MnO/Mn2O3 Aerogels as Effective Materials for Supercapacitor Applications
Abstract
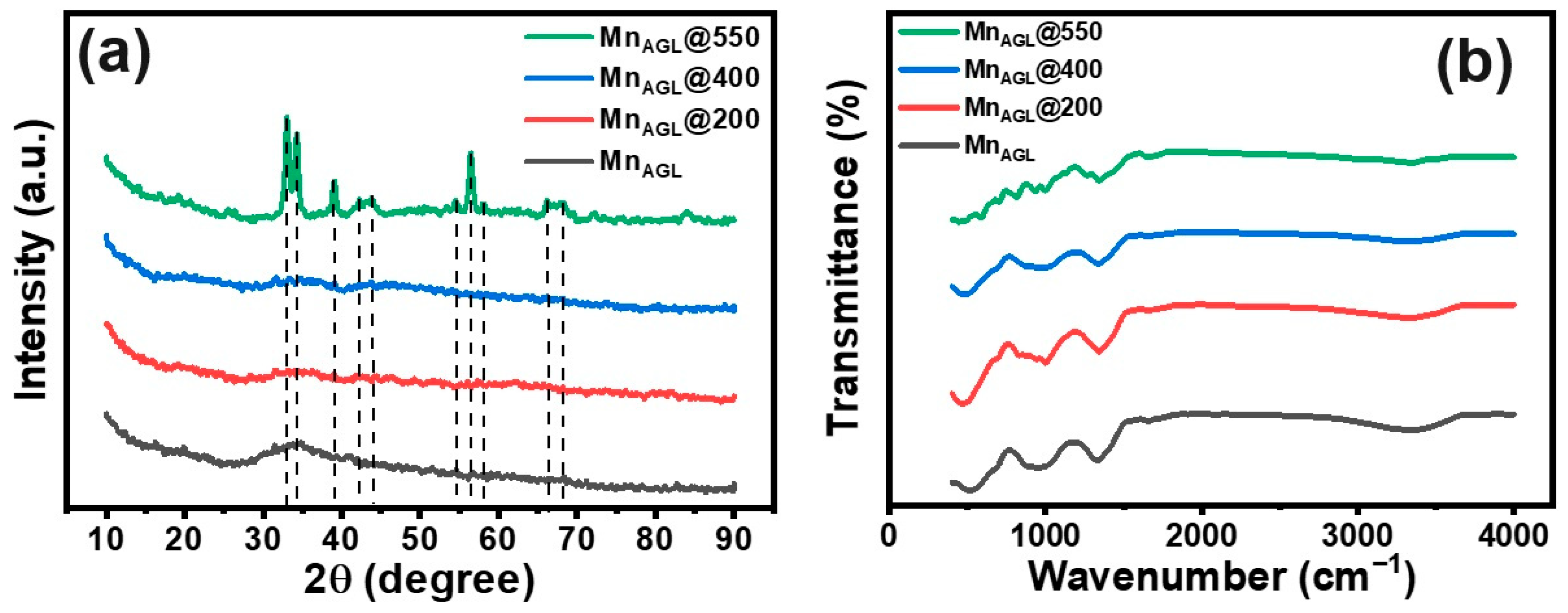
:1. Introduction
2. Experimental
2.1. Electrochemistry
2.1.1. EC Performance of MnAGL@400-Modified Nickel Foam (MnAGL@400/Nickel Foam (NF)) Electrode
2.1.2. Fabrication of MnAGL@400-Based Asymmetric SC
2.1.3. Calculation of Csp, ED, and PD
3. Results and Discussion
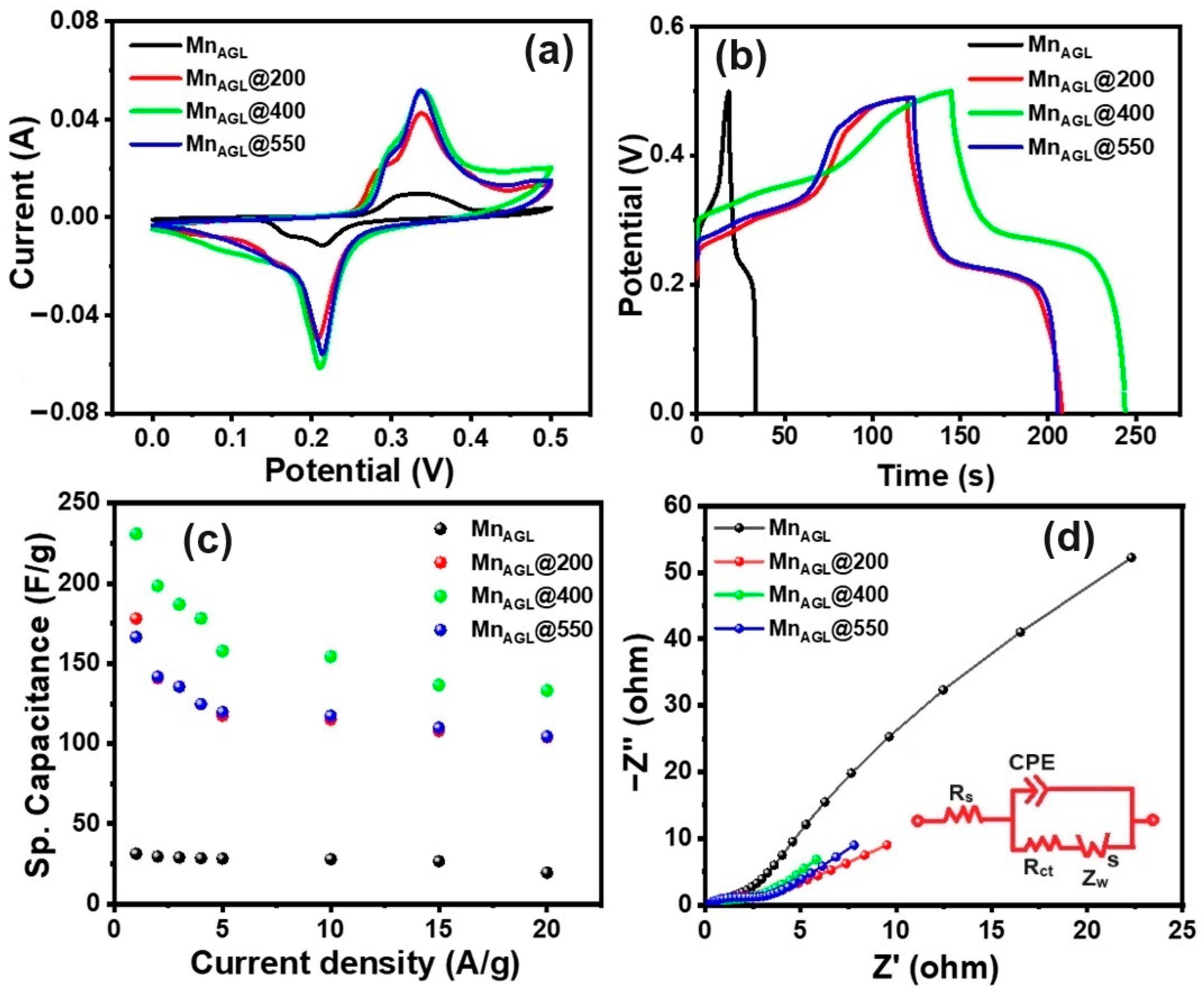
3.1. Electrochemical Studies
3.2. Galvanostatic Charge–Discharge Studies
3.3. Electrochemical Impedance Spectroscopic Studies
3.4. Device Fabrication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nijsse, F.J.M.M.; Mercure, J.-F.; Ameli, N.; Larosa, F.; Kothari, S.; Rickman, J.; Vercoulen, P.; Pollitt, H. The Momentum of the Solar Energy Transition. Nat. Commun. 2023, 14, 6542. [Google Scholar] [CrossRef] [PubMed]
- Fic, K.; Platek, A.; Piwek, J.; Frackowiak, E. Sustainable Materials for Electrochemical Capacitors. Mater. Today 2018, 21, 437–454. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Dang, V.D.; Thamilselvan, A.; Doong, R.; Pandit, B. Sustainable High-Energy Supercapacitors: Metal Oxide-Agricultural Waste Biochar Composites Paving the Way for a Greener Future. J. Energy Storage 2024, 77, 109723. [Google Scholar] [CrossRef]
- Lichchhavi; Kanwade, A.; Shirage, P.M. A Review on Synergy of Transition Metal Oxide Nanostructured Materials: Effective and Coherent Choice for Supercapacitor Electrodes. J. Energy Storage 2022, 55, 105692. [Google Scholar] [CrossRef]
- Sakib, M.N.; Ahmed, S.; Rahat, S.M.S.M.; Shuchi, S.B. A Review of Recent Advances in Manganese-Based Supercapacitors. J. Energy Storage 2021, 44, 103322. [Google Scholar] [CrossRef]
