Glycerin, a Biodiesel By-Product with Potentiality to Produce Hydrogen by Steam Gasification
Abstract
:1. Introduction
2. Experimental
2.1. Materials
| Analysis | Results (mg·kg−1) | Method | |
|---|---|---|---|
| Non-glyceric organic matter | 6.840 | CEA 1705 | |
| Sulphated ash | 4.2 | EP 2002 | |
| Water | 11 | ASTM E-203-1 | |
| Purity (%) | 77.6 | CEA 212 | |
| Sulphur | 40,000 | EN 228:2004 | |
| Phosphorus | 100 | EN 228:2004 | |
| Metals | Calcium | 3.7 | CEA 1574-B |
| Tin | 2 | ||
| Iron | 10 | ||
| Magnesium | 11 | ||
| Nickel | 2.2 | ||
| Potasium | 18,000 | ||
| Sodium | 130 | ||
2.2. Steam Gasification Experiments
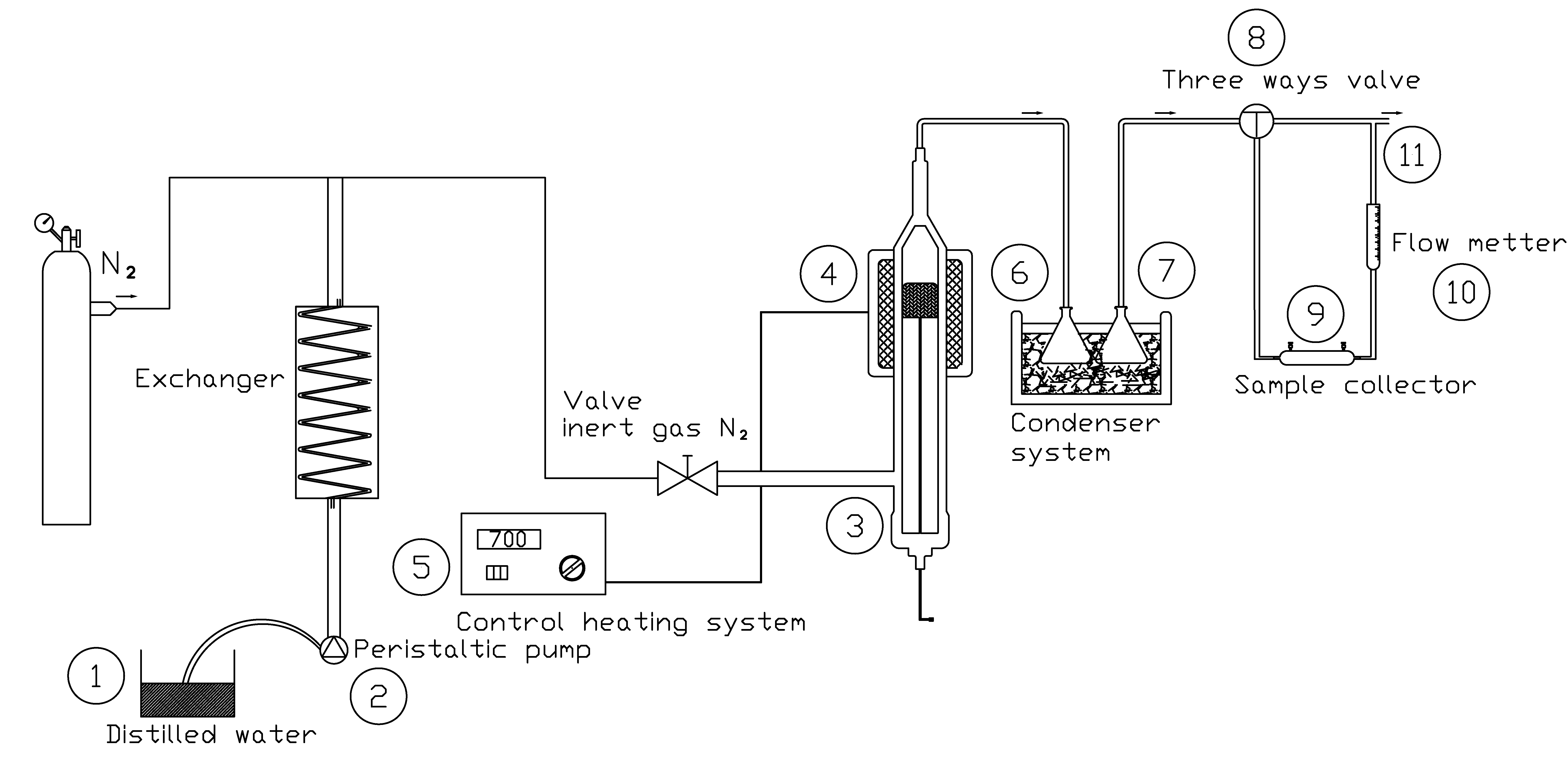
3. Discussion
3.1. Glycerin Characterization
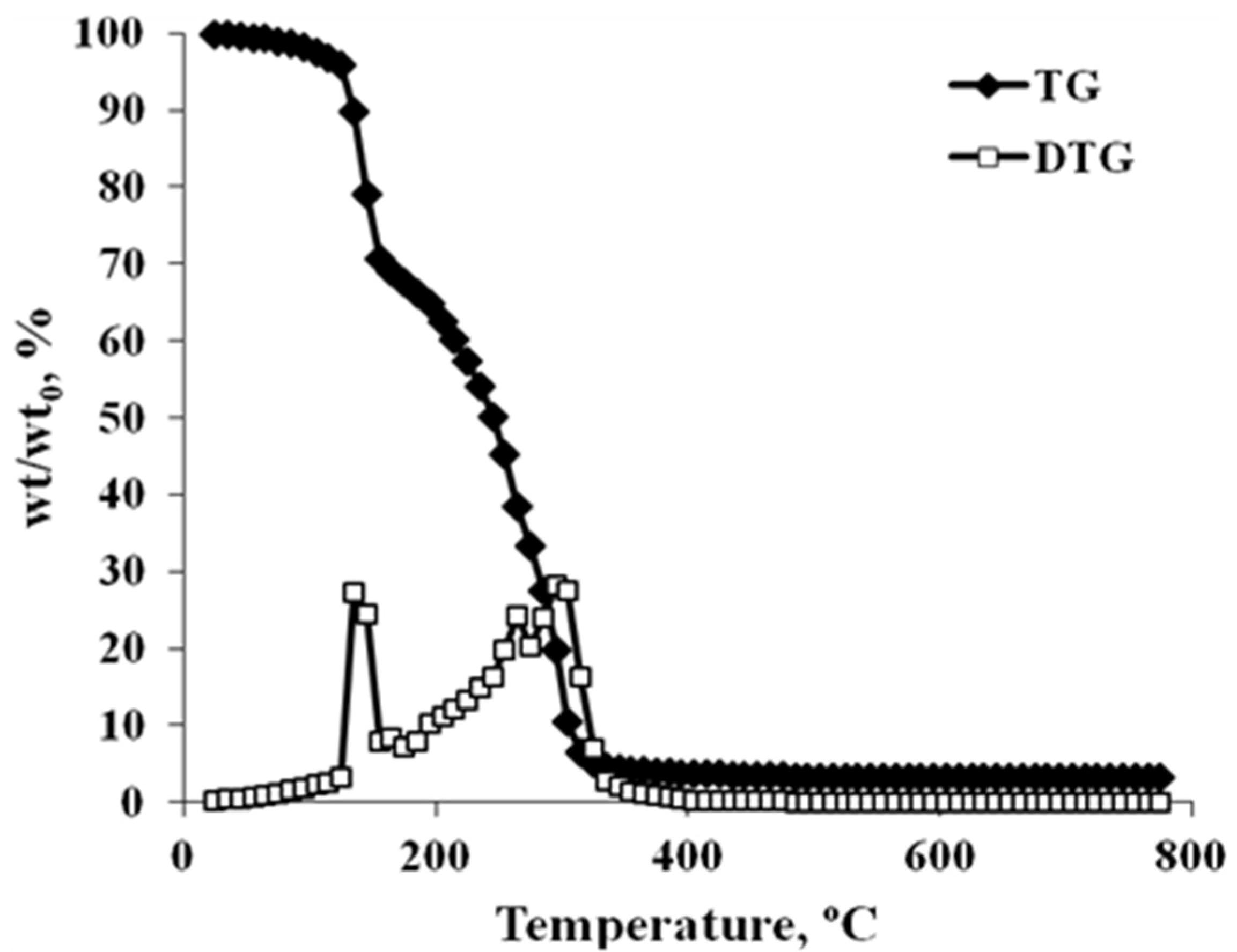
3.2. Steam Gasification of Glycerin
