 10.4 TW from fuel, or 0.32 TW from hydrogen (H
2) and biofuels. If electrolysis were used to supply H
2, about 696 GW of renewable electricity would be required. This result has been included in
Table 5.
As we will see in
Section 6 and
Section 7, in a post-carbon economy demand for woody and non-woody biomass will be superior to the available renewable biomass production if we want to maintain the economic levels of industrialized economies such as those of 2005 or 2012. For this reason, hydrogen would be a more convenient fuel than biofuels in the long term. On the other hand, biofuel-oriented crops may compete with food crops for arable land and should be avoided wherever possible. However, as we will see in
Section 7, about 98 GW of biogas could be saved if future agriculture becomes fully organic and ammonia is no longer industrially produced. A similar situation would result if ammonia were produced directly from hydrogen and air and not from biogas. This would make available a fraction of agricultural wastes able to produce around 98 GW of biofuels. Provided that the present structure of the transport system is not modified, this would amount to 11% of the future aviation demand.
6. Industry
In 2005 the total energy used by industry was 88.2 EJ of fossil fuels and electricity and about 25 EJ of feedstocks,, which is equivalent to 3.59 TW of mean annual power ([
11], Figure 1.5).
Table 5 (column 2) shows the share of the industrial use of energy that year.
The industrial sector is strongly dependent on electricity, coal, gas and oil, in this order (see
Table 4, based on [
51], Table 8.22). However, oil is especially important in the petrochemical and construction sectors. Future decline of many feedstocks coming from oil and coal will force us to substitute these feedstocks for others coming from renewable sources, or to abandon the process.
The degree of substitutability of fossil fuels in the different industrial sub-sectors and their energy cost has been analyzed in detail in the
Appendix. Expressions similar to Equation (A1) have been used for each industry to estimate the energy use in a post-carbon economy. The parameters of
Table 3 have been used for the efficiency of different devices, and the efficiency of the central heating and electro-chemical processes of the specific industry, as well as other processes such as steam generation, have been estimated from the Sankey (energy flow) diagrams produced by the US-DOE for the specific industry [
52], when available. The final result of this analysis is shown in
Table 5, rows 10 to 24. As can be seen in that table, a post-carbon industry would use less energy than the present industry in all the sectors except iron and steel and non-ferrous metals. The energy saving would be specially high in nonmetallic minerals production due to the high heat loss of conventional kilns, which could be minimized by using electric and microwave assisted heating (see
Appendix A6). Many industries, such as transportation equipment, machinery, textiles and others are partially electrified in their central processes and their full electrification should not be a major problem.
The work done in open terrain for wood extraction, construction and mining will have to be fully electrified. Indeed, mobile electric construction machines will have more limitations than fuel powered ones and therefore open field work will have to be planned in a different way. For instance, it will require the installation of temporary grid connections, fleets of (electric) vehicles with lower autonomy and power but in larger number than at present, and different design approaches for the extraction and transport of heavy loads. If we examine a map of the distribution of electricity sub-stations in Spain, which supply power to villages and factories, we can observe that even in less densely populated regions (La Mancha) no point is further than 40 km from a sub-station, and that villages with an electrical supply can be found at a maximum of about 20 km [
53]. Thus, any mining or construction project outside of urban areas will have to include the building of a power connection (of up to 40 km in Spain, maybe longer in some developing countries) to the nearest power sub-station. Once the connection is installed, power shovels could be connected to the grid as do actually many dragline excavators and giant power shovels and bucket wheel excavators used presently in mining. Transport of moderate loads could be made by a fleet of electric vehicles, since the battery recharge could be as frequent as needed, and many electric vehicles would be working while others are recharging. Finally, transport of heavy loads on complex orography could use fuel cell powered vehicles.
Table 4.
Energy input (GW) for the global industrial sector by industry and energy type in 2005 (based on [
51], Table 8.22). Energy from fossil fuel feedstocks is excluded. A zero is displayed when the figure is under 0.05 GW.
Table 4.
