Efficiency of Photosynthetic Microbial Fuel Cells (pMFC) Depending on the Type of Microorganisms Inhabiting the Cathode Chamber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organization of the Experiments
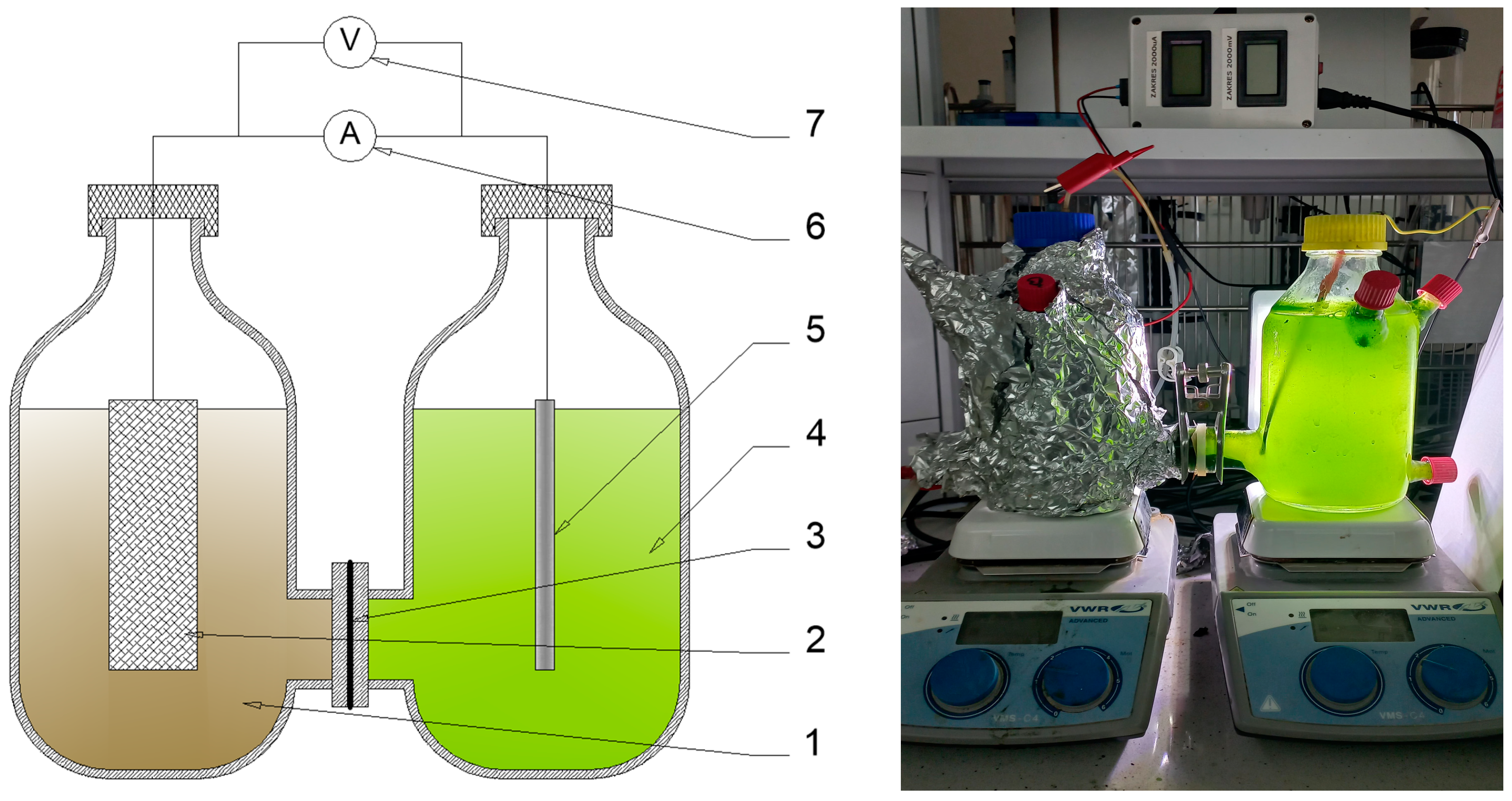
2.2. MFC Configuration
2.3. Start-Up and Condition of the Anode Chamber
2.4. pMFCs1 with Arthrospira Platensis
2.5. pMFCs2 with Chlorella Vulgaris
2.6. Analytical Methods
2.7. Statistical Analyses
3. Results and Discussion
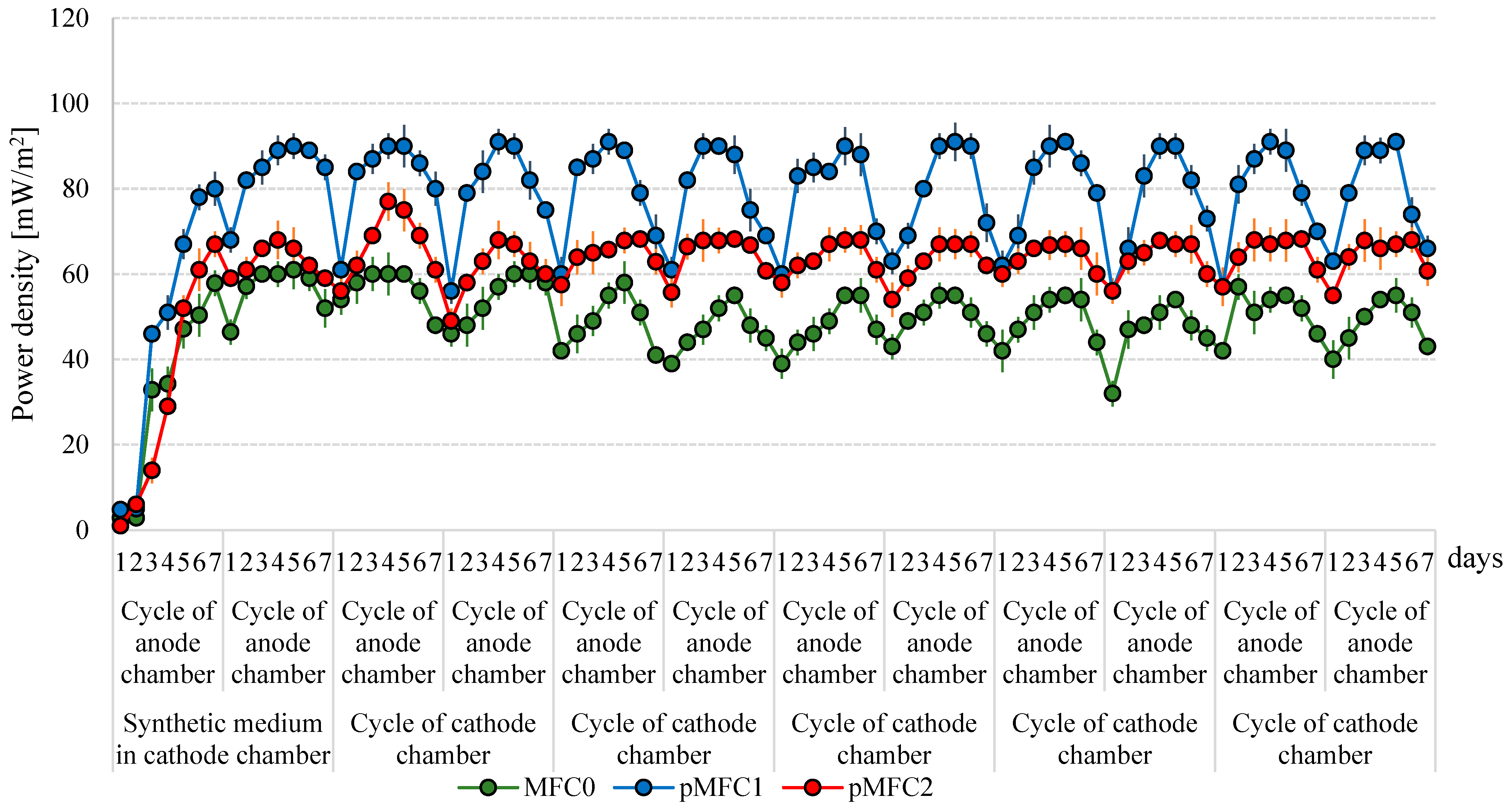
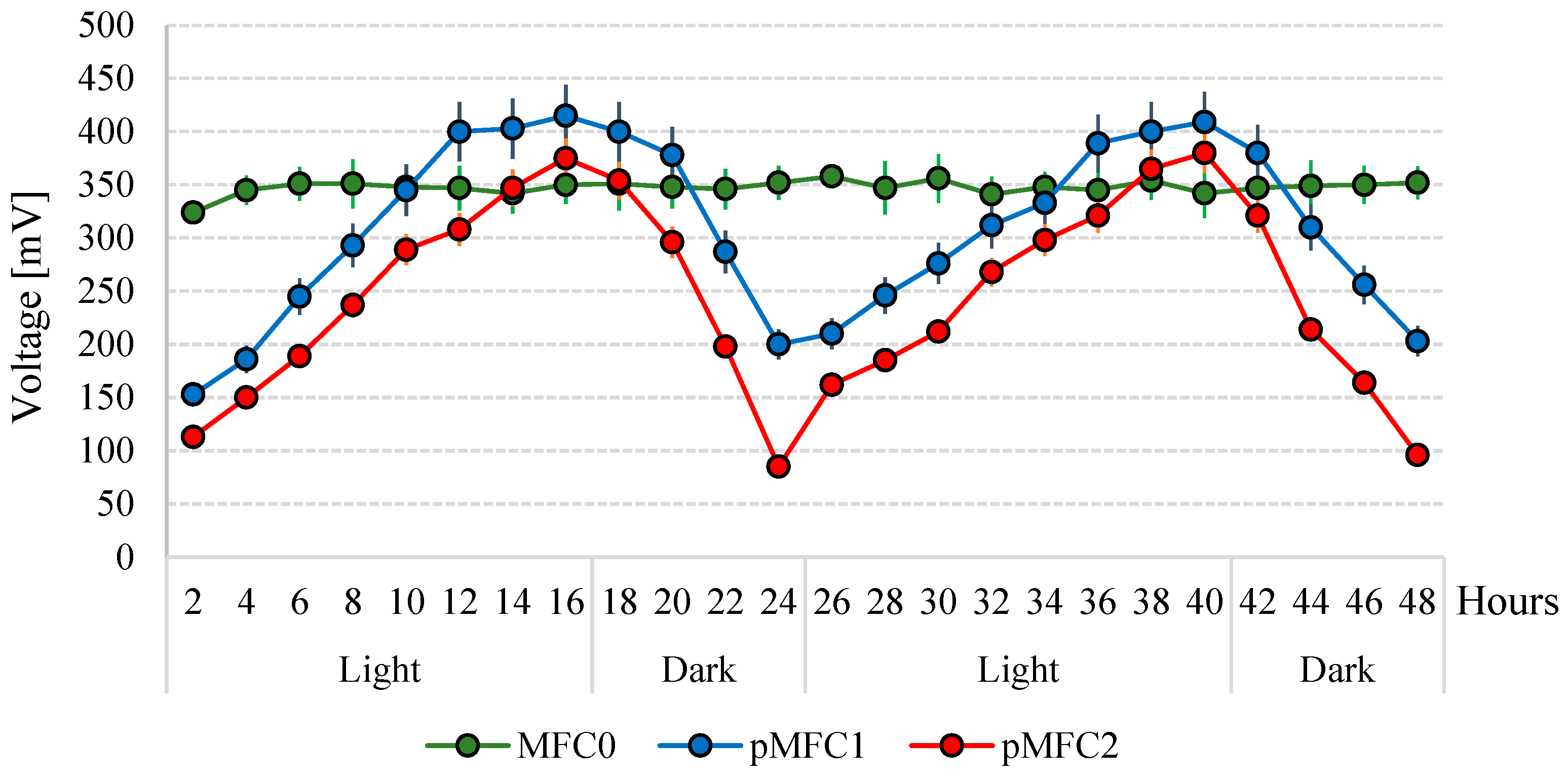
3.1. Power Generation
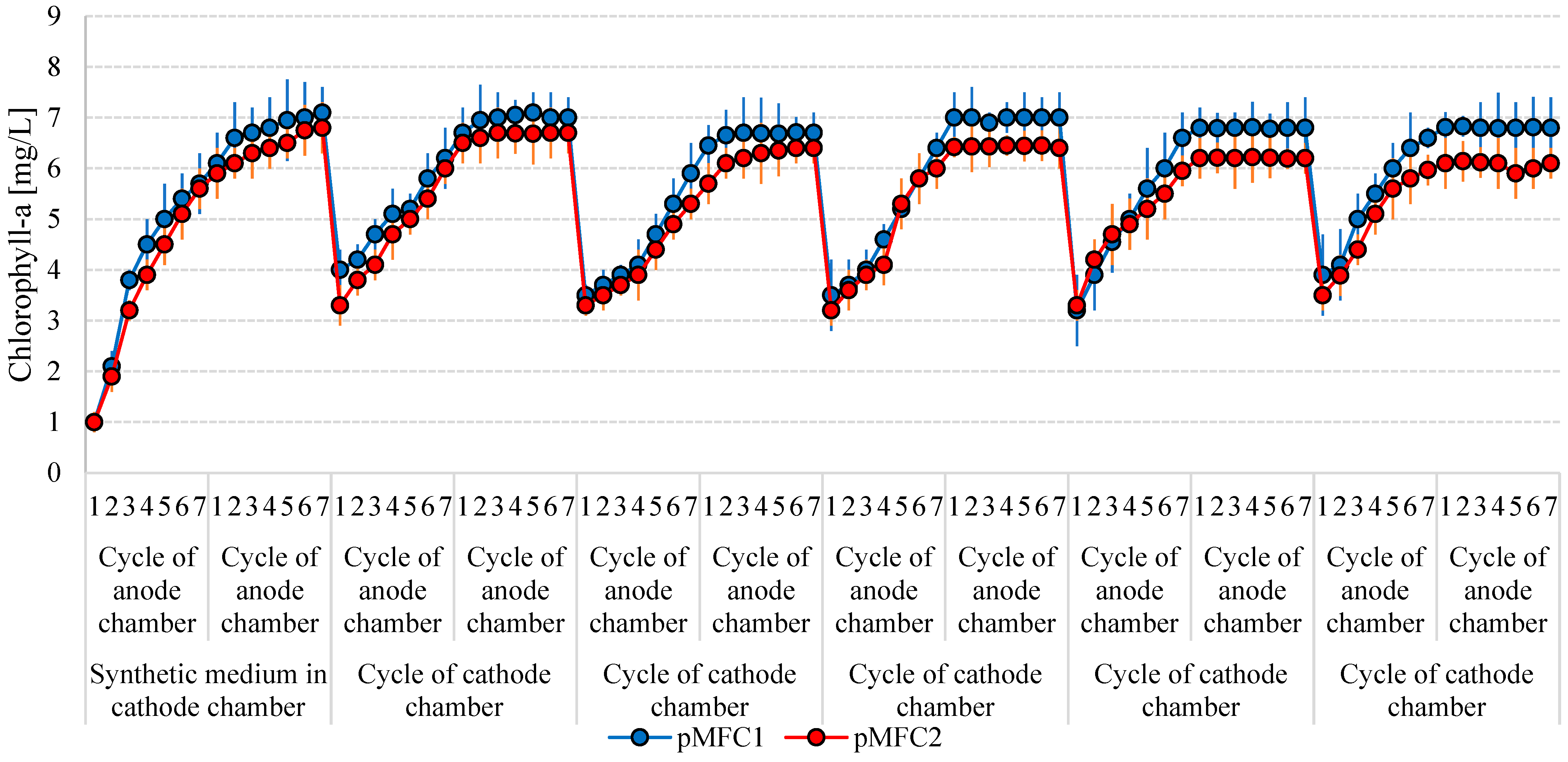
3.2. Microalgae Growth
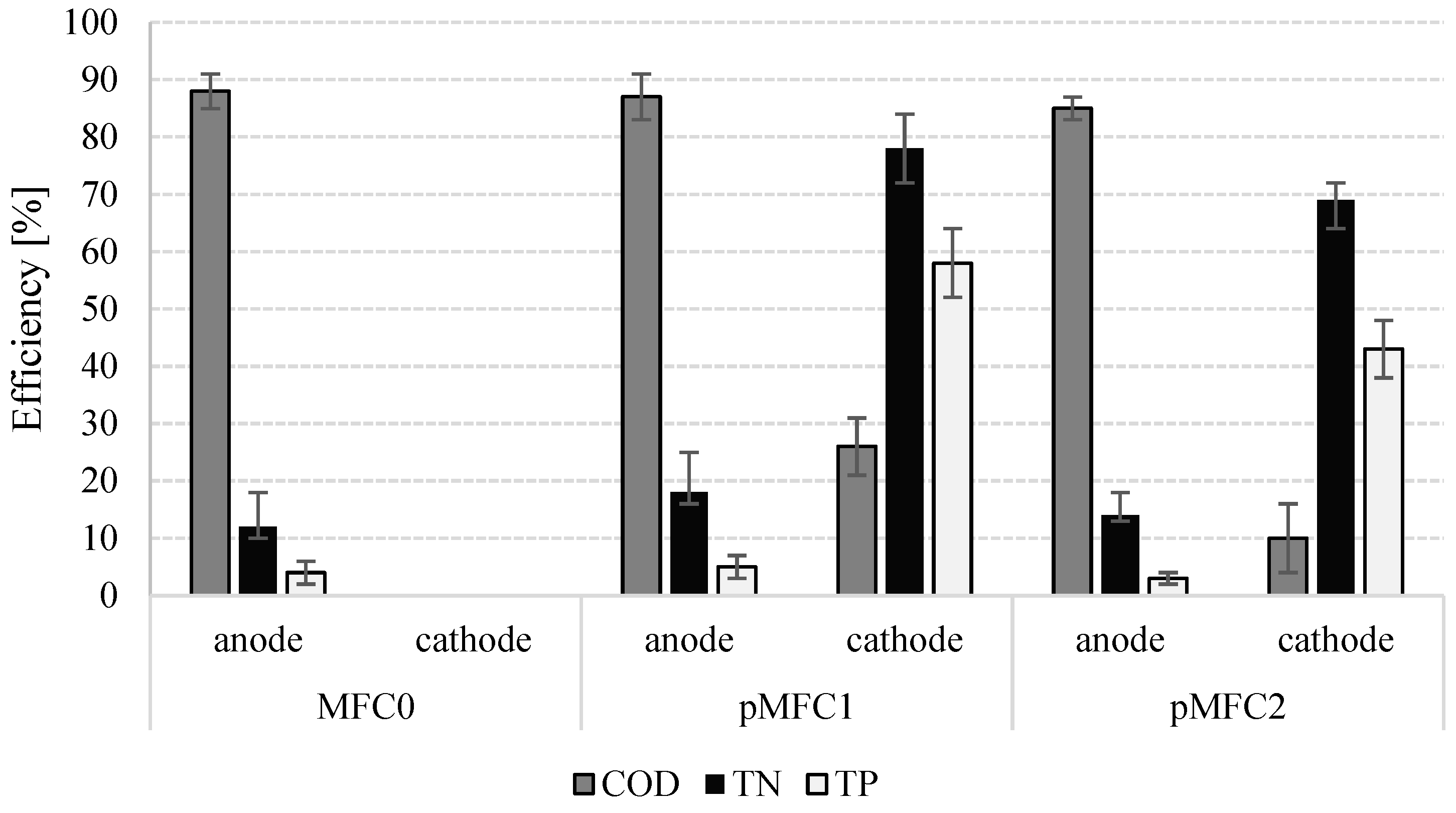
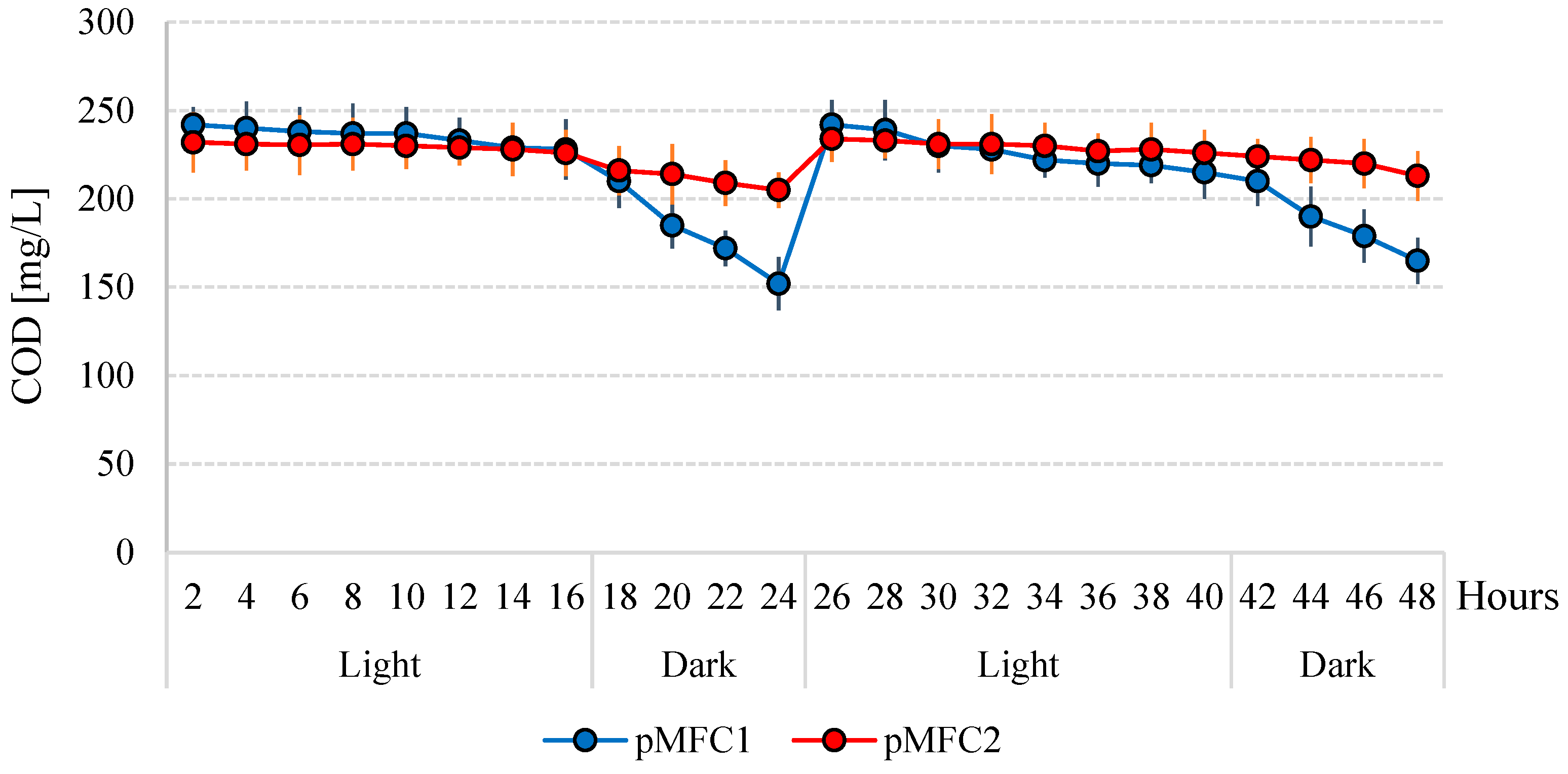
3.3. The Removal of Nutrients and Organics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Logan, B.E. Microbial Fuel Cells, 1st ed.; Wiley-Interscience: Hoboken, NJ, USA, 2008. [Google Scholar]
- Zhao, F.; Harnisch, F.; Schröder, U.; Scholz, F.; Bogdanoff, P. Challenges and constraints of using oxygen cathodes in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 5193–5199. [Google Scholar] [CrossRef]
- Mathuriya, A.S.; Yakhmi, J.V. Microbial fuel cells to recover heavy metals. Environ. Chem. Lett. 2014, 12, 483–494. [Google Scholar] [CrossRef]
- Taşkan, B.; Bakır, M.; Taşkan, E. Enhanced power generation from algal biomass using multi-anode membrane-less sediment microbial fuel cell. Int. J. Hydrog. Energ. 2021, 45, 2011–2022. [Google Scholar] [CrossRef]
- Yang, Z.; Pei, H.; Hou, Q.; Jiang, L.; Zhang, L.; Nie, C. Algal biofilm-assisted microbial fuel cell to enhance domestic wastewater treatment: Nutrient, organics removal and bioenergy production. Chem. Eng. J. 2017, 332, 277–285. [Google Scholar] [CrossRef]
