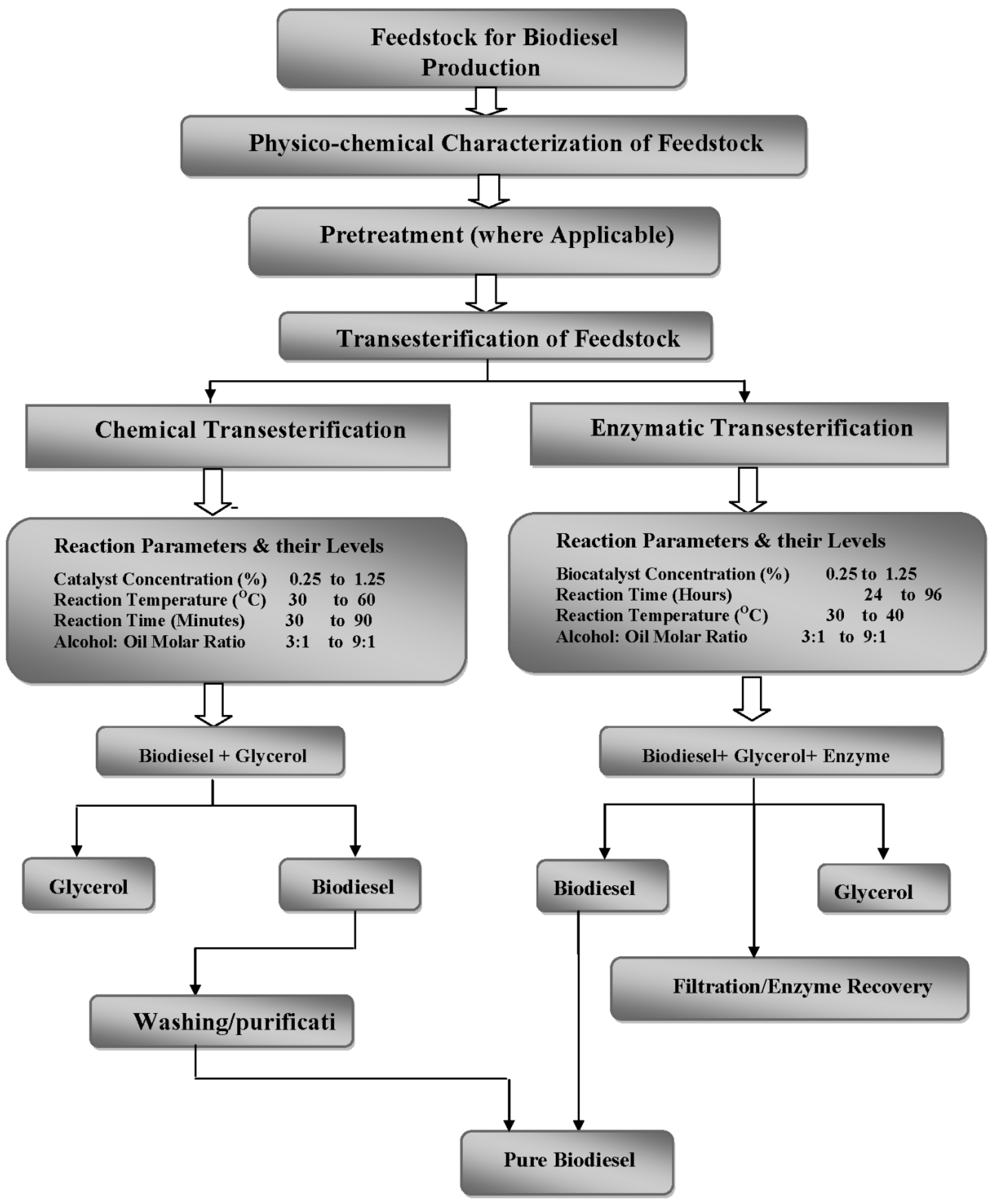
Quality evaluation of feedstock used for biodiesel production is of utmost importance; therefore, the feedstock under study was subjected to physicochemical characterization. According to the present analysis, the acid value, peroxide value, iodine value, density, refractive index, saponification value and unsaponifiable matter were found to be 0.72 ± 0.16 mg KOH/g of oil, 6.51 ± 0.29 meq O
2/kg of oil, 120.4 ± 1.5 g I
2/100 g of oil, 0.919 ± 0.071, 1.465 ± 0.140 191.1 ± 3.3 mgKOH/g of oil and 0.94 ± 0.05%, respectively for sunflower oil, whereas, 23.0 ± 0.09 mgKOH/g of oil, 7.72 ± 1.01 meqO
2/kg of oil, 112.35 ± 1.78 g I
2/100 g of oil, 0.916 ± 0.045, 1.478 ± 0.006, 186.9 ± 2.8 mg KOH/g of oil and 1.87 ± 0.15%, respectively for rice bran oil (
Table 1).
Rashid
et al. [
21] reported an acid value of 0.70 mg KOH/g of oil for sunflower oil, so the acid value of sunflower oil as estimated in the current research work was seen to be comparable with the former results, whereas, the acid value of rice bran oil was found to be lower than that (32.9 mg KOH/g of oil) reported by Rashid
et al. [
22]. The peroxide value of sunflower oil and rice bran oil as measured in this study were somewhat higher than the values of 2.27 meq/kg of oil and 1.73 meq/kg of oil, respectively, as described by Rashid
et al. [
21,
22]. The iodine value for sunflower oil was revealed to be somewhat lesser than the result,
i.e., 127.46 g I
2/100 g of oil reported by Rashid
et al.[
21] for sunflower oil, whereas, for rice bran oil the peroxide value was comparable with the result
i.e., 113.01 g I
2/100 g of oil investigated by Rashid
et al. [
22]. Saponification value of sunflower oil was found to be comparable to the saponification value
i.e., 188.60 mgKOH/g of oil as described by Rashid
et al. [
21]. On the other hand, the saponification value
i.e., 182.91 mgKOH/g described by Rashid
et al. [
22] was somewhat less than the saponification value of rice bran oil used in the present experiments.
