2.1. Comparison of Undoped and 5 mol % MgF2 Doped MgH2 at 335 °C
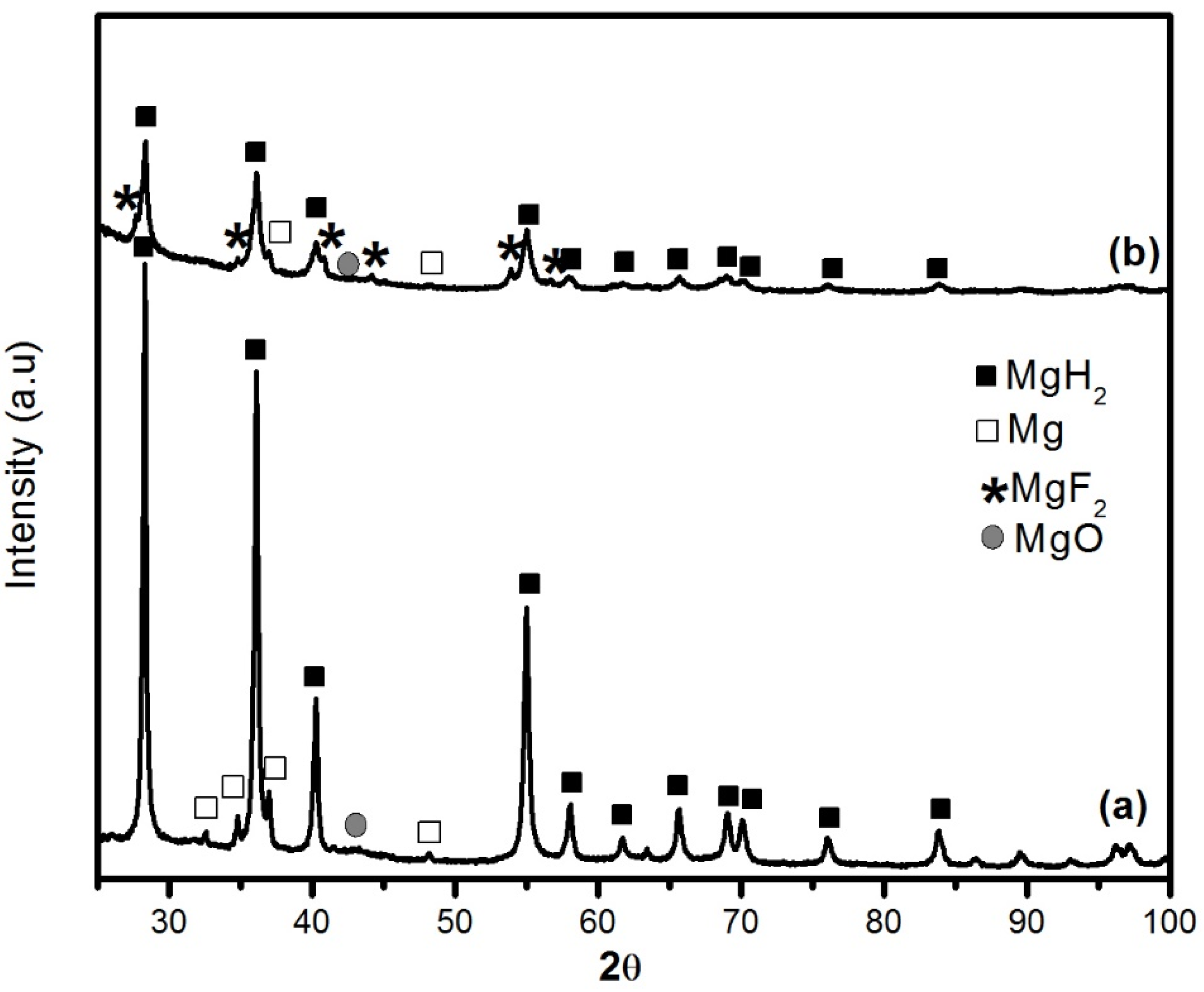
The X-ray diffraction (XRD) patterns of MgH
2 without and with 5 mol % MgF
2 prepared by 1 h ball milling are shown in
Figure 1. For the undoped sample, the diffraction pattern peaks are associated with main phase of β-MgH
2 and some unreacted Mg. There is no evidence of the metastable γ-MgH
2 phase. This is due to the short milling time and low milling intensity. A broad peak centered at 43° is attributed to MgO. The crystallite size of β-MgH
2 is evaluated from Rietveld refinement to be 23.4 ± 0.3 nm. Milling with MgF
2 additive was even more effective for reduction of crystallite size of MgH
2 that was evaluated as 10.9 ± 0.3 nm. During milling there is physical interaction between the different species during the repeating collisions. Therefore, the MgF
2 has also some mechanical effect on MgH
2. Unfortunately, the alloying of brittle-brittle system is poorly understood [
12]. However, from the present experiment it seems that addition of small amount of MgF
2 improves crystallite size reduction but the exact mechanism is still unclear.