Energy input (GW) for the global industrial sector by industry and energy type in 2005 (based on [51], Table 8.22). Energy from fossil fuel feedstocks is excluded. A zero is displayed when the figure is under 0.05 GW.
| Industry | Coal and Derivatives | Crude, NGL, Feedstocks | Petroleum Products | Natural Gas | Geothermal | Solar, Wind, Other | Combustible Renewables and Wastes | Electricity | Heat | Total |
|---|
| Iron and steel | 250.8 | 0 | 20.1 | 77.8 | 0 | 0 | 8.5 | 103.3 | 15.7 | 476.3 |
| Chemical and petrochemical | 58.1 | 0.1 | 80.9 | 150.4 | 0 | 0 | 3.0 | 113.2 | 45.9 | 451.6 |
| Non-ferrous metals | 15.4 | 0 | 10.3 | 19.2 | 0 | 0 | 0.2 | 67.5 | 2.7 | 115.3 |
| Non-metallic minerals | 181.3 | 0 | 48.2 | 66.7 | 0 | 0 | 6.7 | 42.7 | 3.4 | 349.0 |
| Transport equipment | 4.8 | 0 | 4.0 | 13.4 | 0 | 0 | 0 | 18.7 | 4.3 | 45.2 |
| Machinery | 13.2 | 0 | 15.0 | 27.9 | 0 | 0 | 0.1 | 66.3 | 6.1 | 128.6 |
| Mining and quarring | 9.6 | 0 | 18.1 | 12.8 | 0 | 0 | 0 | 26.2 | 3.3 | 70.0 |
| Food and tobacco | 25.2 | 0.1 | 34.4 | 43.4 | 0 | 0 | 35.1 | 40.7 | 11.3 | 190.2 |
| Paper, pulp and printing | 25.2 | 0 | 19.1 | 33.9 | 0.2 | 0 | 65.6 | 53.9 | 6.7 | 204.5 |
| Wood and wood products | 2.8 | 0 | 4.5 | 3.8 | 0 | 0 | 13.1 | 11.1 | 6.8 | 42.0 |
| Construction | 6.8 | 0 | 26.6 | 4.9 | 0 | 0 | 0.2 | 6.8 | 1.6 | 46.9 |
| Textile and leather | 14.3 | 0 | 11.6 | 11.6 | 0 | 0 | 0.3 | 25.1 | 7.6 | 70.5 |
| Non-specified industry | 75.0 | 4.9 | 133.7 | 108.0 | 0.2 | 0.2 | 105.4 | 130.4 | 30.6 | 588.3 |
| Total (GW) | 682.4 | 5.2 | 426.4 | 573.8 | 0.4 | 0.2 | 238.2 | 706.0 | 145.9 | 2778.4 |
Iron and many non-ferrous metals require carbon as a reducing agent in their production, which could be obtained from renewable charcoal in a post-carbon economy. However, demand for charcoal by the petrochemical industry will saturate in a future post-carbon economy (see
Appendix A8) and also its future renewable production will probably not be sufficient to supply the large quantities of coke needed for iron reduction (see
Table 6). Fortunately, sponge iron produced by the direct reduction process is a proven technology that is becoming increasingly popular due to the shortage of high-quality coking coals [
54,
55]. In the process of producing sponge iron, hydrogen is frequently employed as the reductive gas, through the following aggregate reaction:
and this process could be the best suited to a future post-carbon economy. Then, crucible steel may be produced by diffusion of charcoal, a process that is known in the industry as “carburization” [
56].
Other metals that could be primarily produced by direct reduction with hydrogen are copper, tin and nickel (see
Appendix A5). Some metals and ferro-alloys require coal as reducing agent, however, as we will see in
Section 7, present levels of ferro-alloy production could be supplied through the future use of renewable charcoal or methane. Secondary production of the main metals (recycling) already involves the use of electric arc furnaces which can be, in principle, fully electric.
Table 5.