2.1. Optimization of Biodiesel Production
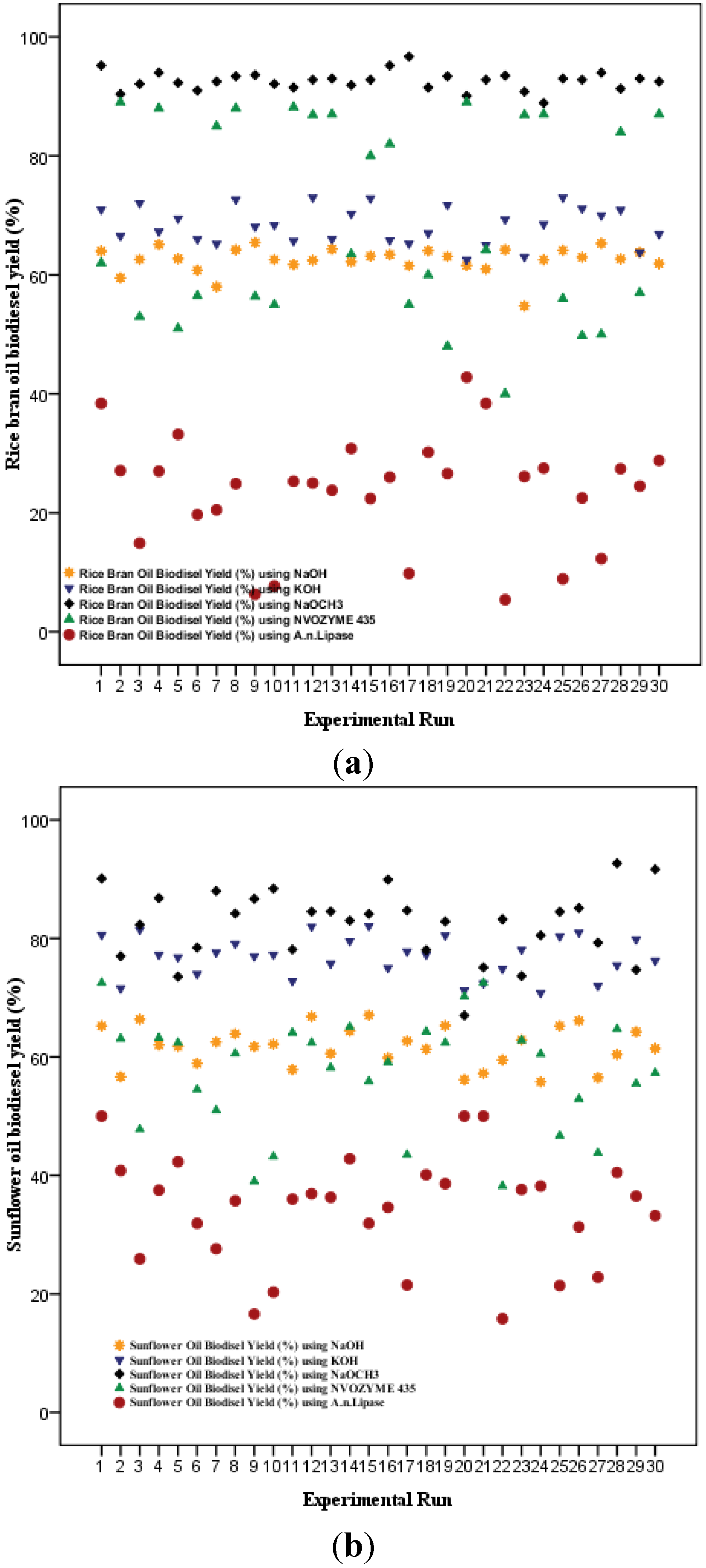
Based upon experimental results, biodiesel yields (%) resulted from chemical and enzymatic transesterification catalyzed by NaOH, KOH and NaOCH
3, NOVOZYME-435 and A.n. Lipase (
Figure 1) ranged from 54.8%–65.3%, 62.5%–73.4%, 88.9%–96.5%, 40.0%–89.3% and 5.4%–41.7%, respectively, for rice bran oil, and 55.8%–65.6%, 70.8%–82.1%, 67.0%–92.5%, 38.2%–72.6% and 15.8%–50.4%, respectively, for sunflower oil.
Figure 1.
Comparative description of biodiesel yields (%) resulted from 30 experiments executed under reaction conditions defined by CCRD for chemical and enzymatic transesterification of (a) rice bran oil and (b) sunflower oil.
Figure 1.
Comparative description of biodiesel yields (%) resulted from 30 experiments executed under reaction conditions defined by CCRD for chemical and enzymatic transesterification of (a) rice bran oil and (b) sunflower oil.
Optimization of the reaction parameters for both chemical and enzymatic transesterification of rice bran and sunflower oils were based on selected response surface models. Therefore, out of linear, 2F1, quadratic and cubic response surface models, the best fitted model was selected based upon
f-values, lack of fit test, R-squared values, adjusted R-squared values, coefficient of variance and adequate precision. Quadratic Models were established to give the best fit for the experimental data of chemical and enzymatic transesterification of ricebran and sunflower oils. Summary statistics of selected response surface quadratic models are given in
Table 2.
Table 2.
Summery statistics of selected models used for optimization of biodiesel production using chemical and enzymatic transesterification.
Table 2.
Summery statistics of selected models used for optimization of biodiesel production using chemical and enzymatic transesterification.
| Feedstock | Catalyst/Enzyme | Selected Model | Model significance (p-value) | C.V (%) | R-squared | Adj. R-squared | Lack of Fit | Adeq Precision |
|---|
| Rice bran oil | NaOH | Qardatic | 0.0003 | 1.78 | 0.8669 | 0.7427 | 0.1333 | 10.523 |
| | KOH | Qardatic | <0.0001 | 1.97 | 0.9040 | 0.8145 | 0.0556 | 10.356 |
| | NaOCH3 | Qardatic | 0.0001 | 0.81 | 0.8848 | 0.7773 | 0.0610 | 11.212 |
| | NOVOZYME-435 | Qardatic | <0.0001 | 2.17 | 0.9958 | 0.9918 | 0.0762 | 46.191 |
| | A. n. Lipase | Qardatic | <0.0001 | 10.72 | 0.9638 | 0.9300 | 0.0561 | 19.765 |
| Sunflower oil | NaOH | Qardatic | 0.0021 | 3.21 | 0.8198 | 0.6517 | 0.0785 | 6.503 |
| | KOH | Qardatic | 0.0020 | 2.58 | 0.8218 | 0.6554 | 0.0677 | 6.629 |
| | NaOCH3 | Qardatic | <0.0001 | 1.60 | 0.9750 | 0.9516 | 0.1729 | 28.066 |
| | NOVOZYME-435 | Qardatic | <0.0001 | 4.87 | 0.9548 | 0.9127 | 0.2229 | 18.502 |
| | A. n. Lipase | Qardatic | <0.0001 | 8.03 | 0.9552 | 0.9133 | 0.1069 | 18.577 |
The significance of suggested quadratic models a, b, c, d and e for chemical and enzymatic transesterification of rice bran and sunflower oils, the main effects, interaction effects and quadratic terms of each model are described in
Table 3,
Table 4,
Table 5 and
Table 6.