Figure 1.
XRD patterns of ball milled samples milled 1 h: (a) pure MgH2 and (b) MgH2 + 5 mol % MgF2.
Figure 1.
XRD patterns of ball milled samples milled 1 h: (a) pure MgH2 and (b) MgH2 + 5 mol % MgF2.
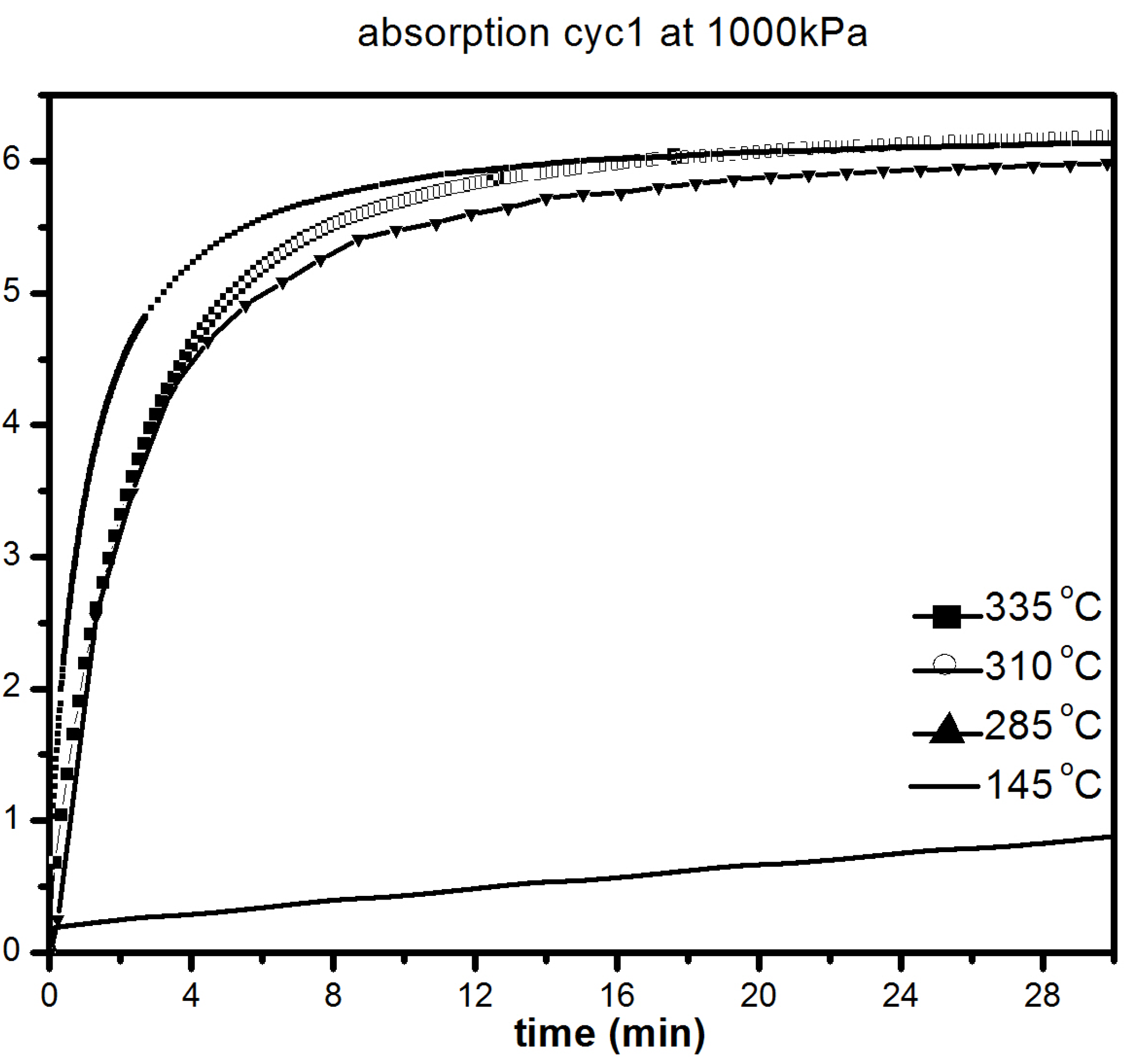
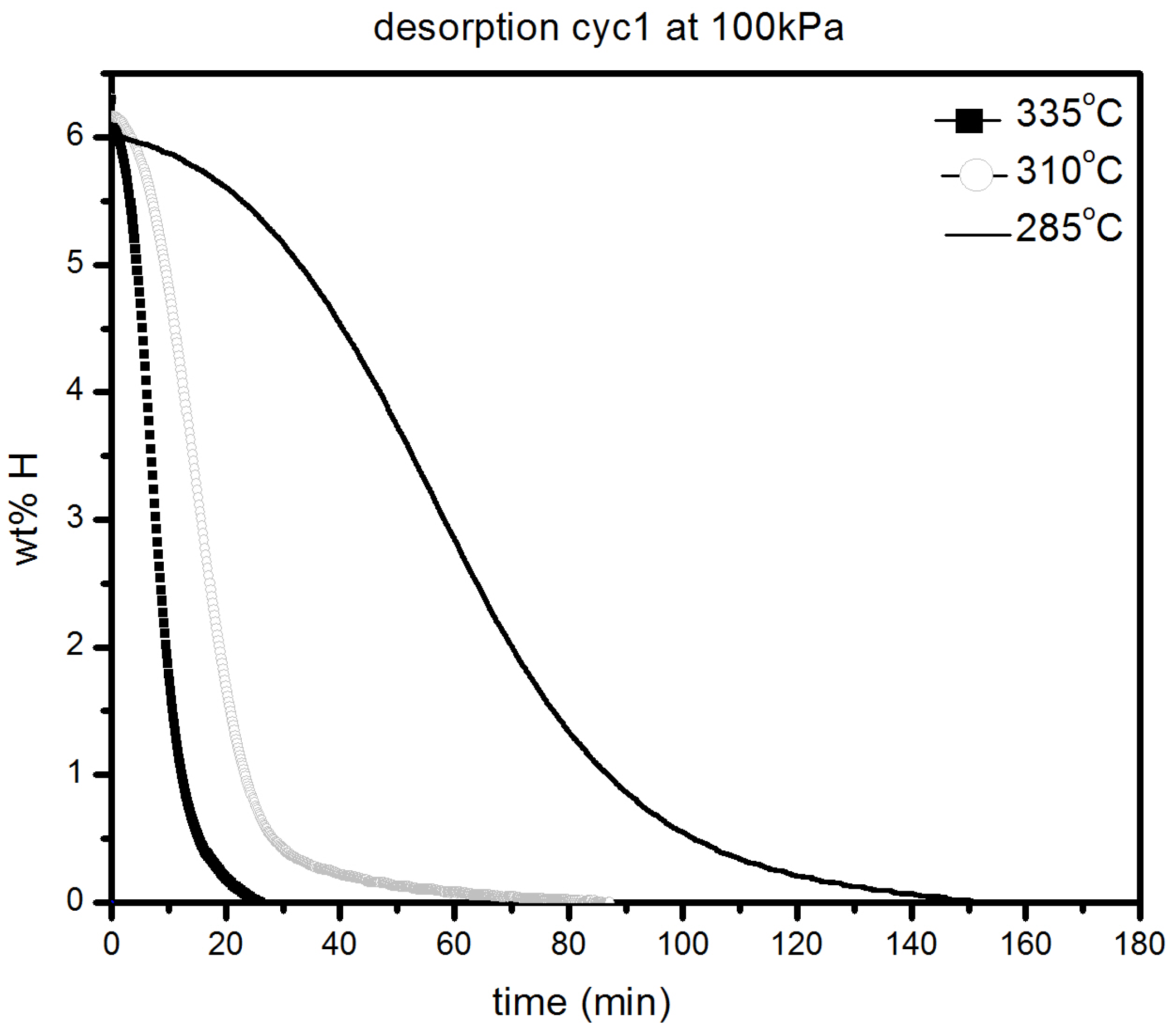
In practical applications, desorption will be performed under a pressure of at least 100 kPa of hydrogen. However, in order to study the behaviour of MgH
2–MgF
2 system, we decided to fully dehydride the samples after ball milling. Therefore, after milling the samples were completely desorbed at 335 °C under dynamic vacuum before investigating their hydrogenation properties. Representative hydrogenation and dehydrogenation characteristics are shown in
Figure 2.
Figure 2.
Hydrogen sorption kinetics at 335 °C of 1 h milled MgH2 without and with 5 mol % MgF2. (a) First absorption under 1000 kPa H2; (b) desorption under 100 kPa H2.
Figure 2.
Hydrogen sorption kinetics at 335 °C of 1 h milled MgH2 without and with 5 mol % MgF2. (a) First absorption under 1000 kPa H2; (b) desorption under 100 kPa H2.