- Shi, X.M.; Liu, H.J.; Zhang, X.W.; Chen, F. Production of biomass and lutein by Chlorella protothecoides at various glucose concentrations in heterotrophic cultures. Process. Biochem. 1999, 34, 341–347. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, C.Y.; Liao, Q.; Zhu, X.; Chang, J.S. Photoheterotrophic growth of Chlorella vulgaris ESP6 on organic acids from dark hydrogen fermentation effluents. Bioresour. Technol. 2013, 145, 331–336. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Kao, C.-J.; Chen, C.-H.; Kuan, T.-C.; Ong, S.-C.; Lin, C.-S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef]
- Rosenbaum, M.; He, Z.; Angenent, L.T. Light energy to bioelectricity: Photosynthetic microbial fuel cells. Curr. Opin. Biotechnol. 2010, 21, 259–264. [Google Scholar] [CrossRef]
- Aouir, A.; Amiali, M.; Bitam, A.; Benchabane, A.; Raghavan, V.G. Comparison of the biochemical composition of different Arthrospira platensis strains from Algeria, Chad and the USA. J. Food Meas. Charact. 2017, 11, 913–923. [Google Scholar] [CrossRef]
- Logan, B.; Wallack, M.; Kim, K.-Y.; He, W.; Feng, Y.; Saikaly, P. Assessment of Microbial Fuel Cell Configurations and Power Densities. Environ. Sci. Technol. Let. 2015, 2, 150803121308002. [Google Scholar] [CrossRef]
- Bazdar, E.; Roshandel, R.; Yaghmaei, S.; Mardanpour, M.M. The effect of different light intensities and light/dark regimes on the performance of photosynthetic microalgae microbial fuel cell. Bioresour Technol. 2018, 261, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Nagendranatha Reddy, C.; Nguyen, H.T.H.; Noori, M.T.; Min, B. Potential applications of algae in the cathode of microbial fuel cells for enhanced electricity generation with simultaneous nutrient removal and algae biorefinery: Current status and future perspectives. Bioresour. Technol. 2019, 292, 122010. [Google Scholar] [CrossRef]
- Colombo, A.; Marzorati, S.; Lucchini, G.; Cristiani, P.; Pant, D.; Schievano, A. Assisting cultivation of photosynthetic microorganisms by microbial fuel cells to enhance nutrients recovery from wastewater. Bioresour. Technol. 2017, 237, 240–248. [Google Scholar] [CrossRef]