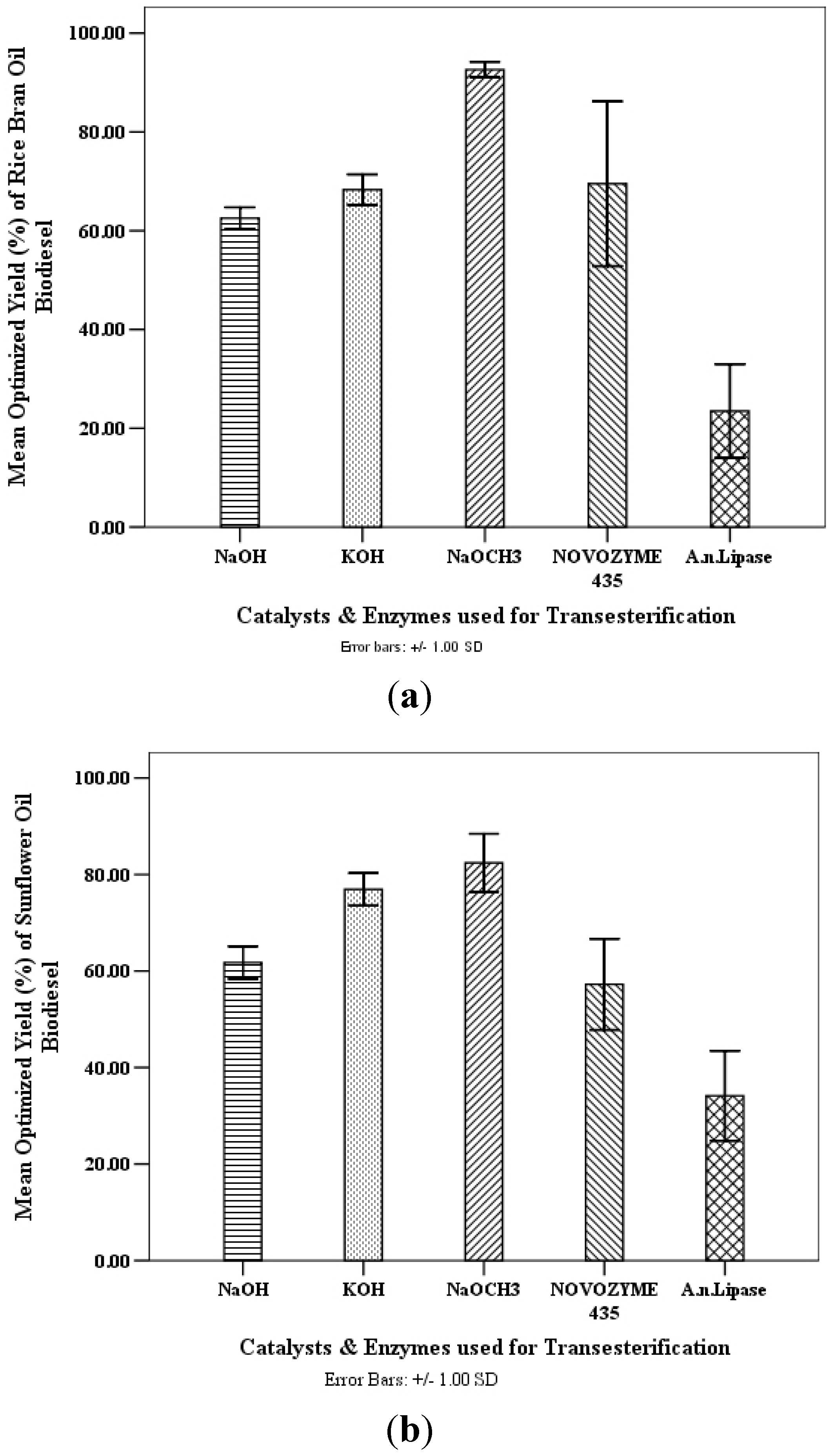
The optimized predicted biodiesel yields using both the vegetable oils were found to be comparable with the experimental results and thus validated the actual biodiesel yields
viz. 65.3 ± 2.0%, 73.4 ± 3.5%, 96.5 ± 1.6%, 89.3 ± 2.0% and 41.7 ± 3.9% using rice bran oil, and 65.6 ± 1.2%, 82.1 ± 1.7%, 92.5 ± 2.8%, 72.6 ± 1.6% and 50.4 ± 2.5% using sunflower oil resulted from transesterification catalyzed by NaOH, KOH and NaOCH
3, NOVOZYME-435 and A.n. Lipase (
Figure 2).
Table 3.
Response Surface quadratic model analysis of variance (ANOVA) table for chemical transesterification of rice bran oil.
Table 3.
Response Surface quadratic model analysis of variance (ANOVA) table for chemical transesterification of rice bran oil.
| Source | df | SS (MS) a | SS (MS) b | SS (MS) c | F Value (p-value) a | F Value (p-value) b | F Value (p-value) c | |
|---|
| Model | 14 | 256.72 (18.34) | 121.66 (8.69) | 65.27 (4.66) | 10.09 (<0.0001) | 6.98 (0.0003) | 8.23 (0.0001) | significant |
| A-Catalyst Concentration | 1 | 53.85 (53.85) | 29.02 (29.02) | 18.20 (18.20) | 29.64 (<0.0001) | 23.31 (0.0002) | 32.13 (<0.0001) | |
| B-Reaction Time | 1 | 5.79 (5.79) | 14.34 (14.34) | 7.37 (7.37) | 3.19 (0.0944) | 11.52 (0.0040) | 13.01 (0.0026) | |
| C-Reaction temperature | 1 | 3.19 (3.19) | 7.01 (7.01) | 4.25 (4.25) | 1.76 (0.2049) | 5.63 (0.0314) | 7.50 (0.0152) | |
| D- Alcohol: Oil Molar Ratio | 1 | 0.84 (0.84) | 5.93 (5.93) | 13.05 (13.05) | 0.46 (0.5069) | 4.76 (0.0454) | 23.05 (0.0002) | |
| AB | 1 | 12.58 (12.58) | 3.79 (3.79) | 0.46 (0.46) | 6.93 (0.0189) | 3.05 (0.1014) | 0.80 (0.3839) | |
| AC | 1 | 0.022 (0.022) | 14.76 (14.76) | 0.016 (0.016) | 0.012 (0.9143) | 11.86 (0.0036) | 0.028 (0.8703) | |
| AD | 1 | 1.26 (1.26) | 0.001806 (0.001806) | 1.89 (1.89) | 0.69 (0.4180) | 0.001451 (0.9701) | 3.34 (0.0877) | |
| BC | 1 | 12.62 (12.62) | 11.82 (11.82) | 0.46 (0.46) | 6.95 (0.0187) | 9.49 (0.0076) | 0.80 (0.3839) | |
| BD | 1 | 0.39 (0.39) | 1.02 (1.02) | 0.53 (0.53) | 0.22 (0.6482) | 0.82 (0.3808) | 0.93 (0.3507) | |
| CD | 1 | 4.34 (4.34) | 8.25 (8.25) | 3.90 (3.90) | 2.39 (0.1432) | 6.63 (0.0211) | 6.89 (0.0191) | |
| A2 | 1 | 81.00 (81.00) | 0.00008601(0.00008601) | 1.73 (1.73) | 44.59 (<0.0001) | 0.00006909 (0.9935) | 3.05 (0.1011) | |
| B2 | 1 | 7.44 (7.44) | 0.089 (0.089) | 0.016 (0.016) | 4.10 (0.0611) | 0.072 (0.7928) | 0.028 (0.8698) | |
| C2 | 1 | 41.39 (41.39) | 0.015 (0.015) | 6.22 (6.22) | 22.79 (0.0002) | 0.012 (0.9154) | 10.97 (0.0047) | |
| D2 | 1 | 83.37 (83.37) | 24.20 (24.20) | 5.23 (5.23) | 45.90 (<0.0001) | 19.44 (0.0005) | 9.23 (0.0083) | |
| Residual | 15 | 27.25 (1.82) | 18.67 (1.24) | 8.50 (0.57) | | | | |
| Lack of Fit | 10 | 24.52 (2.45) | 15.85 (1.58) | 7.61 (0.76) | 4.49 (0.0556) | 2.80 (0.1333) | 4.28 (0.0610) | not significant |
| Pure Error | 5 | 2.73 (0.55) | 2.83 (0.57) | 0.89 (0.18) | | | | |
| Cor Total | 29 | 283.97 | 140.33 | 73.77 | | | | |
Table 4.