Energy consumed by the transport, commercial and residential sectors, agriculture, forestry and fishery and industrial sectors, in 2005 (column 2) and in a post-carbon economy (column 3). Industrial energy use in 2005 is based on Figure 8.1 in [
51]. The energy required for future hydrogen production in some sectors is included in the corresponding box of column 3. An important fraction of energy consumed by the chemical and petrochemical sectors (759 GW) is embedded in feedstocks, as well as 34 GW of coal for chemicals, graphite and steel production, which have been implicitly accounted in “Chemicals and Petrochemicals” (26 GW), or explicitly included in “Non-ferrous metals” (1 GW) and “Iron and steel” (4.8 GW).
Table 5.
Energy consumed by the transport, commercial and residential sectors, agriculture, forestry and fishery and industrial sectors, in 2005 (column 2) and in a post-carbon economy (column 3). Industrial energy use in 2005 is based on Figure 8.1 in [51]. The energy required for future hydrogen production in some sectors is included in the corresponding box of column 3. An important fraction of energy consumed by the chemical and petrochemical sectors (759 GW) is embedded in feedstocks, as well as 34 GW of coal for chemicals, graphite and steel production, which have been implicitly accounted in “Chemicals and Petrochemicals” (26 GW), or explicitly included in “Non-ferrous metals” (1 GW) and “Iron and steel” (4.8 GW).
| Energy end use | Final energy in 2005 (GW) | Final energy in post-carbon economy (GW) |
|---|
| Road | 2100 | 893 |
| Rail | 73 | 52 |
| Shipping | 285 | 830 |
| Air | 330 | 696 |
| Pipelines | 90 | 0 |
| Total transport | 2900 | 2471 |
| Residential and commercial | 3800 | 2954 |
| Agriculture, forestry, fishery | 240 | 188 |
| Wood and wood products | 42 | 35.8 |
| Transport equipment | 45.2 | 39.2 |
| Machinery | 128.6 | 110.7 |
| Construction | 46.9 | 27.1 |
| Mining and quarrying | 72 | 14 |
| Textiles and leather | 72 | 64.1 |
| Renewable feedstocks | - | >541 to >596 * |
| Non-ferrous metals | 115 + 1 | 115 + 1 + 1.6 + 0.2 |
| Food and tobacco | 190 | 162.6 |
| Paper, pulp and print | 216 | 173.2 |
| Non-metallic minerals | 350 | 236 |
| Iron and steel | 476 + 4.8 | 720 + 4.8 |
| Chemicals and petrochemicals | 1057 | 628 to 785 ** |
| Others | 588.3 | 424.5 |
| Total Industry | 3405 | 3292 to 3504 |
| Total | 10,343 | 8912 to 9124 |
The chemical and petrochemical sector is the most energy demanding of the industries, 1057–1075 GW in 2005, including the embedded feedstock ([
51,
57], (
Table 5)). About 50% of this energy, 544 GW, was used to produce high value chemicals (HVC), mainly olefins (ethylene, propylene and butylene) and aromatics (benzene, toluene and xylenes). An important fraction was also used for ammonia production (188 GW), and for methanol production (37 GW). From these primary chemicals, a large number of secondary chemicals and end-products are produced, by processing and polymerization, which consumes 4% of the sector energy (44 GW).
The HVC production is currently based on naphtha, one of the main derivatives of petroleum, which may be replaced by charcoal in the coal to olefins process (see
Appendix A8 for details). However, for obtaining the level of HVCs production of 2004 we would precise 3.7–4.6 times the renewable potential of charcoal production estimated in
Section 7. This suggests that maintaining the current level of petrochemical production may be unfeasible in a post-carbon economy, and that we will have to reduce our present consumption.
Appendix A8 of the
Appendix details the parameters used in the estimation of energy consumed by the chemical and petrochemical sectors, which is showed in
Table 5. Some biological substitutes for the principle petrochemical products are also suggested.
7. Biomass, Charcoal and Biogas Production in a Post-Carbon Economy
Charcoal and biogas will have to be produced in larger quantities in a post-carbon economy than they are at present, in order to compensate for the decline of fossil coal, oil and gas. That increased production should be obtained from renewable biomass production. Governments will have to force this to happen with laws forbidding plant cultivation for biofuels and biogas production, and nonrenewable logging. If they do not enforce this, it is probable that production of biological feedstocks will compete with agriculture, as is presently happening in the USA, Indonesia, Africa and other regions [
58,
59]. Clearing of tropical rainforests for rapeseed and palm oil production is currently destroying biodiversity and releasing 17 to 420 times more CO
2 than the greenhouse reduction that these biofuels would provide by displacing fossil fuels [
58].