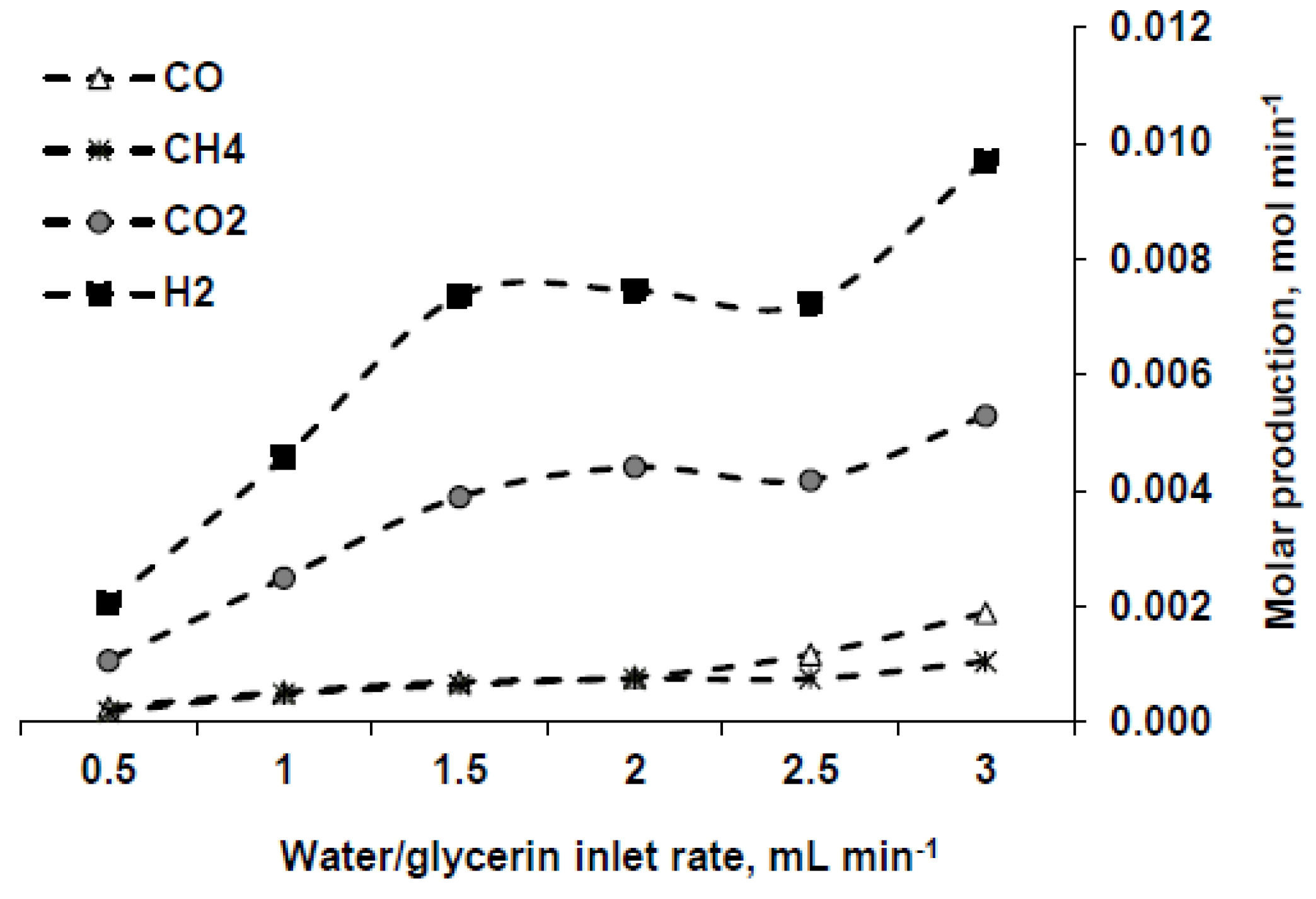
3.2.1. Influence of Water/Glycerin Inlet Rate
| Flow rate (g·min−1) | H2 molar fraction (%) | HHV, kJ·Nm−3 | Power, kJ·min−1 | Energy per mL of glycerin, kJ·mL−1 |
|---|---|---|---|---|
| 0.5 | 67.4 | 9332.8 | 0.6 | 12.5 |
| 1.0 | 63.4 | 9119.1 | 1.2 | 11.6 |
| 1.5 | 60.9 | 8888.2 | 2.1 | 13.7 |
| 2.0 | 59.5 | 8694.7 | 2.8 | 13.8 |
| 2.5 | 58.9 | 8720.6 | 3.8 | 15.0 |
| 3.0 | 59.8 | 8992.2 | 4.7 | 15.7 |
3.2.2. Influence of Temperature
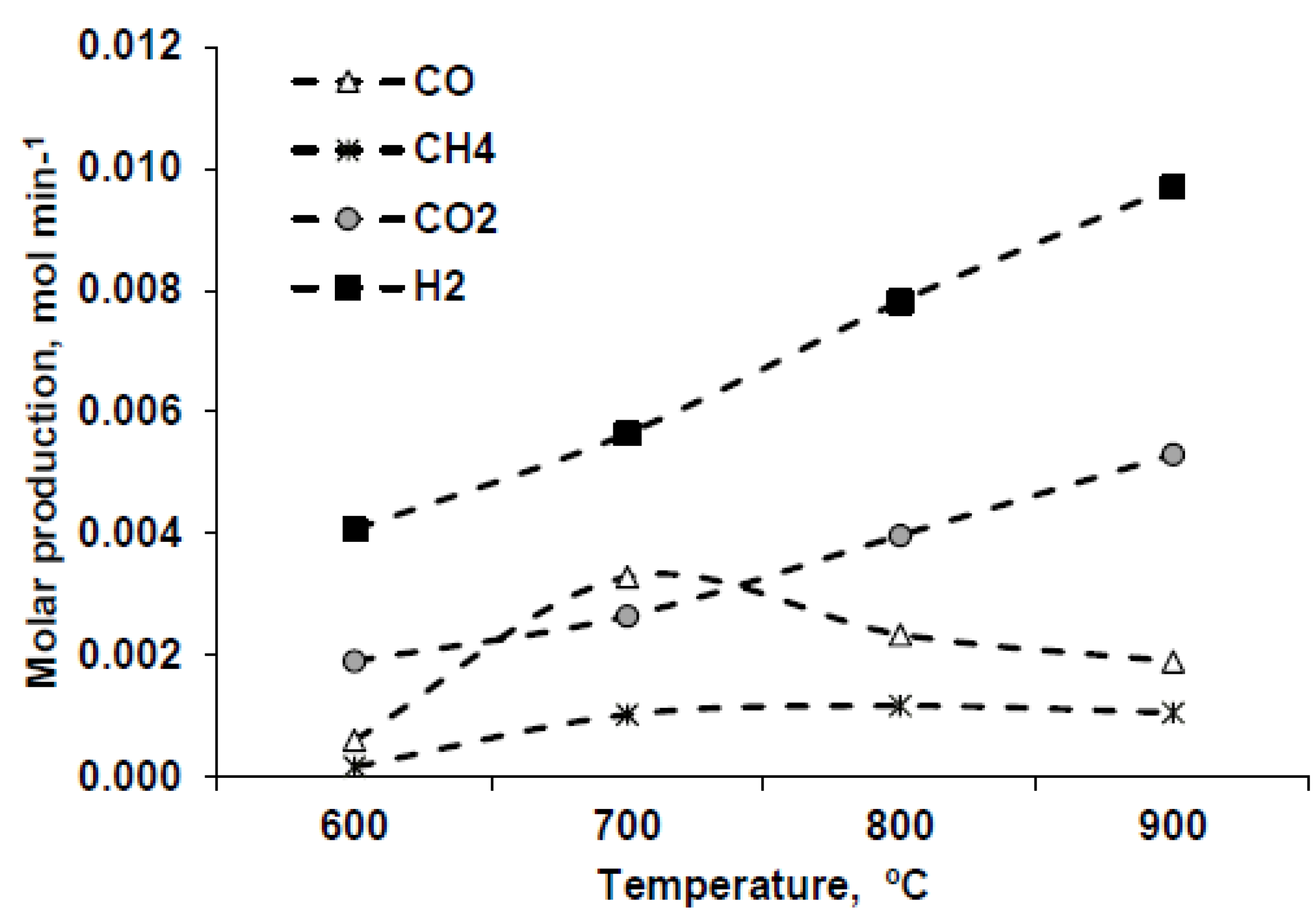
| Temperature, °C | H2 molar fraction (%) | HHV, kJ·Nm−3 | Power, kJ·min−1 | Energy per mL of glycerin, kJ·mL−1 |
|---|---|---|---|---|
| 600 | 51.3 | 11023.5 | 5.4 | 12.3 |
| 700 | 42.8 | 9637.0 | 3.8 | 8.6 |
| 800 | 47.7 | 9243.1 | 4.6 | 11.1 |