Response surface quadratic model analysis of variance (ANOVA) table for enzymatic transesterification of rice bran oil.
Table 4.
Response surface quadratic model analysis of variance (ANOVA) table for enzymatic transesterification of rice bran oil.
| Source | df | SS (MS) d | SS (MS) e | F Value (p-value) d | F Value (p-value) e | |
|---|
| Model | 14 | 2528.43 (180.60) | 8027.32 (573.38) | 28.52 (<0.0001) | 251.65 (<0.0001) | significant |
| A-Enzyme Concentration | 1 | 1123.30 (1123.30) | 130.59 (130.59) | 177.38 (<0.0001) | 57.31 (<0.0001) | |
| B-Reaction Time | 1 | 43.97 (43.97) | 111.41 (111.41) | 6.94 (0.0187) | 48.90(<0.0001)9 | |
| C-Reaction temperature | 1 | 10.35 (10.35) | 0.74 (0.74) | 1.63 (0.2205) | 0.33 (0.5764) | |
| D- Alcohol: Oil Molar Ratio | 1 | 30.92 (30.92) | 109.17 (109.17) | 4.88 (0.0431) | 47.91 (<0.0001) | |
| AB | 1 | 58.52 (58.52) | 89.30 (89.30) | 9.24 (0.0083) | 39.19 (<0.0001) | |
| AC | 1 | 44.22 (44.22) | 58.52 (58.52) | 6.98 (0.0185) | 25.68 (0.0001) | |
| AD | 1 | 12.96 (12.96) | 1.00 (1.00) | 2.05 (0.1731) | 0.44 (0.5177) | |
| BC | 1 | 1.69 (1.69) | 208.80 (208.80) | 0.27 (0.6130) | 91.64 (<0.0001) | |
| BD | 1 | 61.62 (61.62) | 19.36 (19.36) | 9.73 (0.0070) | 8.50 (0.0107) | |
| CD | 1 | 0.72 (0.72) | 30.25 (30.25) | 0.11 (0.7402) | 13.28 (0.0024) | |
| A2 | 1 | 167.44 (167.44) | 0.92 (0.92) | 26.44 (0.0001) | 0.40 (0.5356) | |
| B2 | 1 | 56.84 (56.84) | 40.78 (40.78) | 8.97 (0.0090) | 17.90 (0.0007) | |
| C2 | 1 | 9.82 (9.82) | 0.92 (0.92) | 1.55 (0.2322) | 0.40 (0.5356) | |
| D2 | 1 | 23.49 (23.49) | 78.03 (78.03) | 3.71 (0.0733) | 34.25 (<0.0001) | |
| Residual | 15 | 94.99 (6.33) | 34.18 (2.28) | | | |
| Lack of Fit | 10 | 85.43 (8.54) | 30.22 (3.02) | 4.47(0.0561) | 3.82 (0.0762) | not significant |
| Pure Error | 5 | 9.56 (1.91) | 3.96 (0.79) | | | |
| Cor Total | 29 | 2623.42 | 8061.49 | | | |
Table 5.
Response surface quadratic model analysis of variance (ANOVA) table for chemical transesterification of sunflower oil.
Table 5.
Response surface quadratic model analysis of variance (ANOVA) table for chemical transesterification of sunflower oil.
| Source | df | SS (MS)a | SS (MS)b | SS (MS)c | F Value (p-value) a | F Value (p-value) b | F Value (p-value) c | |
|---|
| Model | 14 | 272.51 (19.46) | 269.00 (19.21) | 1020.82 (72.92) | 4.94 (0.0020) | 4.88 (0.0021) | 41.72 (<0.0001) | significant |
| A-Catalyst Concentration | 1 | 27.95 (27.95) | 28.51 (28.51) | 776.00 (776.00) | 7.09 (0.0177) | 7.24 (0.0168) | 444.01 (<0.0001) | |
| B-Reaction Time | 1 | 25.38 (25.38) | 25.50 (25.50) | 10.08 (10.08) | 6.44 (0.0227) | 6.47 (0.0225) | 5.76 (0.0298) | |
| C-Reaction temperature | 1 | 6.28 (6.28) | 5.38 (5.38) | 70.56 (70.56) | 1.59 (0.2259) | 1.36 (0.2610) | 40.37(<0.0001) | |
| D- Alcohol : Oil Molar Ratio | 1 | 28.95 (28.95) | 23.60 (23.60) | 34.39 (34.39) | 7.35 (0.0161) | 5.99 (0.0272) | 19.68(0.0005) | |
| AB | 1 | 8.12 (8.12) | 8.50 (8.50) | 0.64 (0.64) | 2.06 (0.1716) | 2.16 (0.1627) | 0.37 (0.5529) | |
| AC | 1 | 3.53 (3.53) | 2.12 (2.12) | 10.61 (10.61) | 0.90 (0.3586) | 0.54 (0.4749) | 6.07 (0.0263) | |
| AD | 1 | 16.93 (16.93) | 15.41 (15.41) | 1.37 (1.37) | 4.30 (0.0558) | 3.91 (0.0667) | 0.79 (0.3891) | |
| BC | 1 | 0.44 (0.44) | 0.98 (0.98) | 0.17 (0.17) | 0.11 (0.7422) | 0.25 (0.6252) | 0.097(0.7593) | |
| BD | 1 | 6.71 (6.71) | 5.95 (5.95) | 0.15 (0.15) | 1.70 (0.2116) | 1.51 (0.2380) | 0.086 (0.7735) | |
| CD | 1 | 0.35 (0.35) | 0.029 (0.029) | 2.17 (2.17) | 0.088 (0.7704) | 0.007334 (0.9329) | 1.24 (0.2829) | |
| A2 | 1 | 66.11 (66.11) | 68.00 (68.00) | 84.33 (84.33) | 16.78 (0.0010) | 17.26 (0.0008) | 48.25(<0.0001) | |
| B2 | 1 | 35.33 (35.33) | 39.80 (39.80) | 12.67 (12.67) | 8.97 (0.0091) | 10.10 (0.0062) | 7.25 (0.0167) | |
| C2 | 1 | 84.96 (84.96) | 85.77 (85.77) | 2.98 (2.98) | 21.56 (0.0003) | 21.76 (0.0003) | 1.71 (0.2112) | |
| D2 | 1 | 11.63 (11.63) | 12.07 (12.07) | 10.81 (10.81) | 2.95 (0.1063) | 3.06 (0.1005) | 6.19 (0.0251) | |
| Residual | 15 | 59.10 (3.94) | 59.11 (3.94) | 26.22 (1.75) | | | | |
| Lack of Fit | 10 | 52.62 (5.26) | 52.17 (5.22) | 21.70 (2.17) | 4.06 (0.0677) | 3.76 (0.0785) | 2.40 (0.1729) | not significant |
| Pure Error | 5 | 6.48 (1.30) | 6.94 (1.39) | 4.52 (0.90) | | | | |
| Cor Total | 29 | 331.61 | 328.12 | 0.90 | | | | |
Table 6.