The post-carbon stationary economy that we suggested in the Introduction would avoid these problems since the exploitation of wood and agricultural waste (needed for producing charcoal and biogas) would be stationary and sustainable, and would use agricultural crops to produce biogas. If we focus exclusively on the sustainable exploitation of temperate and tropical forests, up to 3 t/ha/year of woody biomass could be extracted from these forests whilst maintaining stationarity [
60]. The carbon content of tropical wood is 47.3% on average [
61] and similar in temperate wood [
62]. Forests occupied 4000 million Ha in 2012 [
63], assuming that 10% of this extent were sustainably exploited for wood extraction and that 5 t of wood are needed to produce 1 t of charcoal [
64], 240 million tons per year of charcoal could be produced, almost 5 times the present production.
An alternative estimate can be made from the potential of renewable biomass, which is about 103.8 EJ/year, 40% of it coming from woody biomass [
65]. World biomass consumption for cooking, heating, and industrial wood-related activities was 46.3 EJ/year in 2005, 75% of it in developing countries and 25% in developed countries. Assuming that these end-uses are inflexible, and taking into account that 40% of biomass used comes from wood, 18.5 EJ/year of wood consumption must be reserved for these end-uses and 23 EJ/year of wood would be available for other industrial uses, which is equivalent to 1.2 × 10
9 t/year of dry wood if we take 19,000 kJ/kg as the heat content of dry wood. Assuming again 5 tons of wood for 1 t of charcoal produced, 1.2 × 10
9 t/year of wood could be sufficient to produce 240 × 10
6 t/year of charcoal, the same result as in our previous estimation.
Mass yields from a Casamance kiln and a well-managed traditional mound kiln are somewhat higher than the world average, about 25% [
64], therefore the previous potential could be increased to 300 × 10
6 t/year if future governments encouraged the use of best available technologies. Finally, this figure could be increased by an additional 6% if governments of North America and Europe would force the replacement of wood fuel with electric heating (see Table 3 of [
65]).
Methane obtained from biogas could be an alternative source of a reducing agent for ferro-alloys and other metals. In the future, energy crops will probably compete with food production for soils and so should be avoided, however biogas could be obtained from crop residues and urban wastes. Current global production of methane from biogas is only 20–26 × 10
6 t/year, but global potential has been estimated at approximately 900 × 10
9 m
3 [
66] or, equivalently, 600 × 10
6 t/year. If only the European Union, USA and China were able to make the investments needed to develop the new biogas infrastructure, the potential for CH
4 production would be approximately 215 × 10
6 t/year.
As a consequence, present levels of tin, lead, zinc and ferro-alloy production could be supplied through the use of charcoal or methane by a small expansion of the present level of woody biomass and biogas production. This is apparently feasible in both cases.
Charcoal and natural gas production do not involve rock grinding or underground digging and ventilation, both of which are very energy consuming. Therefore, the energy needed to supply the reducing carbon from charcoal should not be larger than that currently needed to extract it from mining.
HVCs production is the most demanding sector regarding charcoal and natural gas and the route from methane to olefins via methanol and dimethyl-ether is the most efficient (see
Appendix A8). After discounting 65.6 × 10
6 t of natural gas needed for ammonia production, 149.4 × 10
6 t of CH
4 remain, which would allow production of 92.6 × 10
6 t of HVCs using that process. If we need to produce 287 × 10
6 t of HVCs (
Appendix A7), 194 × 10
6 t should be produced from charcoal. Given that 4.1 t of carbon are needed to produce 1 t of HVCs (
Appendix A8) and assuming 75% carbon content in charcoal [
67], we find that 1063 × 10
6 t of charcoal would be required.