| 900 | 57.5 | 11023.5 | 5.4 | 12.3 |
3.2.3. Influence of Water/Glycerin Ratio

| Water/glycerin ratio | H2 molar fraction (%) | HHV, kJ·Nm−3 | Power, kJ·min−1 | Energy per mL of glycerin, kJ·mL−1 |
|---|---|---|---|---|
| 6 | 56.5 | 8832,1 | 4,6 | 11,2 |
| 9 | 59.8 | 8992,2 | 4,6 | 15,7 |
| 12 | 62.1 | 8918,0 | 3,4 | 20,6 |
4. Conclusions
- (1)
- Increasing the inlet mixture flow rate is beneficial in order to produce a greater amount of gas and higher power, although it is detrimental if the final goal is to obtain a hydrogen-rich gas.
- (2)
- The addition of water to crude glycerine can be interesting because it provides a greater glycerin reforming. In addition, it moves the equilibria water gas and water gas shift towards the production of hydrogen.
- (3)
- Using higher temperatures is interesting for providing a greater fraction of hydrogen, although it also involves a decrease in the heating value of the gas.
- (4)
- Further research will be devoted to improve the system energy efficiency by studying the incorporation of a heat recovery system and thus taking advantage of the physical exergy of the gas. Also, further studies will address pretreatments of the raw material as well as treatments of the gases in order to mitigate this environmental problem.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IEA Bioenergy Conference. Available online: http://www.ieabioenergy.com (accessed on 2 July 2015).
- Encinar, J.M.; González, J.F.; Rodríguez-Reinares, A. Biodiesel from used frying oil. Variables affecting the yields and characteristics of the biodiesel. Ind. Eng. Chem. Res. 2005, 44, 5491–5499. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, S. Production and characterization of biodiesel derived from Hodgsonia macrocarpa seed oil. Appl. Energy 2015, 146, 135–140. [Google Scholar] [CrossRef]
- Shay, E.G. Diesel fuel from vegetable oils: Status and opportunities. Biomass Bioenergy 1993, 4, 227–242. [Google Scholar] [CrossRef]
- Nagel, N.; Lemke, P. Production of methyl fuel from microalgae. Appl. Biochem. Biotechnol. 1990, 24, 355–361. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Srivastava, A.; Prasad, R. Triglycerides-based diesel fuels. Renew. Sustain. Energy Rev. 2000, 4, 111–133. [Google Scholar] [CrossRef]
- Biofuels Production and Consumption Report; International Energy Agency: The Hague, The Netherlands, 2014.