Response surface quadratic model analysis of variance (ANOVA)table for enzymatic transesterification of sunflower oil.
Table 6.
Response surface quadratic model analysis of variance (ANOVA)table for enzymatic transesterification of sunflower oil.
| Source | df | SS (MS)d | SS (MS)e | F Value (p-value) d | F Value (p-value) e | |
|---|
| Model | 14 | 2403.19 (171.66) | 2470.24 (176.45) | 22.83 (<0.0001) | 22.66 (<0.0001) | Significant |
| A-Enzyme Concentration | 1 | 1184.84 (1184.84) | 1070.56 (1070.56) | 157.55 (<0.0001) | 137.46 (<0.0001) | |
| B-Reaction Time | 1 | 27.10 (27.10) | 29.25 (29.25) | 3.60 (0.0771) | 3.76 (0.0717) | |
| C-Reaction temperature | 1 | 0.096 (0.096) | 2.79 (2.79) | 0.013 (0.9114) | 0.36 (0.5583) | |
| D- Alcohol:Oil Molar Ratio | 1 | 39.19 (39.19) | 62.53(62.53) | 5.21 (0.0374) | 8.03 (0.0126) | |
| AB | 1 | 48.30 (48.30) | 68.48 (68.48) | 6.42 (0.0229) | 8.79 (0.0096) | |
| AC | 1 | 73.96 (73.96) | 60.45 (60.45) | 9.83 (0.0068) | 7.76 (0.0138) | |
| AD | 1 | 30.25 (30.25) | 42.58 (42.58) | 4.02 (0.0633) | 5.47 (0.0336) | |
| BC | 1 | 8.41 (8.41) | 7.43 (7.43) | 1.12 (0.3070) | 0.95 (0.3443) | |
| BD | 1 | 64.00 (64.00) | 29.43 (29.43) | 8.51 (0.0106) | 3.78 (0.0709) | |
| CD | 1 | 4.20 (4.20) | 3.15 (3.15) | 0.56 (0.4663) | 0.40 (0.5343) | |
| A2 | 1 | 63.61 (63.61) | 24.76 (24.76) | 8.46 (0.0108) | 3.18 (0.0948) | |
| B2 | 1 | 83.87 (83.87) | 85.02 (85.02) | 11.15 (0.0045) | 10.92 (0.0048) | |
| C2 | 1 | 0.034 (0.034) | 3.83 (3.83) | 0.004466 (0.9476) | 0.49 (0.4938) | |
| D2 | 1 | 0.70 (0.70) | 0.43 (0.43) | 0.093 (0.7647) | 0.055 (0.8180) | |
| Residual | 15 | 112.81 (7.52) | 116.82 (7.79) | | | |
| Lack of Fit | 10 | 97.47 (9.75) | 93.84 (9.38) | 3.18 (0.1069) | 2.04 (0.2229) | not significant |
| Pure Error | 5 | 15.33 (3.07) | 22.97 (4.59) | | | |
| Cor Total | 29 | 2515.99 | 2587.05 | | | |
Figure 2.
(a) Optimized rice bran oil and (b) Optimized sunflower oil based biodiesel yields (%) for chemical and enzymatic transesterification.
Figure 2.
(a) Optimized rice bran oil and (b) Optimized sunflower oil based biodiesel yields (%) for chemical and enzymatic transesterification.
2.3. FTIR and HPLC Monitoring of Transesterification Reactions
Fourier Transform Infra Red (FTIR) spectroscopic analysis was performed for monitoring the progress of transesterification reactions of rice bran and sunflower oils. IR bands in the region 1425–1447 cm
−1 for CH
3 asymmetric bending and 1188–1200 for O-CH
3 stretching, in all biodiesel IR spectra, clearly demonstrated the transformation of vegetable oils into biodiesel, while these IR bands were absent in the IR spectra of both rice bran oil and sunflower oils, as seen in
Figure 3.
Figure 3.
A typical FTIR Spectrum of biodiesel.
Figure 3.
A typical FTIR Spectrum of biodiesel.
Similarly, IR bands in the region 1370–1400 cm
−1 for O-CH
2 groups in glycerol (moiety of triglycerides, diglycerides, and monoglycerides) were present in the IR spectra of rice bran and sunflower oils only, in accordance with the previous literature [
24,
25].