- Lu, N.; Zhou, S.G.; Zhuang, L.; Zhang, J.T.; Ni, J.R. Electricity generation from starch processing wastewater using microbial. Biochem. Eng. J. 2009, 43, 246–251. [Google Scholar] [CrossRef]
- Rahman, A.; Borhan, M.S.; Shafiqur, R. Evaluation of microbial fuel cell (MFC) for bioelectricity generation and pollutants removal from sugar beet processing wastewater (SBPW). Water Sci. Technol. 2017, 77, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Zhou, M. A photosynthetic algal microbial fuel cell for treating swine wastewater. Environ. Sci. Poll. Res. 2019, 26, 6182–6190. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Ramesh, K.; Booki, M. Algae cathode microbial fuel cells for electricity generation and nutrient removal from landfill leachate wastewater. Int. J. Hydro Energy 2017, 42, 29433–29442. [Google Scholar] [CrossRef]
- Hou, Q.; Cheng, J.; Nie, C.; Pei, H.; Jiang, L.; Zhang, L.; Yang, Z. Features of Golenkinia sp. and microbial fuel cells used for the treatment of anaerobically digested effluent from kitchen waste at different dilutions. Bioresour. Technol. 2017, 240, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Britz, J.T.; van Schalwyk, C.; Hung, Y.T. Treatment of dairy processing wastewaters. In Waste Treatment in the Food Processing Industry; Wang, L.K., Hung, Y.T., Lo, H.H., Yapijakis, C., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–25. [Google Scholar]
- Karadag, D.; Köroğlu, O.E.; Ozkaya, B.; Cakmakci, M. A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process. Biochem. 2015, 50, 262–271. [Google Scholar] [CrossRef]
- Janczukowicz, W.; Zieliński, M.; Dębowski, M. Biodegradability evaluation of dairy effluents originated in selected sections of dairy production. Bioresour. Technol. 2008, 99, 4199–4205. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigun, O.; Onay, T.T. Anaerobic treatment of dairy wastewaters: A review. Process. Biochem. 2005, 40, 2583–2595. [Google Scholar] [CrossRef]