Table 6 summarizes the new production of charcoal, hydrogen and biogas required for the different sectors if a post-carbon society had to reach the same production level as in 2005. The global production of charcoal and biogas that is probably obtainable in a renewable way is also indicated. High quality industrial charcoal has 75% carbon content [
67], and a factor 1.33 has been used to translate carbon required for charcoal used in each sector. All the energy requirements of the marine and aviation sectors in 2005 are assumed to go to propulsion, in the form of hydrogen, and the same efficiency to produce movement is assumed for the energy of hydrogen and kerosene in aviation jets.
Table 6.
New production of charcoal, hydrogen and biogas required for the different sectors if a post-carbon society had to reach the same production levels as in 2005. The second figure in the third column represents the electrical power required to produce the hydrogen.
Table 6.
New production of charcoal, hydrogen and biogas required for the different sectors if a post-carbon society had to reach the same production levels as in 2005. The second figure in the third column represents the electrical power required to produce the hydrogen.
| End-Use Sector | Charcoal (103 t/year)/(GW) | Hydrogen (103 t/year)/(GW) | Biogas (103 t/year)/(GW) |
|---|
| Vehicles fuel cells * | | 17,000/138 | |
| Marine fuel cells | | 60,048/411 | |
| Aviation | | 85,902/588 | |
| Iron and steel | | 64,000/440 | |
| Copper reduction | | 600/4 | |
| Tin reduction | 40 | or 5/0.03 | |
| Nickel reduction | | 81.8/0.6 | |
| Lead reduction | 710 | | |
| Zinc reduction | 1600 | | |
| Ferro-alloys | 30,100 | | |
| Graphite | 1334 | | |
| Ammonia | | 24,706/169 | or 65,634 |
| High value chemicals | 1,063,230 | | 149,366 |
| Total | 1,097,010 | 252,343/1,751 | 215,000 |
| Global potential | 240,000/221 to 300,000/276 | Not applicable | 215,000/320 |
| Percent of global potential | 366 to 457% | Not applicable | 100% |
As can be seen in this table, charcoal needed for a future post-carbon economy is about 4 times above the global potential. This implies that, if the charcoal demand of metal industries is respected, only 45%–49% of the HVCs produced in 2004 could be produced in such a future economy, 92.6 × 106 t from biogas and 37.7–48.7 × 106 t from charcoal.
The routes from carbon and natural gas to HVCs are also more energetically expensive: 45 GJ per ton of HVC produced and 29 GJ per ton of HVC produced, respectively, to be compared with 18 GJ per ton of HVCs produced with the conventional naphtha route (embedded energy not included). If we take into account these factors, the quantities of available charcoal and biogas and the quantities of charcoal and gas to be used for HVC production (
Table 6), and compare with the energy used in 2004 to produce HVCs ([
51], Table 8.5) we find that 544 GW would be required, to be added to the energy cost of producing ammonia, ethanol and all the petrochemical products. We will assume that, in a post-carbon economy, ammonia production will initially be the same as in 2005 (177 GW); but that it will decrease to 15% of this figure following the implementation of a future organic agriculture. 15% of ammonia is expected to continue to be necessary for textile fiber processing, nitric acid production, refrigeration for bulk food storage, water purification, antimicrobial agents, rubber production, metal plating, and other uses [
68,
69]. We will assume also that other petrochemical activities different from HVC and ammonia production will diminish in the same proportion (45%) as the decrease of HVCs. With these assumptions, the total consumption of a future petrochemical sector amounts to 785 GW, decreasing to 628 GW when full organic agriculture is implemented.
8. Discussion and Conclusions
A future post-carbon economy is the only sustainable solution to the present challenges of energy security and rising environmental impacts. Such a future economy should be based on a mix of decentralized and centralized renewable sources. Some studies have warned of the necessity and difficulty of the transition to such a society, and others argue that such a transition is incompatible with the maintenance of an industrial society.
The present analysis shows that a future post-carbon society seems capable of sustaining an industrial developed economy, if the investments needed to implement the appropriate process substitutions are done. Actually, a post-carbon economy based on direct use of renewable electricity is able to provide similar services to those of the 2005 economy.