It is observed that at 335 °C under 1000 kPa H2 pressure MgH2 + 5 mol % MgF2 system absorbs 6.2 wt % hydrogen in 30 min in comparison to only 5.3 wt % absorption by pure MgH2. This shows a large improvement in absorption capacity is achieved, yielding 92% of the theoretical capacity in comparison to 70% for the pure MgH2. In addition, significant improvement in desorption kinetics is achieved with complete desorption of the hydride phase in less than 20 min in presence of MgF2. Thus, the beneficial effect of MgF2 is clearly evident on the hydriding/dehydriding aspect of MgH2.
Figure 3 shows the diffraction patterns of the doped sample in its desorbed and reabsorbed states. The desorbed pattern shows a small amount of un-desorbed MgH
2. The interesting fact is that MgF
2 is still present in the sample. This could be expected because it is known that for MgH
2-transition metal (TM) fluoride systems, milling or dehydrogenation induces the formation of MgF
2 and TM hydride [
4,
11]. Thus, MgF
2 is a stable compound and does not react to form MgH
2.
Figure 3.
XRD patterns of MgH2 + 5 mol % MgF2 (a) after desorption at 335 °C under 100 kPa H2 and (b) after re-hydrogenation at 335 °C under 1000 kPa H2.
Figure 3.
XRD patterns of MgH2 + 5 mol % MgF2 (a) after desorption at 335 °C under 100 kPa H2 and (b) after re-hydrogenation at 335 °C under 1000 kPa H2.
This is confirmed by the diffraction pattern of fully hydrided sample. The phases present are MgH
2 and MgF
2 along with small amount of unreacted Mg. Compared to the patterns of
Figure 1 we see that the peaks of patterns of
Figure 3 are not as broad, implying that the crystallite size increased. From Rietveld analysis we found that the crystallite size of Mg in the dehydrided pattern is 49.1 ± 0.8 nm while the crystallite size of MgH
2 in the reabsorbed pattern is 64 ± 2 nm. This shows that there is grain growth compared to the as-milled sample. This may be due to the high temperature of hydrogenation and also because of desorption/absorption itself.