- Xia, A.; Murphy, J.D. Microalgal Cultivation in Treating Liquid Digestate from Biogas Systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef]
- Mtaki, K.; Kyewalyanga, M.S.; Mtolera, M.S.P. Supplementing wastewater with NPK fertilizer as a cheap source of nutrients in cultivating live food (Chlorella vulgaris). Annals. Microbiol. 2021, 71, 7. [Google Scholar] [CrossRef]
- Hamidian, N.; Zamani, H. Biomass production and nutritional properties of Chlorella sorokiniana grown on dairy wastewater. J. Water Process Eng. 2022, 47, 102760. [Google Scholar] [CrossRef]
- Don, C.D.Y.Y.A.; Babel, S. Circulation of anodic effluent to the cathode chamber for subsequent treatment of wastewater in photosynthetic microbial fuel cell with generation of bioelectricity and algal biomass. Chemosphere 2021, 278, 130455. [Google Scholar]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, Z.H.; Li, C.; Zeng, G.M.; Ma, D.H.; Zhou, L. A novel algal biofilm membrane photobioreactor for attached microalgae growth and nutrients removal from secondary effluent. Bioresour. Technol. 2015, 179, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Pires, J.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2016, 24, 403–415. [Google Scholar] [CrossRef]
- Hena, S.; Fatihah, N.; Tabassum, S.; Ismail, N. Three stage cultivation process of facultative strain of Chlorella sorokiniana for treating dairy farm effluent and lipid enhancement. Water Res. 2015, 80, 346–356. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.; Doulia, D.; Eleni, A. Adsorption of pesticides on porous polymeric adsorbents. Chem. Eng. Sci. 2005, 60, 1177–1186. [Google Scholar] [CrossRef]
- Aiba, S.; Ogawa, T. Assessment of growth yield of a blue-green alga: Spirulina platensis in axenic and continuous culture. J. Gener. Microbiol. 1977, 102, 179–182. [Google Scholar] [CrossRef]