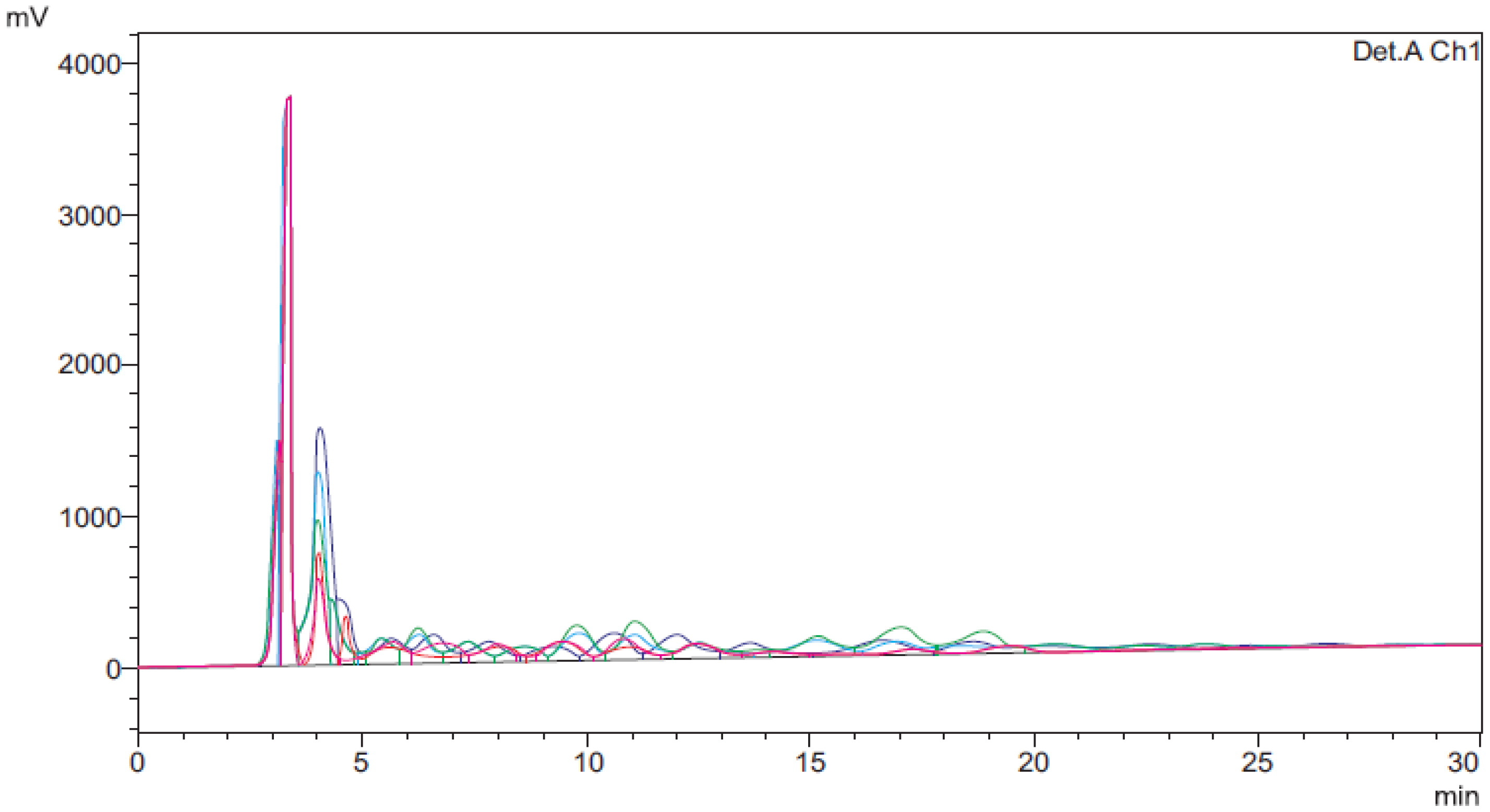
High Performance Liquid Chromatographic (HPLC) analysis further ascertained the transformation of vegetable oils to biodiesel as a result of transesterification (chemical and enzymatic). From the HPLC chromatograms (
Figure 4) taken after regular time interval during chemical and enzymatic transesterification reactions of the vegetable oils under study, it was revealed that there was a gradual shift of dominance from triglycerides to fatty acid methyl esters towards the end of transesterification reaction. The peak for fatty acid methyl esters was observed at reaction time 3–5 min. Chromatographic results were in accordance with the findings of Mumtaz
et al. [
26].
Figure 4.
A typical HPLC chromatogram showing comparative description during transesterification of oil for the production of biodiesel.
Figure 4.
A typical HPLC chromatogram showing comparative description during transesterification of oil for the production of biodiesel.
2.4. Compositional Analysis of Rice Bran Oil and Sunflower Oil Based Biodiesel
Major fatty acid methyl esters investigated in rice bran oil based biodiesel consisted of myristic acid methyl esters (C14:0), palmitic acid methyl esters (C16:0), stearic acid methyl esters (C18:0), oleic acid methyl esters (C18:1), linoleic acid methyl esters (C18:2) and linolenic acid methyl esters (C18:3) with composition 0.40%, 15.6%, 2.0%, 41.0%, 33.5% and 0.5%, respectively (
Table 8). Rashid
et al. [
22] reported fatty acid methyl esters profile of RBOFAMEs consisting of palmitic acid methyl esters (C16:0), stearic acid methyl esters (C18:0), oleic acid methyl esters (C18:1), linoleic acid methyl esters (C18:2) and linolenic acid methyl esters (C18:3) as major fatty acid methyl esters with composition
i.e., 18.8%, 1.40%, 43.1%, 32.2% and 1.8%, respectively. These results were comparable with current study for RBOFAME with oleic acid methyl esters content somewhat lesser and linoleic acid methyl esters (C18:2) somewhat higher comparative to the description of Rashid
et al. [
22]. Comparatively, palmitic acid methyl esters (C16:0), stearic acid methyl esters (C18:0), oleic acid methyl esters (C18:1), linoleic acid methyl esters (C18:2) and arachidic acid methyl esters (20:0) were depicted to be the major fatty acid methyl esters in SFOFAMEs with composition 6.80%, 5.10%, 23.5%, 64.0% and 0.16%, respectively (
Table 8). Rashid
et al. [
21] reported palmitic acid methyl esters (6.85%), stearic acid methyl ester (2.11%), oleic acid methyl ester (14.20%) and linoleic acid methyl ester (75.98%) as the major fatty acid methyl esters for sunflower oil based biodiesel. Palmitic acid methyl esters content as estimated in the current research work was seen to be comparable, stearic acid methyl ester; oleic acid methyl esters were somewhat higher, whereas linoleic acid methyl esters content was lesser than the investigation of Rashid
et al. [
21] for sunflower oil based biodiesel.
Table 8.
Major fatty acid methyl esters of different biodiesels.
Table 8.
Major fatty acid methyl esters of different biodiesels.
| Sr. No. | Fatty Acid Methyl Ester | Retention Times | RBOFAMEs | SFOFAMEs |
|---|
| 1 | Myristic Acid (C14:0) | 12.0920 | 0.40 ± 0.02 | - |
| 2 | Palmitic acid (C16:0) | 14.5991 | 15.6 ± 0.26 | 6.80 ± 0.15 |
| 3 | Stearic acid (C18:0) | 17.8101 | 2.00 ± 0.07 | 5.10 ± 0.09 |
| 4 | Oleic acid (C18:1) | 18.896 | 41.00 ± 1.04 | 23.50 ± 1.20 |
| 5 | Linoleic acid(C18:2) | 20.3148 | 33.50 ± 0.02 | 64.00 ± 1.38 |
| 6 | Linolenic acid(C18:3) | 22.0776 | 0.50 ± 0.05 | - |
| 7 | Arachidic acid (20:0) | 23.4130 | 0.20 ± 0.01 | 0.16 ± 0.01 |
2.5. Fuel Properties of Biodiesel
Fuel properties of synthesized biodiesel were estimated (
Table 9) and ascertained to be compatible with ASTM biodiesel standers (D6751a) and European biodiesel standers (EN-14214).