Figure 4 shows the SEM images of MgH
2 + 5 mol % MgF
2 composite in (
Figure 4a) desorbed state and (
Figure 4b) after re-hydrogenation at 335 °C in comparison with pure MgH
2 (
Figure 4c). The images show that ball milling with additive leads to effective decrease in particle size. In addition, energy dispersive X-ray (EDX) mapping done at higher magnification (
Figure 5) shows that agglomerates consist of smaller MgH
2 particles and additive.
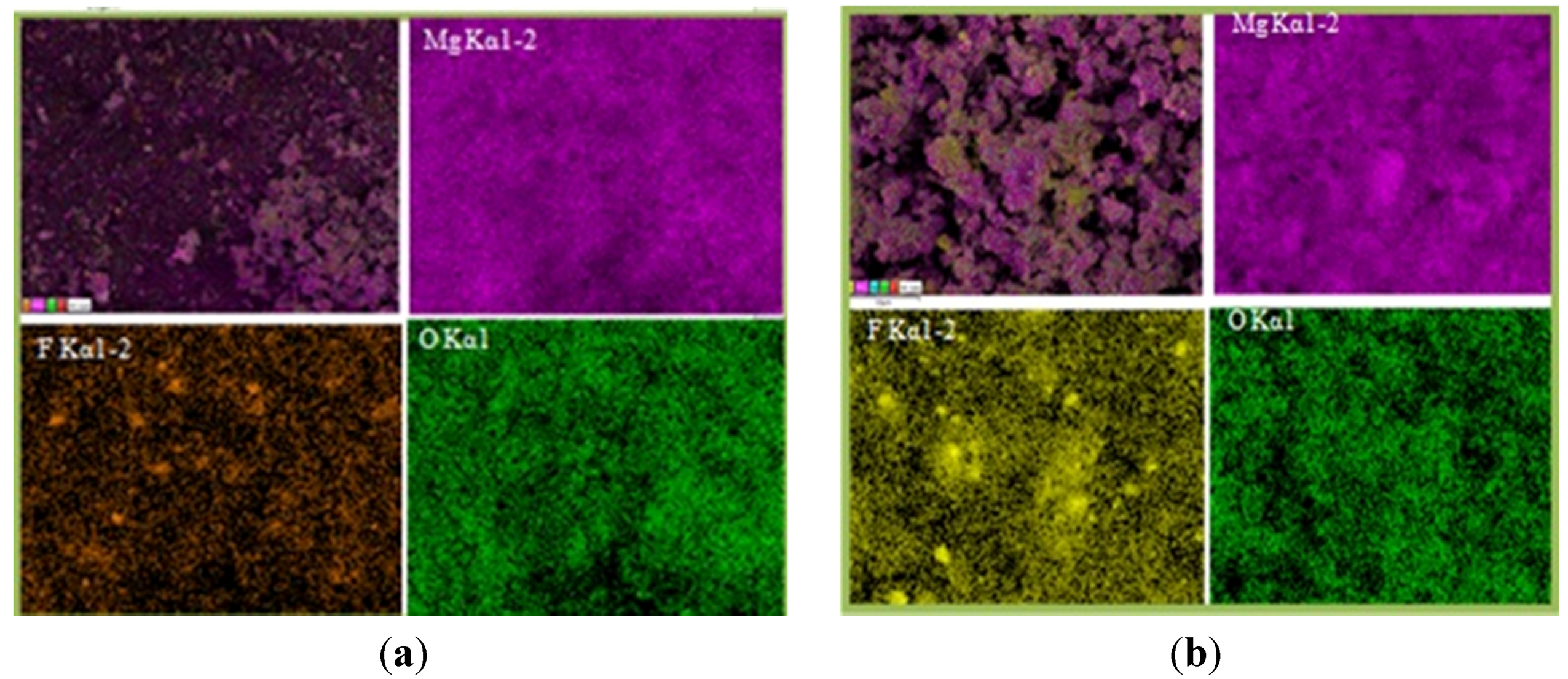
Elemental mapping made on MgH
2 + 5 mol % MgF
2 in both the desorbed state (
Figure 5a) and re-hydrogenated state (
Figure 5b) confirms homogenous distribution of MgF
2.