- Guo, Z.; Phooi, W.B.A.; Lim, Z.J.; Tong, Y.W. Control of CO2 input conditions during outdoor culture of Chlorella vulgaris in bubble column photobioreactors. Bioresour. Technol. 2015, 186, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.; Prasad Uday, U.S.; Bandyopadhyay, T.K.; Ray, R.N.; Bhunia, B. Performance improvement of microbial fuel cell (MFC) using suitable electrode and bioengineered organisms: A review. Bioengineered 2017, 8, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, H.; Han, J.; Yang, X.; Qiao, Z.; Yin, T. Performance improvement of microbial fuel cells through assembling anodes modified with nanoscale materials. Nanomater. Nanotechnol. 2022, 12. [Google Scholar] [CrossRef]
- Commault, A.S.; Laczka, O.; Siboni, N.; Tamburic, B.; Crosswell, J.R.; Seymour, J.R.; Ralph, P.J. Electricity and biomass production in a bacteria-Chlorella based microbial fuel cell treating wastewater. J. Power Sour. 2017, 356, 299–309. [Google Scholar] [CrossRef]
- Don, C.D.Y.Y.A.; Babel, S. Effects of organic loading on bioelectricity and micro-algal biomass production in microbial fuel cells using synthetic wastewater. J. Water Process Eng. 2020, 39, 101699. [Google Scholar] [CrossRef]
- Rago, L.; Cristiani, P.; Villa, F.; Zecchin, S.; Colombo, A.; Cavalca, L.; Schievano, A. Influences of dissolved oxygen concentration on biocathodic microbial communities in microbial fuel cells. Bioelectrochemistry 2017, 116, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Bayona-Morcillo, P.J.; Plaza, B.M.; Gómez-Serrano, C.; Rojas, E.; Jiménez-Becker, S. Effect of the Foliar Application of Cyanobacterial Hydrolysate (Arthrospira platensis) on the Growth of Petunia x Hybrida under Salinity Conditions. J. Appl. Phycol. 2020, 32, 4003–4011. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; El-Ashram, S.; Yilmaz, S.; Naiel, M.A.E.; Abdul Kari, Z.; Hamid, N.K.A.; Dawood, M.A.O.; Nowosad, J.; Kucharczyk, D. The Effectiveness of Arthrospira platensis and Microalgae in Relieving Stressful Conditions Affecting Finfish and Shellfish Species: An Overview. Aquac. Rep. 2022, 24, 101135. [Google Scholar] [CrossRef]