- Frank, B.; Bendz, K.; Krautgartner, R.; Lieberz, S. EU Biofuels Annual 2013. USDA Foreign Agricultural Service. Available online: http://gain.fas.usda.gov/Recent%20GAIN%20Publications/Biofuels%20Annual_The%20Hague_EU-27_8-13-2013.pdf (accessed on 20 April 2015).
- Winfield, A.J. Glycerine—A key cosmetic ingredient. Talanta 1993, 40, 291–292. [Google Scholar] [CrossRef]
- Brar, S.K.; Sydney, E.B.; le Bihan, Y.; Soccol, C.R.; Sarma, S.J. Microbial hydrogen production by bioconversion of crude glycerol: A review. Int. J. Hydrog. Energy 2012, 37, 6473–6490. [Google Scholar] [CrossRef]
- Valliyappan, T.; Bakhshi, N.N.; Dalai, A.K. Pyrolysis of glycerol for the production of hydrogen or syngas. Bioresour. Technol. 2008, 99, 4476–4483. [Google Scholar] [CrossRef] [PubMed]
- Stein, Y.S.; Antal, M.J., Jr.; Jones, M.A., Jr. Study of the gas-phase pyrolysis of glycerol. J. Anal. Appl. Pyrolysis 1983, 4, 283–296. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Campanario, F.J.; Aguilera, P.G.; Ollero, P. Hydrogen production from supercritical water reforming of glycerol over Ni/Al2O3–SiO2 catalyst. Energy 2015, 84, 634–642. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, L. Preliminary investigation on hydrogen-rich gas production by co-steam-reforming of biomass and crude glycerin. Int. J. Hydrog. Energy 2012, 37, 765–773. [Google Scholar] [CrossRef]
- Valliyappan, T. Hydrogen or Syn Gas Production from Glycerol Using Pyrolysis and Steam Gasification Processes; University of Saskatchewan: Saskatoon, SK, Canada, 2004. [Google Scholar]
- Adhikari, S.; Fernando, S.D.; Haryanto, A. Hydrogen production from glycerin by steam reforming over nickel catalysts. Renew. Energy 2008, 33, 1097–1100. [Google Scholar] [CrossRef]
- Ramesh, S.; Eun-Hyeok, Y.; Jae-Sun, J.; Dong Ju, M. Copper decorated perovskite an efficient catalyst for low temperature hydrogen production by steam reforming of glycerol. Int. J. Hydrog. Energy 2015, 40, 11428–11435. [Google Scholar] [CrossRef]
- Engo, G. Aprovechamiento Energético de la Glicerina, Final Degree Project; University of Extremadura: Plasencia, Spain, 2011. (In Spanish) [Google Scholar]
- González, J.F.; Román, S.; Bragado, D.; Calderón, M. Investigation on the reactions influencing biomass air and air/steam gasification for hydrogen production. Fuel Process. Technol. 2008, 89, 764–772. [Google Scholar] [CrossRef]
- González, J.F.; Román, S.; Engo, G.; Encinar, J.M.; Martínez, G. Reduction of tars by dolomite cracking during two-stage gasification of olive cake. Biomass Bioenergy 2011, 35, 4324–4330. [Google Scholar] [CrossRef]
- Álvarez-Murillo, A.; Ledesma, B.; Román, S.; Sabio, E.; Gañán, J. Biomass pyrolysis toward hydrocarbonization. Influence on subsequent steam gasification processes. J. Anal. Appl. Pyrolysis 2015, 113, 380–389. [Google Scholar] [CrossRef]
- Román, S.; Ledesma, B.; González, J.F.; Al-Kassir, A.; Engo, G.; Álvarez-Murillo, A. Two stage thermal regeneration of exhausted activated carbons. Steam gasification of effluents. J. Anal. Appl. Pyrolysis 2013, 103, 201–206. [Google Scholar] [CrossRef]
- Román, S.; Ledesma, B.; Álvarez-Murillo, A.; González, J.F. Comparative study on the thermal reactivation of spent adsorbents. Fuel Process. Technol. 2013, 116, 358–365. [Google Scholar] [CrossRef]
- González, J.F.; Engo, G.; Román, S.; Arranz, J.I.; Encinar, J.M. Providing an added value to biodiesel by-products: Pyrolysis of glycerin. Thermogravimetric Study and Sulphur Analysis. In Proceedings of the International Conference on Renewable Energies and Power Quality, Las Palmas de Gran Canaria, Spain, 13–15 April 2011.