High energy milling leads to uniform dispersion of MgF
2 phase in MgH
2 matrix which may act as a catalytic layer and contributes in improving sorption properties. Chemical analysis performed by EDX spectroscopy during transmission electron microscopy (TEM) investigation of the desorbed sample gave the average atomic composition of different elements as 9.8% O, 14.7% F and 75.7% Mg which is very close to the nominal composition (86% Mg and 14% F). The presence of oxygen in EDX pattern in comparison to its small trace in XRD pattern could be due to small crystallite size of MgO making it peak difficult to distinguish from the background. A similar EDX investigation was performed on a sample that has been submitted to five dehydrogenation/hydrogenation cycles. Because abundances vary from point to point, we average over four different localisations. We found that, after cycling, the atomic composition of different elements was 15% ± 4% O, 11% ± 4% F and 74% ± 6% Mg. Within experimental error, these values are similar to the ones before cycling. However, this may be an indication that cycling induces a loss of MgF
2 and increase of MgO. Typical TEM micrographs presented in
Figure 6 shows the morphology of the desorbed sample.
The image shows presence of large number of particles agglomerated together with no visibility clear particle boundaries. These observations are quite similar to those reported recently by Grzech
et al. [
13]. High resolution pictures taken over region-1 in
Figure 6 and its corresponding selected area electron diffraction (SAED) patterns shows reflections at
d-spacing 2.45, 1.90, 1.60, 1.36 and 1.22 Å which are characteristic of Mg (101), (102), (110), (112) and (202) planes respectively along with reflections at d values 2.10 and 1.48 Å, corresponding to MgO (200) and (220) planes. Thus, the surface consists of small crystallites of MgO (forming well defined ring and represented by red rings) surrounding the large crystallites of Mg (seen as discontinuous spots and represented by white rings). While the multiple SAED patterns acquired from the region-2 were well indexed as a mixture of large crystallite Mg and small crystallite of MgF
2 (seen as well-defined rings and colored yellow). The absence of oxide in region-2 is evidence that presence of fluoride limits MgO only to the surface. Both structural and morphological studies support the presence of MgF
2 phase even after complete hydrogen absorption/desorption cycle at 335 °C.
Figure 4.
SEM images for MgH2 + 5 mol % MgF2 in (a) desorbed state and (b) re-hydrogenated state in comparison to (c) pure MgH2.
Figure 4.
SEM images for MgH2 + 5 mol % MgF2 in (a) desorbed state and (b) re-hydrogenated state in comparison to (c) pure MgH2.
Figure 5.
Elemental mapping showing particle morphology and distribution of 1 h milled MgH2 + 5 mol % MgF2: (a) after desorption and (b) after re-hydrogenation at 335 °C.
Figure 5.
Elemental mapping showing particle morphology and distribution of 1 h milled MgH2 + 5 mol % MgF2: (a) after desorption and (b) after re-hydrogenation at 335 °C.
Figure 6.
Transmission electron microscopy (TEM) micrograph of MgH2 + 5 mol % MgF2 sample after desorption at 335 °C with selected area electron diffraction (SAED) patterns and simulations. Region 1 is composed of Mg (white rings) covered with MgO layer (red rings) in simulated data while Region 2 shows diffraction rings corresponding to Mg (white rings) and MgF2 (yellow rings).
Figure 6.
Transmission electron microscopy (TEM) micrograph of MgH2 + 5 mol % MgF2 sample after desorption at 335 °C with selected area electron diffraction (SAED) patterns and simulations. Region 1 is composed of Mg (white rings) covered with MgO layer (red rings) in simulated data while Region 2 shows diffraction rings corresponding to Mg (white rings) and MgF2 (yellow rings).