- Celekli, A.; Yavuzatmaca, M.; Bozkurt, H. Modeling of biomass production by Spirulina platensis as function of phosphate concentrations and pH regimes. Bioresour. Technol. 2009, 100, 3625–3629. [Google Scholar] [CrossRef] [PubMed]
- Celekli, A.; Yavuzatmaca, M. Predictive modeling of biomass production by Spirulina platensis as function of nitrate and NaCl concentrations. Bioresour. Technol. 2009, 100, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Kougia, E.; Arapoglou, D.; Chentir, I.; Andreou, V.; Tzovenis, I. Production of Arthrospira platensis: Effects on Growth and Biochemical Composition of Long-Term Acclimatization at Different Salinities. Bioengineering 2023, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.I.; Murata, N. Salt stress inhibits photosystems II and I in cyanobacteria. Photosynth Res. 2008, 98, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, S.; Nagarajan, P. Effect of salinity on biomass and biochemical constituents of Spirulina platensis (Geitler). Intern J. Plant Protect. 2014, 7, 71–73. [Google Scholar]
- Dębowski, M.; Zieliński, M.; Vdovychenko, A.; Kazimierowicz, J. The Use of the Autotrophic Culture of Arthrospira platensis for CO2 Fixation from Biogas Combustion. Processes 2024, 12, 396. [Google Scholar] [CrossRef]
- Dudek, M.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M.; Quattrocelli, P.; Nowicka, A. The Cultivation of Biohydrogen-Producing Tetraselmis subcordiformis Microalgae as the Third Stage of Dairy Wastewater Aerobic Treatment System. Sustainability 2022, 14, 12085. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Sirohi, R.; Udayan, A.; Yadav, P.; Raj, A.; Sim, S.J.; Pandey, A. Sustainable Microalgal Biomass Production in Food Industry Wastewater for Low-Cost Biorefinery Products: A Review. Phytochem. Rev. 2022, 3, 969–991. [Google Scholar] [CrossRef] [PubMed]