Density and Kinematic viscosity: Comparable density values (g/cm
3)
i.e., 0.880 ± 0.015 and 0.840 ± 0.015 g/cm
3 were obtained for both rice bran and sunflower oils, respectively. Engine efficiency is significantly linked with the fuel viscosity (fuel’s resistance to flow) and is also associated with fuel atomization. Viscosity is temperature dependant fuel property [
27] and acceptable ASTM (standard D 6751) defined range at 40 °C is 1.9–6.0 mm
2/s. The estimated kinematic viscosity ((mm
−2/s) 40 °C) for RBOFAMEs and SFOFAMEs were found to be 5.40 ± 0.34, 4.31 ± 0.23 and 4.68 ± 0.31 mm
−2/s, respectively as described in
Table 4, clearly indicating that kinematic viscosity ((mm
−2/s) 40 °C) values were within the ASTM (standard D 6751) limit for kinematic value.
Pour Point and Cloud Point: Pour point represents the minimum temperature where fuel still has ability to move before its solidification (gel formation). The estimated pour point (°C) for RBOFAMEs and SFOFAMEs were depicted to be −2.17 ± 0.46 and −3.74 ± 0.41 °C, respectively whereas, estimated cloud point values for RBOFAME and SFOFAME were revealed to be 6.5 ± 0.2 and 4.6 ± 0.5 °C, respectively (
Table 4). Cloud point defines the temperature where saturates solidify and crystal formation causes cloudy appearance of liquid fatty material. High cloud point usually results in fuel line clogging [
28].
Table 9.
Fuel properties of rice bran and sunflower oil based biodiesel.
Table 9.
Fuel properties of rice bran and sunflower oil based biodiesel.
| Sr. No. | Fuel Property | RBOFAME | SFOFAME | ASTM D6751 | EN14214 |
|---|
| 1 | Density (g/cm3) | 0.88 ± 0.01 | 0.84 ± 0.01 | - | - |
| 2 | Kinematic viscosity (mm−2/s) 40 °C | 5.40 ± 0.34 | 4.68 ± 0.31 | 1.9–6.0 | 3.5–5.0 |
| 3 | Pour Point (°C) | −2.17 ± 0.46 | −3.74 ± 0.41 | - | - |
| 4 | Cloud point (°C) | 6.53 ± 0.23 | 4.57 ± 0.46 | - | - |
| 5 | Flash point (°C) | 177.66 ± 3.01 | 180.1 ± 1.7 | 93 min | 120 min |
| 6 | Fire point (°C) | 186.3 ± 1.5 | 185.3 ± 2.7 | - | - |
| 7 | Ash content (%) | 0.009 ± 0.004 | 0.015 ± 0.006 | 0.020 max | 0.020 max |
| 8 | Cetane Number | 61.39 ± 2.6 | 50.54 ± 1.48 | 47 min | 51 min |
| 9 | Higher Heating value (MJ/kg) | 40.79 ± 1.43 | 43.90 ± 1.42 | - | - |
| 10 | Oxidative stability (h) | 1.92 ± 0.07 | 2.00 ± 0.11 | 03 min | 06 min |
Flash Point and Fire Point: Fuel’s tendency regarding the formation of flammable mixtures when exposed to air is usually described by the flash point and is considered as an essential fuel property to express the hazards associated with fuel flammability because of the presence of extremely flammable and volatile constituents. Higher flash point usually eliminates the risk of fire. The flash point (°C) values for ESOFAMEs and SFOFAMEs were depicted to be 177.7 ± 3.0 and 180.1 ± 1.8 °C, respectively, as given in
Table 4. The flash point values were in good agreement with the prescribed flash point limits in ASTM D6751
i.e., 93 °C minimum for both rice bran oil and sunflower oil based biodiesel and also with EN 14214
i.e., 120 °C minimum. On the other hand, fire point represents temperature at which a fuel caught fire. The investigated fire point values (°C) for RBOFAMEs and SFOFAMEs were found to be 186.3 ± 1.6 and 185.3 ± 2.7 °C, respectively (
Table 4).
Cetane number and higher heating value: Fuel ignition ability is directly related to the cetane number of fuel. Cetane number is considered as main indicator for ignition quality of diesel engines. Cetane number is inversely linked with the ignition delay time and also related with the chain length and branching, usually, higher cetane number is associated with longer chain length with least branching and vice versa. Fatty acids are recognized with higher cetane number values. The investigated cetane number RBOFAMEs and SFOFAMEs were revealed to be 61.39 ± 2.69 and 50.54 ± 1.49, respectively which were depicted to be within the prescribed limit for cetane number as described in ASTM D6751. Higher heating value of a fuel describes the energy produced during its complete burning and is considered as valuable fuel property that determines the compatibility of biodiesel as alternative to conventional fossil fuel. In current study the higher heating values for RBOFAMEs and SFOFAMEs were depicted to be 40.79 ± 1.43, and 43.90 ± 1.42 MJ/kg, respectively as shown in
Table 4.
Oxidative stability and Ash Content: Oxidative stability values
i.e., 1.92 ± 0.07 and 2.00 ± 0.11 h for RBOFAMEs and SFOFAMEs, respectively. The prescribed limits specified in ASTM D6751 and EN 14214 are >3 and 6 h, respectively. Ash content represents the level of inorganic contaminants present in fuel. The ash contents were 0.009% ± 0.004% and 0.015% ± 0.006% for RBOFAMEs and SFOFAMEs, respectively (
Table 4). ASTM D6751 and EN 14214 standard limits for ash content of biodiesel is 0.02 maximum, the present ash content values were depicted to be within the prescribed limits of ASTM and EN.