2.2. Hydrogenation Characteristics of MgH2 + 5 mol % MgF2 at Lower Temperatures
The catalytic effect of 5 mol % MgF
2 on hydrogen sorption properties of MgH
2 was further investigated at lower temperatures.
Figure 7 shows the absorption kinetics at 335, 310, 285 and 145 °C under 1000 kPa of hydrogen.
Figure 7.
First absorption under 1000 kPa H2 at different temperatures of 1 h milled MgH2 + 5 mol % MgF2. The insert is a compete absorption curve at 145 °C.
Figure 7.
First absorption under 1000 kPa H2 at different temperatures of 1 h milled MgH2 + 5 mol % MgF2. The insert is a compete absorption curve at 145 °C.
It should be pointed out that the samples were initially desorbed at 335 °C in order to ensure that full desorption was achieved before all absorption measurements. We notice only a slight loss in absorption capacity with reduction of temperature from 335 °C (6.2 wt % H
2) to 285 °C (5.8 wt % H
2). As seen in
Figure 7, there was slight loss in kinetics and capacity in the temperature range 335–285 °C with the material reaching its complete capacity in less than 30 min. However, at 145 °C, the kinetics are much slower, but after 20 h, a capacity of 5.5 wt % is reached, as shown in
Figure 7b. Desorption kinetic under 100 kPa H
2 at 285, 310 and 335 °C are shown in
Figure 8.
Figure 8.
Desorption under 100 kPa H2 at different temperatures of 1 h milled MgH2 + 5 mol % MgF2.
Figure 8.
Desorption under 100 kPa H2 at different temperatures of 1 h milled MgH2 + 5 mol % MgF2.
As expected, the kinetics are getting slower as temperature decreases but is still relatively fast even at 285 °C were complete desorption takes place in less than 3 h. These results reveal that even by sole addition of alkaline metal fluorides, improvements in hydrogenation characteristics of magnesium hydride can be achieved.
2.3. Cyclic Stability of MgH2 + 5 mol % MgF2 at T = 310 °C
Micro structural results have confirmed that MgF