- Kisielewska, M.; Zieliński, M.; Dębowski, M.; Kazimierowicz, J.; Romanowska-Duda, Z.; Dudek, M. Effectiveness of Scenedesmus sp. Biomass Grow and Nutrients Removal from Liquid Phase of Digestates. Energies 2020, 13, 1432. [Google Scholar] [CrossRef]
- Abazarian, E.; Gheshlaghi, R.; Mahdavi, A.M. Impact of light/dark cycle on electrical and electrochemical characteristics of algal cathode sediment microbial fuel cells. J. Power Sour. 2020, 475, 228686. [Google Scholar] [CrossRef]
- Ullah, Z.; Sheikh, Z.; Zaman, W.Q.; Zeeshan, M.; Miran, W.; Li, J.; Khan, M.A.N.; Saleem, S.; Shabbir, S. Performance comparison of a photosynthetic and mechanically aerated microbial fuel cell for wastewater treatment and bioenergy generation using different anolytes. J. Water Proc. Eng. 2023, 56, 104358. [Google Scholar] [CrossRef]
- Nio Song, A.; Banyo, Y. Leaf chlorophyll concentration as an indicator of water deficiency in plants. Sci. J. Sci. 2011, 15, 166. [Google Scholar] [CrossRef]
- Zieliński, M.; Kazimierowicz, J.; Dębowski, M. The Possibility of Deploying CO2 from Biogas Combustion to Improve the Productivity of a Periodical Chlorella vulgaris Culture. Front Biosci. Elit. 2023, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Don, C.D.Y.Y.A.; Babel, S. Effects of ammonium concentration in the catholyte on electricity and algal biomass generation in a photosynthetic microbial fuel cell treating wastewater. Bioresour. Technol. Rep. 2021, 16, 100867. [Google Scholar] [CrossRef]
- Jones, H.R. Pollution Control in the Dairy Industry; Noyes Data Corporation: Park Ridge, NJ, USA, 1974. [Google Scholar]