- Lu, W.; Li, Y.; Yang, M.; Jiang, X.; Zhang, Y.; Xing, Y. Construction of Hierarchical Mn2O3@MnO2 Core–Shell Nanofibers for Enhanced Performance Supercapacitor Electrodes. ACS Appl. Energy Mater. 2020, 3, 8190–8197. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, M.; Dou, Y.; Chen, S.; Liu, P.; Yin, H.; Zhao, H. Manganese Oxides Transformed from Orthorhombic Phase to Birnessite with Enhanced Electrochemical Performance as Supercapacitor Electrodes. J. Mater. Chem. A 2020, 8, 3746–3753. [Google Scholar] [CrossRef]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese Oxide-Based Materials as Electrochemical Supercapacitor Electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef] [PubMed]
- Nechikott, A.A.; Nayak, P.K. Electrochemical Capacitance Properties of Pre-Sodiated Manganese Oxide for Aqueous Na-Ion Supercapacitors. RSC Adv. 2023, 13, 14139–14149. [Google Scholar] [CrossRef]
- Zhu, S.; Li, L.; Liu, J.; Wang, H.; Wang, T.; Zhang, Y.; Zhang, L.; Ruoff, R.S.; Dong, F. Structural Directed Growth of Ultrathin Parallel Birnessite on β-MnO2 for High-Performance Asymmetric Supercapacitors. ACS Nano 2018, 12, 1033–1042. [Google Scholar] [CrossRef]
- Siddique, M.A.B.; Bithi, U.H.; Ahmed, A.N.; Gafur, M.A.; Reaz, A.H.; Roy, C.K.; Islam, M.M.; Firoz, S.H. Preparation of Manganese Oxide Nanoparticles with Enhanced Capacitive Properties Utilizing Gel Formation Method. ACS Omega 2022, 7, 48007–48017. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Chen, J.; Zheng, Y.; Li, P.; Huang, J.; Chen, Z. Topological Design for Isotropic Metamaterials Using Anisotropic Material Microstructures. Eng. Anal. Bound. Elem. 2024, 162, 28–44. [Google Scholar] [CrossRef]
- Tian, L.-Y.; Huang, P.-B.; Lv, J.-Q.; Zhang, Y.-H.; Zhao, L.-N.; Liu, L.; Wang, P.-F.; Wu, Y.-H.; Shi, Q.; Shi, F.-N. Metal-Organic Frameworks Based on Ternary Transition Metal Ions for High-Performance Lithium Ion Batteries. J. Solid State Chem. 2024, 335, 124717. [Google Scholar] [CrossRef]
- Ramkumar, R.; Rajkumar, C.; Do, H.; Kim, H.; Kim, W.K. A Remarkable Oxygen Vacancy-Rich Rare Earth Aerogel with High Activity towards Electro and Catalytic Reduction of 5-Nitroquinoline. J. Clean. Prod. 2023, 423, 138683. [Google Scholar] [CrossRef]