- Aslam, Z.; Shawabkeha, R.A.; Hussein, I.A.; Al-Baghli, N.; Eic, M. Synthesis of activated carbon from oil fly ash for removal of H2S from gas stream. Appl. Surf. Sci. 2015, 327, 107–115. [Google Scholar] [CrossRef]
- Materazzi, M.; Lettieri, P.; Mazzei, L.; Taylor, R.; Chapman, C. Reforming of tars and organic sulphur compounds in a plasma-assisted process for waste gasification. Fuel Process. Technol. 2015, 137, 259–268. [Google Scholar] [CrossRef]
- Carmona, M.; Valverde, J.L.; Pérez, A.; Warcholb, J.; Rodriguez, J.F. Purification of glycerol/water solutions from biodiesel synthesis by ion exchange: sodium removal Part I. J. Chem. Technol. Biotechnol. 2009, 84, 738–744. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Pardal, A. Rape oil transesterification over heterogeneous catalysts. Fuel Process. Technol. 2010, 91, 1530–1536. [Google Scholar] [CrossRef]
- Crnkovic, P.M.; Koch, C.; Ávila, I.; Mortari, D.A.; Cordoba, A.M.; dos Santos, A.M. Determination of the activation energies of beef tallow and crude glycerin combustion using thermogravimetry. Biomass Bioenergy 2012, 44, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Dou, B.; Dupont, V.; Williams, P.T.; Chen, H.; Ding, Y. Thermogravimetric kinetics of crude glycerol. Bioresour. Technol. 2009, 100, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, D.; de Oliveira, E.R.; Araújo Sales, M.J. Polystyrene/thermoplastic starch blends with different plasticizers. Preparation and thermal characterization. J. Therm. Anal. Calorim 2007, 87, 635–638. [Google Scholar] [CrossRef]
- Franco, C.; Pinto, P.; Gulyurtlu, I.; Cabrita, I. The study of reactions influencing the biomass steam gasification process. Fuel 2003, 82, 835–842. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Borup, L. Thermodynamic equilibrium calculations of hydrogen production from the combined processes of dimethyl ether steam reforming and partial oxidation. J. Power Sources 2006, 155, 340–352. [Google Scholar] [CrossRef]
- Werle, S. Gasification of a Dried Sewage Sludge in a Laboratory Scale Fixed Bed Reactor. Energies 2015, 8, 8562–8572. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suero, S.R.; Ledesma, B.; Álvarez-Murillo, A.; Al-Kassir, A.; Yusaf, T. Glycerin, a Biodiesel By-Product with Potentiality to Produce Hydrogen by Steam Gasification. Energies 2015, 8, 12765-12775. https://doi.org/10.3390/en81112339
Suero SR, Ledesma B, Álvarez-Murillo A, Al-Kassir A, Yusaf T. Glycerin, a Biodiesel By-Product with Potentiality to Produce Hydrogen by Steam Gasification. Energies. 2015; 8(11):12765-12775. https://doi.org/10.3390/en81112339
Chicago/Turabian StyleSuero, Silvia Román, Beatriz Ledesma, Andrés Álvarez-Murillo, Awf Al-Kassir, and Talal Yusaf. 2015. "Glycerin, a Biodiesel By-Product with Potentiality to Produce Hydrogen by Steam Gasification" Energies 8, no. 11: 12765-12775. https://doi.org/10.3390/en81112339
APA StyleSuero, S. R., Ledesma, B., Álvarez-Murillo, A., Al-Kassir, A., & Yusaf, T. (2015). Glycerin, a Biodiesel By-Product with Potentiality to Produce Hydrogen by Steam Gasification. Energies, 8(11), 12765-12775. https://doi.org/10.3390/en81112339