Characterization of Anaerobic Degradability and Kinetics of Harvested Submerged Aquatic Weeds Used for Nutrient Phytoremediation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of Submerged Aquatic Weed Species Used for Anaerobic Digestion Based on Batch Biochemical Methane Potential Tests
| Species | Cellulose (mg/g-TS) | Hemicellulose (mg/g-TS) | Lignin (mg/g-TS) | Protein (mg/g-TS) | Lipid (mg/g-TS) | Carbon/nitrogen ratio |
|---|---|---|---|---|---|---|
| P. malaianus | 212 | Not detected | 116 | 350 ± 39 | 34.2 | 12.5 ± 0.3 |
| P. perfoliatus | 200 | Not detected | 165 | 298 ± 32 | 26.1 | 8.5± 0.2 |
| C. demersum | 185 | 82.0 | 186 | 315 ± 121 | 31.8 | 10.4 ± 0.2 |
| P. dentatus | 195 | Not detected | 155 | 266 ± 39 | 41.0 | 8.7 ± 0.1 |
| H. verticillata | 178 | 66.0 | 129 | 261 ± 86 | 15.1 | 9.6 ± 0.4 |
| E. densa | 202 | Not detected | 50 | 294 ± 24 | 29.1 | 10.2 ± 0.2 |
| P. inbaensis | 210 | Not detected | 83 | 280 ± 32 | 49.4 | 12.9 ± 0.4 |
| M. aquaticum | 200 | Not detected | 59 | 286 ± 33 | 53.8 | 9.8 ± 0.3 |
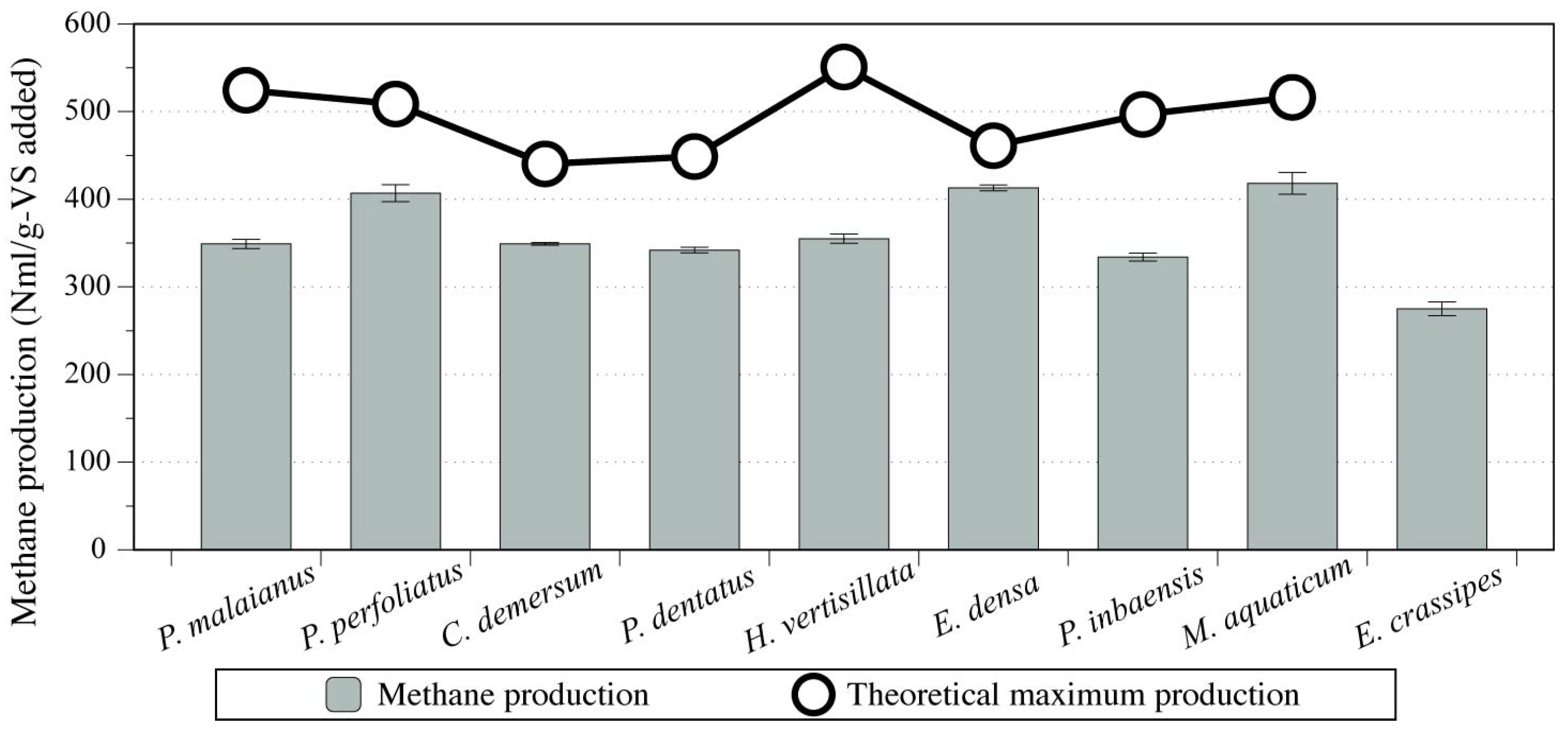
2.2. Performance of Mesophilic Anaerobic Digestion Treating E. Densa in Semi-Continuous Operation
2.2.1. Characteristics of Feedstock (E. Densa)
2.2.2. Time Course of Gas Production and Digestibility during the Experiment
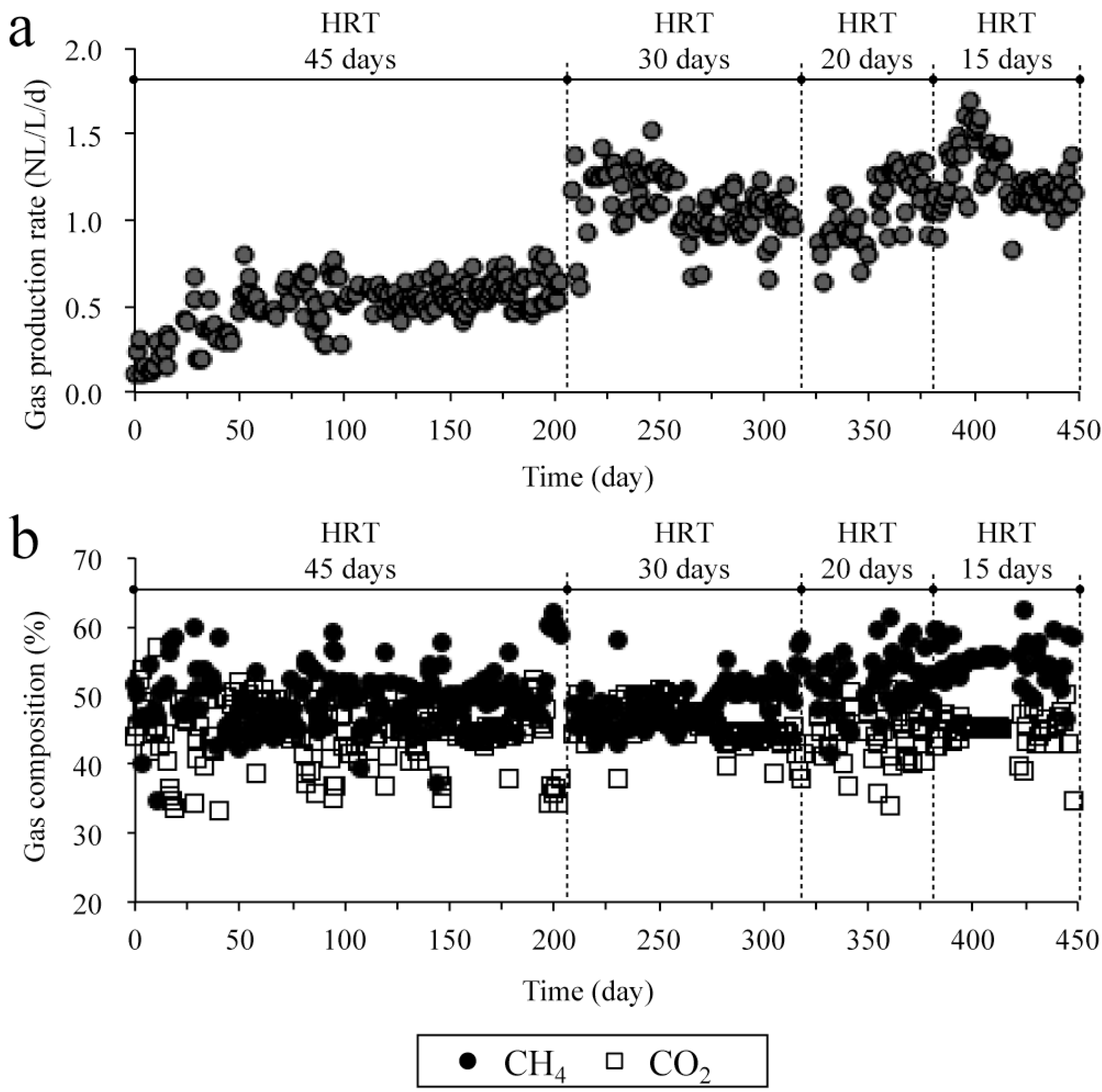
2.2.3. Characteristics of Organic Compounds Degradation
| Parameter | HRT (days) | |||
|---|---|---|---|---|
| 45 | 30 | 20 | 15 | |
| Methane production rate (NL/L/d) | 0.38 ± 0.06 | 0.53 ± 0.04 | 0.67 ± 0.08 | 0.64 ± 0.04 |
| Methane yield (NmL/g-VS added) | 231 ± 24 | 201 ± 10 | 171 ± 21 | 126 ± 7 |
| COD reduction (%) | 52.7 ± 8.2 | 44.6 ± 2.4 | 38.2 ± 0.9 | 33.1 ± 1.8 |
| Protein reduction (%) | 62.1 ± 6.7 | 53.9 ± 3.3 | 47.8 ± 3.7 | 38.7 ± 13.2 |
| NH4+ release (%) | 23.4 ± 3.3 | 22.0 ± 5.7 | 26.8 ± 2.7 | 18.0 ± 3.2 |
| PO43− release (%) | 2.5 ± 1.5 | 2.5 ± 0.5 | 3.9 ± 0.9 | 3.8 ± 1.7 |
2.2.4. Rate Limiting Step and Effect of HRT on the Reaction Steps

3. Materials and Methods
3.1. Biochemical Methane Potential Tests
3.2. Semi-Continuous Anaerobic Digestion Experiment of E. Densa
3.3. Analytical Methods
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Tripathi, B.D.; Upadhyay, A.R. Dairy effluent polishing by aquatic macrophytes. Water Air Soil Pollut. 2003, 143, 377–385. [Google Scholar] [CrossRef]
- Nahlik, A.M.; Mitsch, W.J. Tropical treatment wetlands dominated by free-floating macrophytes for water quality improvement in Costa Rica. Ecol. Eng. 2006, 28, 246–257. [Google Scholar] [CrossRef]
- Van Donk, E.; Gulati, R.D.; Iedema, A.; Meulemans, J.T. Macrophyte-related shifts in the nitrogen and phosphorus contents of the different trophic levels in a biomanipulated shallow lake. Arch. Hydrobiol. 1993, 251, 19–26. [Google Scholar]
- O’Sullivan, C.; Rounsefell, B.; Grinham, A.; Clarke, W.; Udy, J. Anaerobic digestion of harvested aquatic weeds: water hyacinth (Eichhornia crassipes), cabomba (Cabomba caroliniana) and salvinia (Salvinia molesta). Ecol. Eng. 2010, 36, 1459–1468. [Google Scholar] [CrossRef]
- Escobar, M.M.; Voyevoda, M.; Fühner, C.; Zehnsdorf, A. Potential uses of Elodea nuttallii-harvested biomass. Energy Sustain. Soc. 2011, 1, 1–8. [Google Scholar]
- Haga, H.; Ishikawa, K. Spatial distribution of submerged macrophytes in the southern basin of Lake Biwa in the summer of 2007, in comparison with that in 2002. Jpn. J. Limnol. 2011, 72, 81–88. (In Japanese) [Google Scholar] [CrossRef]
- Rajendran, K.; Aslanzadeh, S.; Taherzadeh, M.J. Household biogas digesters—A review. Energies 2012, 5, 2911–2942. [Google Scholar] [CrossRef]
- Chynoweth, D.P.; Dolenc, D.A.; Ghosh, S.; Henry, M.P.; Jerger, D.E.; Srivastava, V.J. Kinetics and advanced digester design for anaerobic digestion of water hyacinth and primary sludge. Biotechnol. Bioeng. Symp. 1982, 12, 381–398. [Google Scholar]
- Abbasi, S.A.; Nipaney, P.C.; Schaumberg, G.D. Bioenergy potential of eight common aquatic weeds. Biol. Wastes 1990, 34, 359–366. [Google Scholar] [CrossRef]