- Ramkumar, R.; Dhakal, G.; Tamang, T.L.; Yu, S.; Veerakumar, P.; Shim, J.-J.; Kim, W.K. Reverse-Engineered Borohydride Hydrolysis: Vacancy-Rich Cobalt Aerogel Nanowafers as High-Efficiency Electrode Materials for Supercapacitor Applications. J. Energy Storage 2024, 86, 111338. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Ren, B.; Zhao, J.; Zhang, L.; Dong, X.; Liu, Z. Manganese Oxide Doping Carbon Aerogels Prepared with MnO2 Coordinated by N, N-Dimethylmethanamide for Supercapacitors. J. Colloid Interface Sci. 2019, 537, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, L.; Hu, Y.; Lu, Y.; He, J.; Wang, S.; Yang, Q.; Shi, Z.; Xiong, C. Manganese Oxide/Nitrogen-Doped Carbon Aerogels from Cellulose Nanofibrils for High-Performance Supercapacitor Electrodes. Diam. Relat. Mater. 2022, 122, 108813. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Q.; Li, Q.; Yu, H.; Liu, Y. Composite Aerogels of Carbon Nanocellulose Fibers and Mixed-Valent Manganese Oxides as Renewable Supercapacitor Electrodes. Polymers 2019, 11, 129. [Google Scholar] [CrossRef]
- Qiu, X.; Xu, D.; Ma, L.; Wang, Y. Preparation of Manganese Oxide/Graphene Aerogel and Its Application as an Advanced Supercapacitor Electrode Material. Int. J. Electrochem. Sci. 2017, 12, 2173–2183. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, X.; Chen, M. Pore Structure Improvement of Lignin Composite Carbon Aerogels by Introducing Manganese Ion and Its Application in Supercapacitors. Mater. Res. Express 2019, 6, 065036. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Wang, X.; Xie, K.; Wang, Y. Effect of Manganese Valence on Specific Capacitance in Supercapacitors of Manganese Oxide Microspheres. Chem. Eur. J. 2021, 27, 9152–9159. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhang, Y.; Li, F.; Zhang, L.; Ruoff, R.S.; