Biogas Production from Thin Stillage on an Industrial Scale—Experience and Optimisation
Abstract
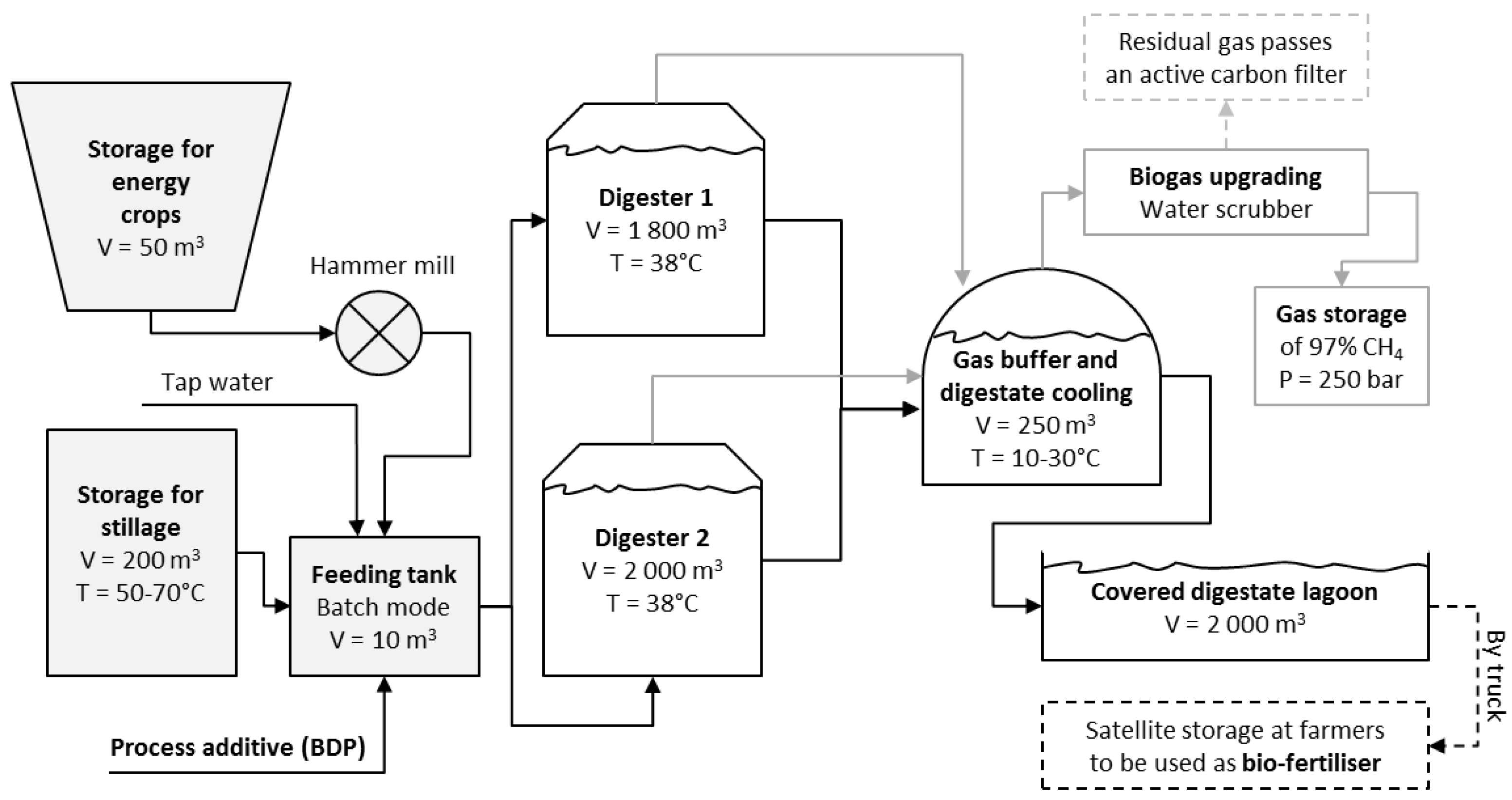
:1. Introduction
2. Results and Discussion
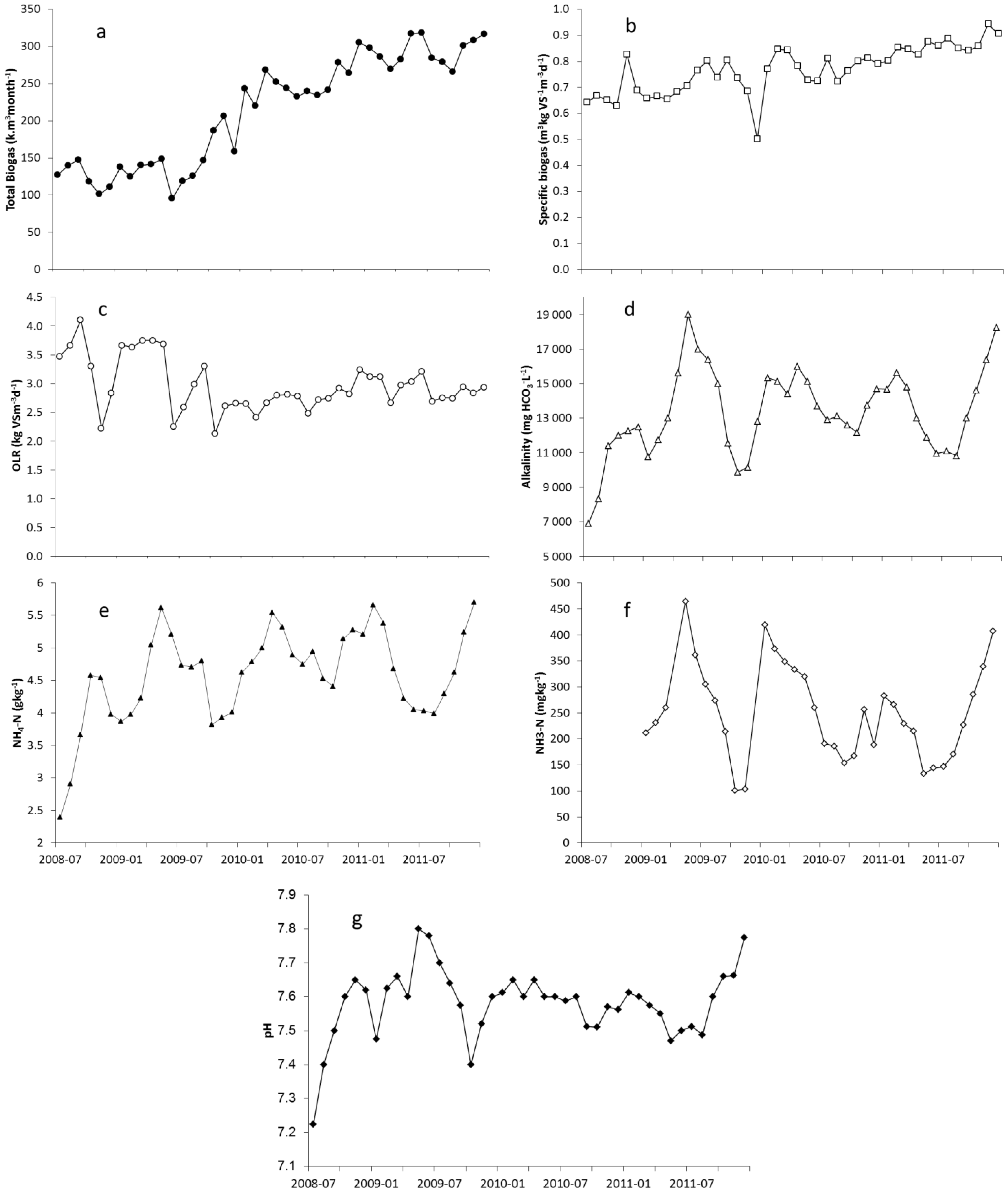
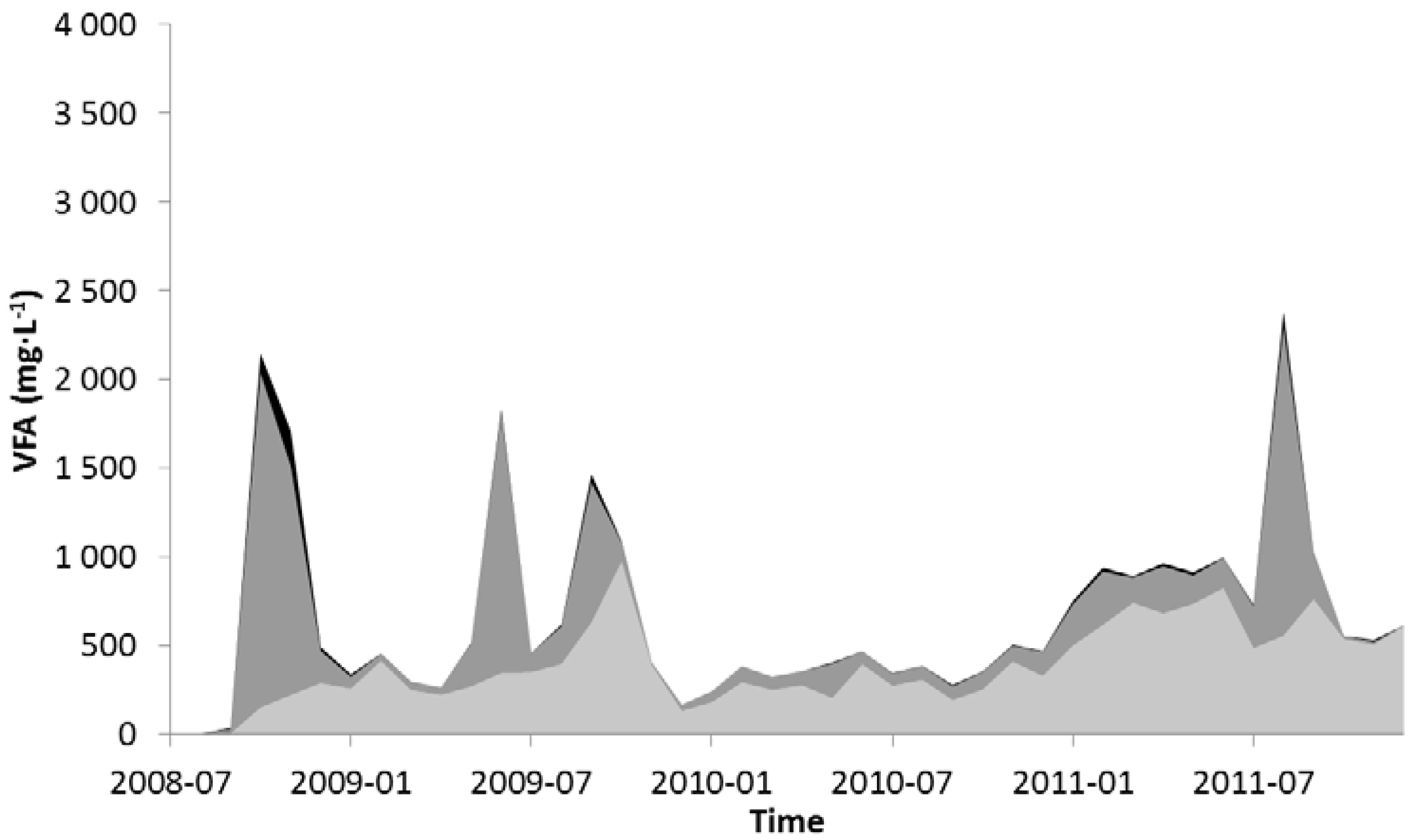
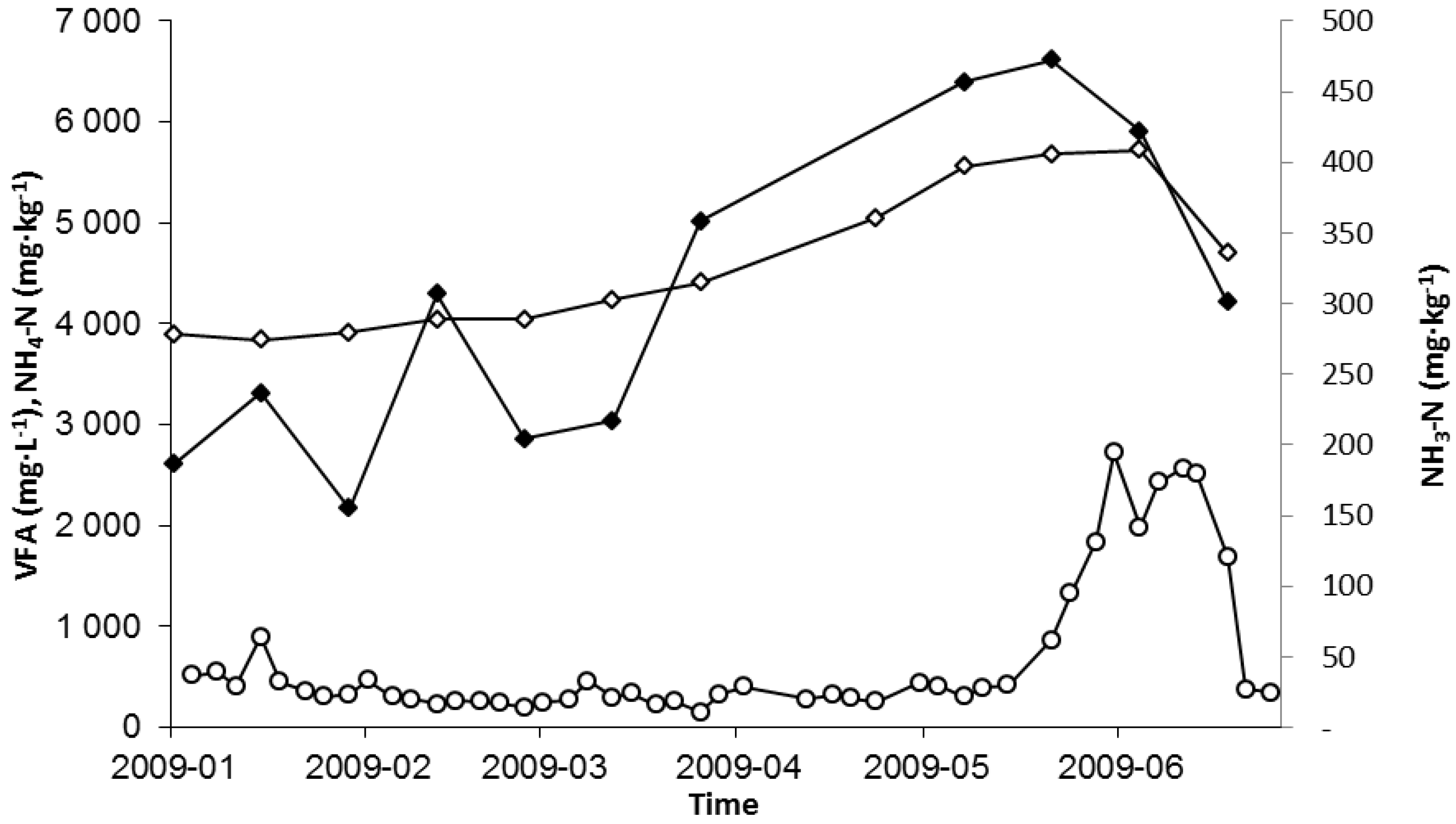
2.1. Nitrogen
| Measure | Cause | Mode of action |
|---|---|---|
| Mesophilic temperature | Nitrogen toxicity | Keep the fraction of NH3-N as low as possible |
| Addition of hydrochloric acid (HCl) and Fe3+ |
|
|
| Optimised dilution of feedstock |
|
|
| - |
|
|
| - |
|
|
| Addition of iron (Fe2+/Fe3+) | High levels of H2S |
|
| Addition of cobalt (Co2+) |
| Obtain high process stability by supplying microorganisms with this critical trace element |
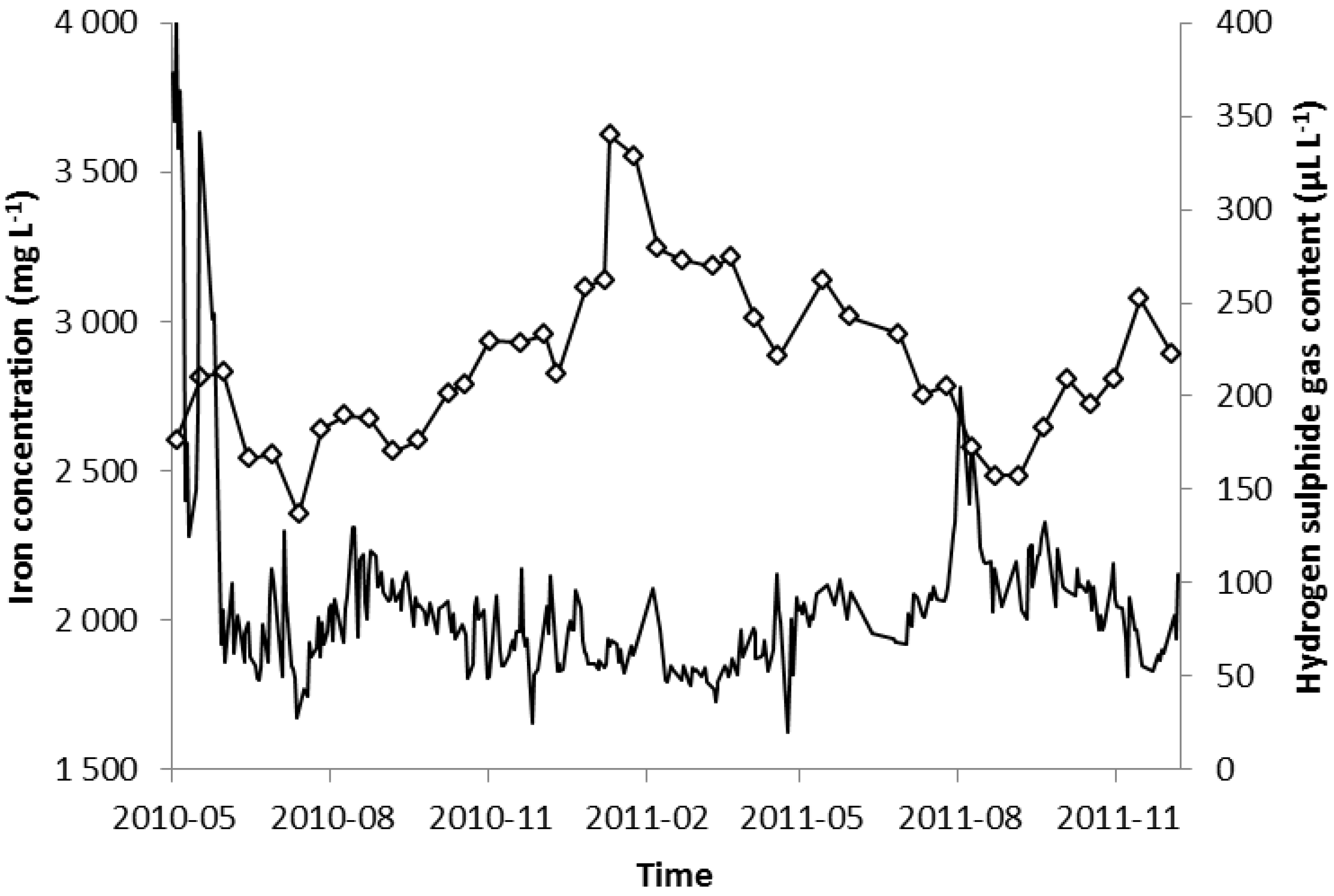
2.2. Hydrogen Sulphide
2.3. Trace Elements and Co-Digestion
2.4. Further Measures
3. Experimental Section
Analyses
4. Conclusions
Nomenclature:
| HRT | hydraulic retention time |
| COD | chemical oxygen demand |
| OLR | organic loading rate |
| TS | total solids |
| VS | volatile solids |
| VFA | volatile fatty acids |
| DNA | deoxyribonucleic acid |
| qPCR | quantitative polymerase chain reaction |
| SAO | syntrophic acetate oxidation |
| SAOB | syntrophic acetate-oxidising bacteria |
| SRB | sulphate-reducing bacteria |
Acknowledgments
Conflicts of Interest
References
- Abubaker, J.; Risberg, K.; Pell, M. Biogas residues as fertilisers—Effects on wheat growth and soil microbial activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- EurObserv’ER. Biogas barometers. J. Énergies Renouv. 2012, 212, 66–79. [Google Scholar]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- EurObserv’ER. Biofuels barometer. J. Énergies Renouv. 2012, 210, 42–62. [Google Scholar]
- Schmidt, T.; Pröter, J.; Scholwin, F.; Nelles, M. Anaerobic digestion of grain stillage at high organic loading rates in three different reactor systems. Biomass Bioenergy 2013, 55, 285–290. [Google Scholar] [CrossRef]
- Borjesson, P.; Tufvesson, L.M. Agricultural crop-based biofuels—Resource efficiency and environmental performance including direct land use changes. J. Clean. Prod. 2011, 19, 108–120. [Google Scholar] [CrossRef]
- Kim, Y.; Mosier, N.S.; Hendrickson, R.; Ezeji, T.; Blaschek, H.; Dien, B.; Cotta, M.; Dale, B.; Ladisch, M.R. Composition of dry-grind ethanol by-products: DDGS, wet cake and thin stillage. Bioresour. Technol. 2008, 99, 5165–5176. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, A.C.; Riedesel, K.J.; Owens, J.M. Stillage characterization and anaerobic treatment of ethanol stillage from conventional and cellulosic feedstock. Biomass Bioenergy 2000, 19, 63–102. [Google Scholar] [CrossRef]
- Martin, M.; Eklund, M. Improving the environmental performance of biofuels with industrial symbiosis. Biomass Bioenergy 2011, 35, 1747–1755. [Google Scholar] [CrossRef]
- Agler, M.T.; Garcia, M.L.; Lee, E.S.; Schlicher, M.; Angenent, L.T. Thermophilic anaerobic digestion to increase the net eneregy balance of corn grain ethanol. Environ. Sci. Technol. 2008, 42, 6723–6729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Schnürer, A.; Zeller, G.; Svensson, B.H. Mesophilic syntrophic acetate oxidation during methane formation in biogas reactors. FEMS Microbiol. Ecol. 1999, 29, 249–261. [Google Scholar] [CrossRef]
- Schnürer, A.; Nordberg, A. Ammonia, a selective agent for methane production by syntrophic acetate oxidation at mesophilic temperature. Water Sci. Technol. 2008, 57, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Westerholm, M. Biogas Production through the Syntrophic Acetate-Oxidising Pathway. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2012. [Google Scholar]
- Karlsson, R. Personal Communication, Lantmännen Agroetanol AB: Norrköping, Sweden, 2013.
- Rabus, R.; Hansen, T.A.; Widdel, F. Dissimilatory Sulfate- and Sulfur-Reducing Prokaryotes. In The prokaryotes; Springer: Singapore, 2006; Volume 2, pp. 659–768. [Google Scholar]
- Moestedt, J.; Nilsson Påledal, S.; Schnürer, A. The effect of substrate and operational parameters on the abundance of sulphate-reducing bacteria in industrial anaerobic biogas digesters. Bioresour. Technol. 2013, 132, 327–332. [Google Scholar] [CrossRef] [PubMed]
- McCartney, D.M.; Oleszkiewicz, J.A. Sulfide inhibition of anaerobic degradation of lactate and acetate. Water Res. 1991, 25, 203–209. [Google Scholar] [CrossRef]
- Van der Veen, A.; Fermoso, F.G.; Lens, P.N.L. Bonding form of metals and sulfur fractionation in methanol-grown anaerobic granular sludge. Eng. Life Sci. 2007, 7, 480–489. [Google Scholar] [CrossRef]
- Dererie, D.Y.; Tobro, S.; Momeni, M.H.; Hansson, H.; Blomqvist, J.; Passoth, V.; Schnurer, A.; Sandgren, M.; Ståhlberg, J. Improved bio-energy yields via sequential ethanol fermentation and biogas digestion of steam exploded oat straw. Bioresour. Technol. 2011, 102, 4449–4455. [Google Scholar] [CrossRef] [PubMed]
- Nasr, N.; Elbeshbishy, E.; Hafez, H.; Nakhla, G.; El Naggar, M.H. Comparative assessment of single-stage and two-stage anaerobic digestion for the treatment of thin stillage. Bioresour. Technol. 2012, 111, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Alkan-Ozkaynak, A.; Karthikeyan, K.G. Anaerobic digestion of thin stillage for energy recovery and water reuse in corn-ethanol plants. Bioresour. Technol. 2011, 102, 9891–9896. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.L.; Angenent, L.T. Interaction between temperature and ammonia in mesophilic digesters for animal waste treatment. Water Res. 2009, 43, 2373–2382. [Google Scholar] [CrossRef] [PubMed]
- Eskicioglu, C.; Kennedy, K.J.; Marin, J.; Srehler, B. Anaerobic digestion of whole stillage from dry-grind corn ethanol plant under mesophilic and thermophilic conditions. Bioresour. Technol. 2011, 102, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Schnürer, A.; Houwen, F.P.; Svensson, B.H. Mesophilic syntrophic acetate oxidation during methane formation by a triculture at high ammonium concentration. Arch. Microbiol. 1994, 162, 70–74. [Google Scholar] [CrossRef]
- Sun, L.; Müller, B.; Westerholm, M.; Schnürer, A. Syntrophic Acetate Oxidation in Industrial CSTR Biogas Digesters. Department of Microbiology, BioCenter, Swedish University of Agricultural Sciences: Uppsala, Sweden, Unpublished work. 2013. [Google Scholar]
- Karlsson, A.; Ejlertsson, J. Addition of HCl as a means to improve biogas production from protein-rich food industry waste. Biochem. Eng. J. 2012, 61, 43–48. [Google Scholar] [CrossRef]
- Strik, D.; Domnanovich, A.M.; Holubar, P. A pH-based control of ammonia in biogas during anaerobic digestion of artificial pig manure and maize silage. Process Biochem. 2006, 41, 1235–1238. [Google Scholar] [CrossRef]
- Calli, B.; Mertoglu, B.; Inanc, B.; Yenigun, O. Effects of high free ammonia concentrations on the performances of anaerobic bioreactors. Process Biochem. 2005, 40, 1285–1292. [Google Scholar] [CrossRef]
- Poggi-Varaldo, H.M.; Rodríguez-Vázquez, R.; Fernandez-Villagómez, G.; Esparza-García, F. Inhibition of mesophilic solid-substrate anaerobic digestion by ammonia nitrogen. Appl. Microbiol. Biotechnol. 1997, 47, 284–291. [Google Scholar] [CrossRef]
- Siles, J.A.; Brekelmans, J.; Martin, M.A.; Chica, A.F.; Martin, A. Impact of ammonia and sulphate concentration on thermophilic anaerobic digestion. Bioresour. Technol. 2010, 101, 9040–9048. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.I.C.; Capela, M.I.; Lens, P. Sulfate reduction during the acidification of sucrose at pH 5 under thermophilic (55 °C) conditions. I: Effect of trace metals. Bioresour. Technol. 2010, 101, 4269–4277. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Svensson, B.H.; Karlsson, A. The feasibility of trace element supplementation for stable operation of wheat stillage-fed biogas tank reactors. Water Sci. Technol. 2011, 64, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Westerholm, M.; Hansson, M.; Schnürer, A. Improved biogas production from whole stillage by co-digestion with cattle manure. Bioresour. Technol. 2012, 114, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.J.; Zhang, Y.; Jiang, Y.; Heaven, S. Trace element requirements for stable food waste digestion at elevated ammonia concentrations. Bioresour. Technol. 2012, 104, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.; Einarsson, P.; Schnürer, A.; Sundberg, C.; Ejlertsson, J.; Svensson, B.H. Impact of trace element addition on degradation efficiency of volatile fatty acids, oleic acid and phenyl acetate and on microbial populations in a biogas digester. J. Biosci. Bioeng. 2012, 114, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Nordell, E.; Moestedt, J.; Wiberg, L.; Karlsson, M. A Trace Metal Formulation that Reinforces the Effects of a Cobalt Additive. In Proceedings of the Fourth International Symposium on Energy from Biomass and Waste, Venice, Italy, 12–15 November 2012; Volume 4.
- Ek, A.; Hallin, S.; Vallin, L.; Schnürer, A.; Karlsson, M. Slaughterhouse Waste Co-Digestion—Experiences from 15 Years of Full-Scale Operation. In Proceedings of the World Renewable Energy Congress, Linköping, Sweden, 8–13 May 2011; pp. 64–71.
- Nordell, E.; Karlsson, M. Post Digestion of Biogas Production Residues at Mid-Range Mesophilic Temperature. In Proceedings of the International Symposium on Anaerobic Digestion of Solid Waste and Energy Crops, Vienna, Austria, 28 August–1 September 2011.
- Ejlertsson, J. Method, a Device, and an Additive for Digesting Organic Matter. U.S. Patent 20070184542 A1, 9 August 2007. [Google Scholar]
- Petersson, A. English Summary of SPCR 120—Certification Rules for Digestate from Biowaste by the Quality Assurance System of Swedish Waste Management; Swedish Gas Centre: Borås, Sweden, 2013. [Google Scholar]
- Jonsson, S.; Borén, H. Analysis of mono- and diesters of o-phthalic acid by solid-phase extractions with polystyrene—Divinylbenzene-based polymers. J. Chromatogr. A 2002, 963, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Geets, J.; Borremans, B.; Diels, L.; Spingael, D.; Vangronsveld, J.; van der Leilie, D.; Vanbroekhoven, K. DsrB gene-based dgge for community and diversity surveys of sulfate-reducing bacteria. J. Microbiol. Methods 2006, 66, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Yao, L.; van Dongen, U.; Kuenen, J.G.; Muyzer, G. Analysis of diversity and activity of sulfate-reducing bacterial communities in sulfidogenic bioreactors using 16S rRNA and dsrB genes as molecular marjers. Appl. Environ. Microbiol. 2007, 73, 594–604. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moestedt, J.; Påledal, S.N.; Schnürer, A.; Nordell, E. Biogas Production from Thin Stillage on an Industrial Scale—Experience and Optimisation. Energies 2013, 6, 5642-5655. https://doi.org/10.3390/en6115642
Moestedt J, Påledal SN, Schnürer A, Nordell E. Biogas Production from Thin Stillage on an Industrial Scale—Experience and Optimisation. Energies. 2013; 6(11):5642-5655. https://doi.org/10.3390/en6115642
Chicago/Turabian StyleMoestedt, Jan, Sören Nilsson Påledal, Anna Schnürer, and Erik Nordell. 2013. "Biogas Production from Thin Stillage on an Industrial Scale—Experience and Optimisation" Energies 6, no. 11: 5642-5655. https://doi.org/10.3390/en6115642