However, global electrification would put the finite supplies of copper, nickel, lithium and platinum under rising stress. Therefore, a post-carbon economy will have to adapt, sooner rather than later, to a stationary consumption of energy and materials. Further growth in the throughput of that future economy will be dependent on improvements in efficiencies of charcoal and biogas production, as well as on dematerialization of processes.
About 10.3–10.4 TW of final energy was required in 2005 for all the economic end uses. As can be seen in
Table 5, the conclusion of our analysis is that a 100% RE economy trying to provide the same services as the economy of 2005 would require initially about 9.1 TW, and 8.9 TW after BAT in charcoal production and implementation of full organic agriculture. Thus, a 100% renewable economy would require 87% of the energy consumed in 2005 to provide similar services, except for the petrochemical sector, which would be similar to the one of 1985. No savings due to efficiency improvement have been assumed, however demand-side energy-conservation measures could allow savings of about 5%–15% of energy demand [
31], which would amount to 0.5–1.3 TW in a post-carbon economy.
Once we have obtained these general figures, a comparison with the recent or near future economy becomes easier. As an example, total final consumption of energy in 2012 was 8979 Mtoe [
70], which is equivalent to 11.9 TW. If a post-carbon economy would require 87% of that energy, 10.4 TW would supply similar services to those supplied by that recent economy. Thus, 12 TW of renewable electricity and feedstocks and 1 TW of biomass (that is the maximum supply compatible with a prudent use of the mineral reserves) would be more than sufficient to supply similar services than the present economy, except for the petrochemical sector. On the other hand, economic growth of developing countries is expected to increase the world energy consumption in the next decades, which has been estimated in 460 to 520 EJ/year in 2030 [
71]. This range is equivalent to 14.6–16.5 TW, or 12.7–14.4 TW if the services were supplied for a post-carbon economy. If we accept that a sustainable level of final energy supply is not much above 12 TW of electricity and new feedstocks and 1 TW of traditional biomass, the conclusion is that in 2030 the economy may be on the limit of what is sustainable.
On the other hand, world's population continues increasing and, in a future globalized society, industrial economy will have to grow accordingly to world's population in order not to get a per capita decline. The medium variant of World Population Prospects of the UN [
72] estimates that world population will increase from 6520 million in 2005 to 8500 million in 2030, 9725 million in 2050 and 11200 million in 2100. In order to maintain the same amount of energy per capita than in 2005 to this higher number of people, energy production would have to be 13.4 TW, 15.4 TW and 17.7 TW in 2030, 2050 and 2100, respectively or, in RE equivalents, 11.7 TW, 13.4 TW and 15.4 TW. Thus, even maintaining a constant energy use per person, expected population growth may take us to unsustainable levels of energy production after 2050. Under present growth expectations, if some technological alternatives to copper do not appear on time, RE might probably not be enough to maintain an industrial society as we presently understand it in the long term, and all these over-shoot scenarios mentioned above would imply a forced de-growth in a future post-carbon economy.
Over the scale of several decades to one century we cannot discard technological innovations that enable replacement of copper and lead to a possibly larger energy supply, and even unexpected techno-explosions. However, even in that case, we cannot know if such technological surprises will permit sustained exponential energy growth, linear growth or only logistic growth in the long term. Therefore, in a post-carbon economy we should be prepared to work under all the range of possibilities, from stationary energy supply to exponential growth. Such an economy would adapt its growth to the availability of new energy sources and feedstocks, and not the opposite, and in this context any improvement in economic efficiency would allow the energy available (e.g., 13 TW) to satisfy a larger service demand. This will probably require structural transformations of capitalism such as we know it [
73]; it is high time to stop considering any change in capitalism as the end of the world.
Provided that future economy is able to adapt to a stationary energy supply and that population can be limited to less than 9700 million, the above analysis shows that the main economic processes can, in principle, be replaced by sustainable alternatives based on electricity, charcoal, biogas and hydrogen. And contrarily to the most pessimistic expectations, those services that cannot be replaced are not as crucial as to cause a return to a pre-industrial society.
RE are physical energies and in many economic sectors, such as transport, people demand essentially physical services as well. Conversion of RE into chemical energy and then back again into physical energy wastes an important fraction of the initial RE produced, and should be avoided through direct use of electricity in transport [
31]. This implies avoiding the use of hydrogen in transport except where connection to the grid or battery use are impossible, e.g., in aviation, shipping, and specialist (emergency) vehicles.
Even so, if land transport at present levels were based on a fleet of electric cars, trucks and motorcycles, and 50% of batteries were made of lithium (Li) and 50% of nickel (Ni), 29% and 40% of the present reserves of Li and Ni would be used, respectively, as well as 22% of platinum-palladium reserves. The limited reserves of these metals constraints the number of trucks that could be powered by fuel cells to a fraction not much above 10%. Given that reserves cannot be indefinitely expanded [
2], the viability of the present level of land freight transport may depend on future development of high capacity batteries and higher use of rail freight. Electrification of the entire global fleet is a feasible option, but the number of vehicles that a future post-carbon society may sustain is roughly the number we have currently. A larger number would endanger the availability of Li and Ni for other economic sectors. Given that demand for vehicles will probably increase in the future due to demographic and economic growth, especially in developing countries, the present land transport might not be fully sustained. However, a reorganization with a larger emphasis on rail transport, would alleviate the increased demand on these metals and thereby probably maintaining sufficient mobility for people and freight.
The energy demand of transport in a future post-carbon economy will be very similar to the present one. On one side, aviation and shipping will notably increase energy use due to the necessity to produce electrolytic hydrogen to be consumed in jet engines and fuel cells, respectively. But this enhanced demand will be compensated for savings coming from road transport, since electric motors have greater efficiency than combustion ones. Actually, future aviation would have energy consumption larger than trains and close to that of road transport, if present transport structure remained in a post-carbon economy. Given that aviation is less energy efficient than vehicles and trains for the transport of people and goods, the use of jet aircraft may diminish in importance in the future. Thus, present levels of air transport may not be fully sustained. A similar mechanism could enhance the use of rail transport in a substitution of cars and trucks.
Fishing and mercantile activities frequently require long periods of navigation, which make battery-based motors inappropriate. Fuel cells may be the best solution in these circumstances. They are a relatively inefficient way to use electricity, however the size of the fishing and merchant fleets is small in comparison with that of land vehicles, and they would not imply an insurmountable problem in terms of energy demand.
Open field work in farming, mining and construction sometimes requires high power tractors that should also be supplied by fuel cell vehicles, while other generic farm work could be done using many smaller electric tractors which would recharge their batteries in the grid. Thus, full connection of farms to the electric grid would become necessary in the future economy. For similar reasons, any project involving open field construction will have to plan for the building of a connection to the grid. This reorganization of open field work does not necessarily create an insurmountable problem.
Mining in a post-carbon economy seems sustainable in the short and medium term, but is unsustainable in the long term due to the decline of ore grades [
74]. Thus, a fully sustainable post-carbon economy should increasingly base its mineral production on recycling. The form that this transition may take is a crucial subject that would require discussion in a separate paper.
The future industrial sector will initially demand more energy than the present one to deliver the same services (see
Table 5), due to the increased use of charcoal, biogas and hydrogen. However, if we subtract the energy embodied in future feedstocks (see
Table 6), we find that the electricity needed for industry is 2751–2908 GW, which is smaller than the 2005 industrial energy demand (3403 GW). A post-carbon industry would use similar or less energy than the present industry in all the sectors except iron and steel and non-ferrous metals. The energy savings would be especially high in nonmetallic minerals production due to the severe heat loss associated to flue gases in high temperature furnaces, which could be minimized by using electric heating.
To make these estimations, the efficiency of electricity for machinery, vehicles and heating has been assumed to be equal as reported in current systems. When Sankey (energy flow) diagrams are available and fuels are the main energy input for heating in an industry, the present efficiency of industrial heating has been calculated from these Sankey diagrams. When this method is not applicable, the reported efficiency of current furnaces is used. A similar procedure has been used to estimate the efficiency of steam production in different industries. Actually, the different industrial and economic sectors are complex and the precise form and efficiency that their processes will have in a post-carbon economy remain uncertain. For these reasons, the energy demand calculations made here cannot be considered as precise projections but as indicative estimates that should be improved through continuous research.