- Jalal, K.C.A.; Alam Md, A.M.; Kamaruzzaman, Y.; Akbar, J.; Hossain, T. Removal of Nitrate and Phosphate from Municipal Wastewater Sludge by Chlorella vulgaris, Spirulina platensis and Scenedesmus quadricauda. IIUM Eng. J. 1970, 12, 125–132. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Liu, J.; Lee, H.; Li, C.; Li, N.; Ren, N. Sequestration of CO2 discharged from anode by algal cathode in microbial carbon capture cells (MCCs). Biosens. Bioelectron. 2010, 25, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, O.; Escalante, F.M.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.; Mohammad, A.; Kalbasi, M.; Mousavi, S.; Ghobadian, B. Investigation of Mixotrophic, Heterotrophic and Autotrophic Growth of Chlorella vulgaris Under Agricultural Waste Medium. Prep. Biochem. Biotechnol. 2015, 46, 150–156. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Z.; Su, X.; Zhao, P.; Zhou, J.; He, Q.; Ai, H. Cost-effective domestic wastewater treatment and bioenergy recovery in an immobilized microalgal-based photoautotrophic microbial fuel cell (PMFC). Chem. Eng. J. 2019, 372, 956–965. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieliński, M.; Rusanowska, P.; Dudek, M.; Starowicz, A.; Barczak, Ł.; Dębowski, M. Efficiency of Photosynthetic Microbial Fuel Cells (pMFC) Depending on the Type of Microorganisms Inhabiting the Cathode Chamber. Energies 2024, 17, 2296. https://doi.org/10.3390/en17102296
Zieliński M, Rusanowska P, Dudek M, Starowicz A, Barczak Ł, Dębowski M. Efficiency of Photosynthetic Microbial Fuel Cells (pMFC) Depending on the Type of Microorganisms Inhabiting the Cathode Chamber. Energies. 2024; 17(10):2296. https://doi.org/10.3390/en17102296
Chicago/Turabian StyleZieliński, Marcin, Paulina Rusanowska, Magda Dudek, Adam Starowicz, Łukasz Barczak, and Marcin Dębowski. 2024. "Efficiency of Photosynthetic Microbial Fuel Cells (pMFC) Depending on the Type of Microorganisms Inhabiting the Cathode Chamber" Energies 17, no. 10: 2296. https://doi.org/10.3390/en17102296