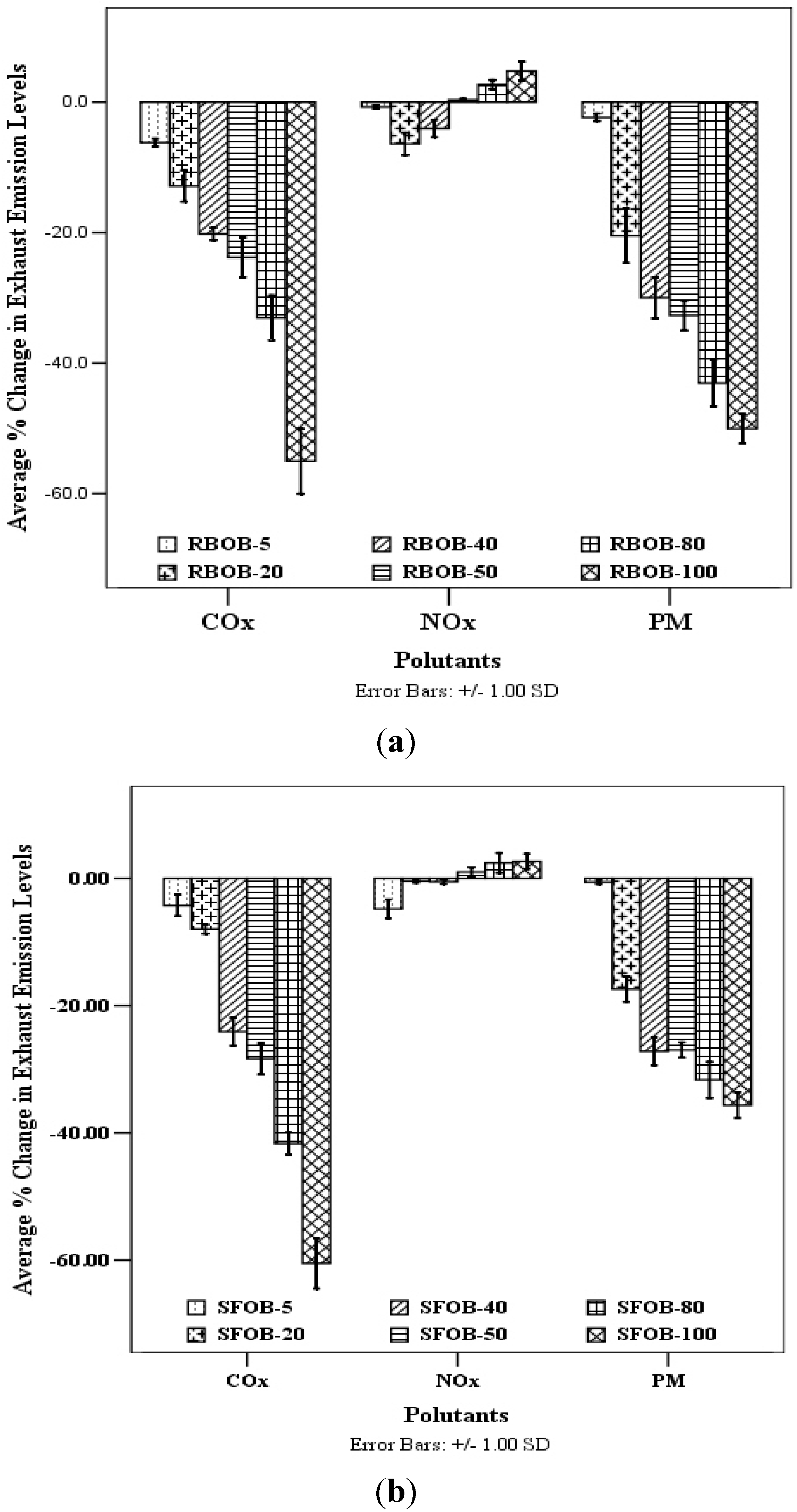
Exhaust Emission Profile of Rice bran and Sunflower oil based Biodiesel: The results showed an apparent % reduction in both CO and particulate matter (PM) emissions from engine exhaust operated on rice bran oil based biodiesel and its blends comparative to engine exhaust emissions based on petro diesel (
Figure 5). On the average basis, % change in CO emission levels from engine exhaust operated on RBOB-5, RBOB-20, RBOB-40, RBOB-50, RBOB-80 and RBOB-100 were found to be −6.2 ± 0.6, −2.9 ± 2.4, −20.2 ± 0.9, −23.8 ± 3.0,−33.1 ± 3.4 and −55.1 ± 5.0%, respectively, whereas, % change in PM emissions were revealed to be −2.3± 0.6, −20.5 ± 4.2, −30.0 ± 3.2, −32.7 ± 2.3, −43.1 ± 3.6 and −50.1 ± 2.2%, respectively comparative to conventional petro diesel. On the other hand, NO
x emissions showed an irregular trend, NO
x emissions from engine exhaust operated on RBOB-50, RBOB-80 and RBOB-100 were found to be higher than engine exhaust emissions operated on conventional petro diesel with % change in NO
x emissions
i.e., 0.33 ± 0.21, 2.7 ± 0.7 and 4.8 ± 1.4%, respectively, whereas, NO
x emissions from engine exhaust operated on RBOB-5, RBOB-20, RBOB-40 were found to be lesser than conventional petro diesel with % change −0.73 ± 0.25, −6.4 ± 1.7 and −4.03 ± 1.33%, respectively comparative to petro diesel. On the other hand, in case of sunflower oil based biodiesel, % change in CO emission levels was found to be −4.27 ± 1.7, −7.9 ± 0.7, −24.1 ± 2.2, −28.3 ± 2.5, −41.7 ± 1.7 and −60.5 ± 3.9%, respectively using SFOB-5, SFOB-20, SFOB-40, SFOB-50, SFOB-80 and SFOB-100, whereas, % change in PM emissions was found to be −0.6 ± 0.3, −17.4 ± 1.9, −27.2 ± 2.2, −27.0 ± 1.2, −31.7 ± 2.8 and −35.6 ± 2.0%, respectively, compared to conventional diesel fuel. Just like rice bran oil based biodiesel, an irregular trend in NO
x emissions was observed. NO
x emissions from engine exhaust operated on SFOB-50, SFOB-80 and SFOB-100 were found to be higher than engine exhaust emissions operated on conventional petro diesel with % change 1.0 ± 0.7, 2.4 ± 1.5 and 2.7 ± 1.1%, respectively, whereas for SFOB-5, SFOB-20, and SFOB-40, NO
x levels were found to be lesser than conventional petro diesel with % change −4.8 ± 1.5, −0.43 ± 0.25 and −0.5 ± 0.3%, respectively (
Figure 5).
The variations in biodiesel production process along with other physico-chemical, fuel characteristics and exhaust emissions may be because of certain factors including location based variable biodiesel source, fatty acid profile of feedstock (oil) used for the production of biodiesel, contaminants arising during biodiesel production process or from other sources,
etc. Among these the influence of fatty acid profile of various oils is more pronounced towards biodiesel characteristics. Both moieties,
i.e., fatty acid chain along with alcohol functionality, may be significant contributors to the overall characteristics of fatty esters. Structural features including degree of unsaturation, chain length and branching of the fatty acid chain may also affect biodiesel characteristics as per descriptions of Knothe and Steidley [
29].
Figure 5.
% change in exhaust emissions (CO, NOx and PM) from engine exhaust operated on (a) rice bran and (b) sunflower oils based biodiesel blends comparative to engine exhaust operated on conventional petro diesel.
Figure 5.
% change in exhaust emissions (CO, NOx and PM) from engine exhaust operated on (a) rice bran and (b) sunflower oils based biodiesel blends comparative to engine exhaust operated on conventional petro diesel.