- Koyama, M.; Yamamoto, S.; Ishikawa, K.; Ban, S.; Toda, T. Anaerobic digestion of submerged macrophytes: Chemical composition and anaerobic digestibility. Ecol. Eng. 2014, 69, 304–309. [Google Scholar] [CrossRef]
- Benner, R.; Maccubbin, A.E.; Hodson, R.E. Anaerobic biodegradation of thelignin and polysaccharide components of lignocellulose and synthetic lignin bysediment microflora. Appl. Environ. Microbiol. 1984, 47, 998–1004. [Google Scholar] [PubMed]
- Chanakya, H.N.; Borgaonkar, S.; Meena, G.; Jagadish, K.S. Solid-phase biogas production with garbage or water hyacinth. Bioresour. Technol. 1993, 46, 227–231. [Google Scholar] [CrossRef]
- Patel, V.; Desai, M.; Madamwar, D. Thermochemical pretreatment of water hyacinth for improved biomethanation. Appl. Biochem. Biotechnol. 1993, 42, 67–74. [Google Scholar] [CrossRef]
- Srivastava, R.C. Kinetics of fresh water hyacinth digestion in semi-continuous operation. Chem. Eng. J. 1995, 56, 100–113. [Google Scholar]
- Pfeffer, J.T. Temperature effects on anaerobic fermentation of domestic refuse. Biotechnol. Bioeng. 1974, 16, 771–787. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes toimprove ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Magdum, S.S.; More, S.; Nadaf, A. Biochemical conversion of acid-pretreated water hyacinth (Eichhornia crassipes) to alcohol using pichia stipitis NCIM3497. Int. J. Adv. Biotechnol. Res. 2012, 3, 585–590. [Google Scholar]
- Mukherjee, R.; Ghosh, M.; Nandi, B. Improvement of dry matter digestibility of water hyacinth by solid state fermentation using white rot fungi. Indian J. Exp. Biol. 2004, 42, 837–843. [Google Scholar] [PubMed]
- Chuang, Y.-S.; Lay, C.-H.; Sen, B.; Chen, C.-C.; Gopalakrishnan, K.; Wu, J.-H.; Lin, C.-S.; Lin, C.-Y. Biohydrogen and biomethane from water hyacinth (Eichhornia crassipes) fermentation: Effects of substrate concentration and incubation temperature. Int. J. Hydrog. Energy 2011, 36, 14195–14203. [Google Scholar] [CrossRef]
- Pistori, R.E.; Camargo, A.F.; Henry-Silva, G.G. Relative growth rate and doubling time of the submerged aquatic macrophyte Egeria densa Planch. Acta Limnol. Brasiliensia 2004, 16, 77–84. [Google Scholar]