2 phase does not decompose and no new phase formation occurs during hydrogen absorption/desorption measurements for MgH
2 + 5 mol % MgF
2 system. Therefore, cyclic performance of catalyzed magnesium hydride was examined at moderate operating temperature of 310 °C at pressure of 1000 kPa (for absorption) and 10 kPa (for desorption) to evaluate performance stability. Prior to the measurements the sample was completely desorbed at 335 °C.
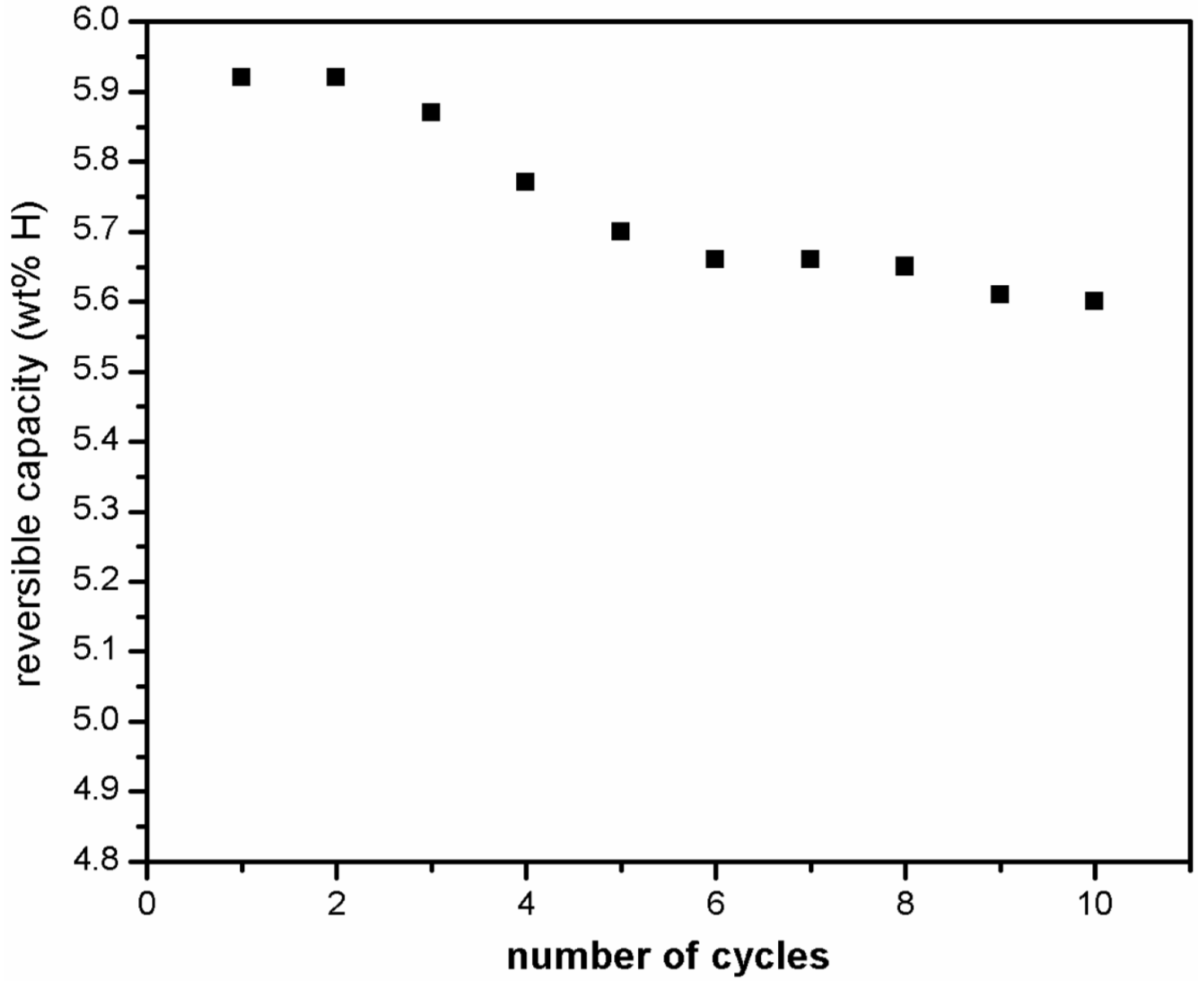
Figure 9 shows that the absorption capacity goes down from 5.9 wt % in first cycle to 5.6 wt % in the 10th cycle.
Figure 9.
Hydrogen absorption kinetics at 310 °C under 1000 kPa of hydrogen of ball milled MgH2 + 5 mol % MgF2 for 10 cycles.
Figure 9.
Hydrogen absorption kinetics at 310 °C under 1000 kPa of hydrogen of ball milled MgH2 + 5 mol % MgF2 for 10 cycles.
The observed loss of 0.3 wt % in capacity was reached within the first five cycles and thereafter the maximum capacity achieved by the system is more or less stabilized. These results show that magnesium hydride exhibits good hydrogen storage capacity and cyclic stability when magnesium fluoride is used as catalyst in comparison to the use of transition metal fluoride like NbF
5 or ZrF
4 where sharp decline in storage capacity was observed by Malka
et al. [
14] in the first 10 hydrogenation cycles recorded at 325 °C. X-ray diffraction patterns of the sample taken after 1st and 10th desorption cycle are presented in
Figure 10. It shows that the β-MgH
2 phase and the catalytic material remain intact while small increase in content of MgO occurs. Thus, the growth of the MgO layer is mostly responsible for an observed loss in capacity.
Figure 10.
X-ray diffraction patterns of ball milled MgH2 + 5 mol % MgF2 after (a) one desorption and (b) 10 desorption cycles at 310 °C.
Figure 10.
X-ray diffraction patterns of ball milled MgH2 + 5 mol % MgF2 after (a) one desorption and (b) 10 desorption cycles at 310 °C.