To compensate for the withdrawal of coal, oil and gas, charcoal and biogas will have to be produced in larger quantities than currently. Most of this demand will originate from the production of High Value Chemicals (HVC) required by the petrochemical industry.
To achieve economic activity similar to that of 2005, 240–300 million tonnes of charcoal per year should be produced from woody biomass. This will require governments to encourage people to abandon the use of wood for heating and cooking purposes, at least in developed countries, where electric alternatives will exist. 215 million tonnes of biogas will also be required for ammonia production, at least until organic agriculture is fully developed. This quantity can be obtained through the current production by developed countries and China alone, given that many developing countries may need their biogas for rural self-consumption.
Use of direct iron reduction with hydrogen in steel production will alleviate an important fraction of the future carbon (and therefore charcoal) demand (
Table 6). Even so, the estimated potential of renewable charcoal production is insufficient to maintain the services that the world economy produced in 2005. If 100% of the biogas renewable potential were used to produce 100% of ammonia and 32% of the HVCs consumed in 2005, and all the available charcoal is used for the coal to olefins process (after setting aside the demands of the metal sectors), 130 to 141 × 10
6 t of HVCs could be produced. This amounts to 45%–49% of HVC production of 2004.
Olefin production was 220 × 10
6 t in 2012 [
75], 10.9% higher than in 2004 ([
51], Table 8.5). If HVC production had the same relative increase, 318 Mt were produced in 2012. If the calculations of
Table 6 are made with this value, only 40%–43% of HVCs produced in 2012 can be produced in a post-carbon economy. This range could be increased by a few percent if ammonia were produced from hydrogen (
Appendix, Equation (A6)) and not from biogas. In any case, the present growth in HVC demand takes us progressively away from what is sustainable from a post-carbon perspective.
Given the limitations of the renewable production of natural gas and charcoal, systematic utilization of these products as fuel should be legally discouraged in the future, since electricity is a more efficient energy supply for end uses, and these products are critical for supplying feedstocks to the petrochemical industry.
The petrochemical industry cannot be fully maintained at its present size in a post-carbon economy and, to a first approach, should shrink to a size of 40%–43% of that of the 2012 petrochemical sector, which is the size that the sector had in 1985–1986 [
76]. Fortunately, many of the products currently provided by the petrochemical industry can be replaced with similar products based on natural feedstocks although their supply rate must adapt to their natural availability. Substitution of natural feedstocks such as those mentioned in
Table A2 (Appendix) for HVC consumption would allow an increase of the size of the petrochemical sector above the mentioned 40%–43% factor. In particular, there is a global potential of non-woody biomass that is not fully utilized. Starch and non-woody biomass are rich in xylenes and glucose, sugars required for the production of polylactic acid (PLA). Thus, production of polystyrene from PLA would alleviate the demand for charcoal, saving a fraction of it that could be used for olefin production.
Polyurethanes, polyesters, sealants, many pharmaceutical drugs and some dyes cannot be replaced by natural substitutes and should be produced from HVC and, ultimately, from charcoal. However, given the insufficient availability of renewable charcoal, these products would be produced at a lower rate than at present; up to 2.5 times lower, making them scarce in a future post-carbon society. The services that these products offer include cushioning foams, foams that do not melt when heated, and some synthetic fibers with special qualities.
More critical can be some pharmaceutical drugs whose lack would affect quality of life. Scarcity of these drugs may worsen the treatment of some specific health conditions, although it will probably not affect life expectancy. Indeed, the main factor influencing life expectancy is the incidence of infectious diseases, which are mainly related to poor basic hygiene and poverty [
77] and not to specific drugs.
While electricity, charcoal and biogas may be the base of a sustainable transport and industry, they will be necessary but not sufficient for future sustainable farming. Agriculture is not sustainable at present given its dependence on fossil fuels and minerals such as phosphorous and potassium. In a post-carbon economy, organic farming may be the only sustainable solution able to (almost) fully recycle these essential nutrients. A detailed discussion of future sustainable farming would require another paper. However, sustainable farming will be one of the key elements of a future post-carbon society.