- Yarrow, M.; Marin, V.H.; Finlayson, M.; Tironi, A.; Delgado, L.E.; Fischer, F. The ecology of Egeria densa Planchon (Liliopsida: Alismatales): A wetland ecosystem engineer. Rev. Chil. Hist. Nat. 2009, 82, 299–313. [Google Scholar] [CrossRef]
- Kobayashi, T.; Xu, K.Q.; Li, Y.Y.; Inamori, Y. Evaluation of hydrogen and methane production from municipal solid wastes with different compositions of fat, protein, cellulosic materials and the other carbohydrates. Int. J. Hydrog. Energy 2012, 37, 15711–15718. [Google Scholar] [CrossRef]
- Kobayashi, T.; Xu, K.Q.; Li, Y.Y.; Inamori, Y. Effect of sludge recirculation on characteristics of hydrogen production in a two-stage hydrogen–methane fermentation process treating food wastes. Int. J. Hydrog. Energy 2012, 37, 5602–5611. [Google Scholar] [CrossRef]
- Kobayashi, T.; Wu, Y.P.; Xu, K.Q.; Li, Y.Y. Effect of mixing driven by siphon flow: Parallel experiments using the anaerobic reactors with different mixing modes. Energies 2013, 6, 4207–4222. [Google Scholar] [CrossRef]
- Martinez, A.; Church, D.C. Effect of various mineral elements on in vitro rumen cellulose digestion. J. Anim. Sci. 1970, 31, 982–990. [Google Scholar] [PubMed]
- Qiang, H.; Lang, D.L.; Li, Y.Y. High-solid mesophilic methane fermentation of food waste with an emphasis on Iron, Cobalt, Nickel requirements. Bioresour. Technol. 2012, 103, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.; Inoue, Y.; Hosomi, M.; Murakami, A. Growth inhibition of blue–green algae by allelopathic effects of macrophytes. Water Sci. Technol. 1999, 39, 47–53. [Google Scholar] [CrossRef]
- Hilt, S. Allelopathic inhibition of epiphytes by submerged macrophytes. Aquat. Bot. 2006, 85, 252–256. [Google Scholar] [CrossRef]
- Sakurai, K.; Li, Y.Y.; Noike, T. Characteristics of mesophilic methane fermentation of high-solid content cow manure. J. Jpn. Soc. Waste Manag. Expert. 2005, 16, 65–73. (In Japanese) [Google Scholar] [CrossRef]
- Vavilin, V.A.; Lokshina, L.Y.; Jokela, J.P.; Rintala, J.A. Modeling solid waste decomposition. Bioresour. Technol. 2004, 94, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradablity of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Veeken, A.; Hamelers, B. Effect of temperature on hydrolysis rates of selected biowaste components. Bioresour. Technol. 1999, 69, 249–254. [Google Scholar] [CrossRef]
- Lu, Z.; Li, J.; Inamori, R.; Xu, K.Q.; Sugiura, N.; Inamori, Y. Comparative study on purification characteristics of various submerged macrophyte species in different seasons. Jpn. J. Water Treat. Biol. 2013, 49, 11–19. [Google Scholar]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association (APHA): Washington, DC, USA, 1998. [Google Scholar]
- Van Soest, P.J.; McQueen, R.W. The chemistry and estimation of fibre. Proc. Nutr. Soc. 1973, 32, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Effland, M.J. Modified procedure to determine acid-insoluble lignin in wood and pulp. Tappi J. 1977, 60, 143–144. [Google Scholar]
- Eastman, J.A.; Ferguson, J.F. Solubilization of particulate organic carbon during the acid phase of anaerobic digestion. J. Water Pollut. Control Fed. 1981, 53, 352–366. [Google Scholar]
- Pavlostathis, S.G.; Giraldo-Gomez, E. Kinetics of anaerobic treatment: A critical review. Crit. Rev. Environ. Control 1991, 21, 411–490. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, T.; Wu, Y.-P.; Lu, Z.-J.; Xu, K.-Q. Characterization of Anaerobic Degradability and Kinetics of Harvested Submerged Aquatic Weeds Used for Nutrient Phytoremediation. Energies 2015, 8, 304-318. https://doi.org/10.3390/en8010304
Kobayashi T, Wu Y-P, Lu Z-J, Xu K-Q. Characterization of Anaerobic Degradability and Kinetics of Harvested Submerged Aquatic Weeds Used for Nutrient Phytoremediation. Energies. 2015; 8(1):304-318. https://doi.org/10.3390/en8010304
Chicago/Turabian StyleKobayashi, Takuro, Ya-Peng Wu, Zhi-Jiang Lu, and Kai-Qin Xu. 2015. "Characterization of Anaerobic Degradability and Kinetics of Harvested Submerged Aquatic Weeds Used for Nutrient Phytoremediation" Energies 8, no. 1: 304-318. https://doi.org/10.3390/en8010304





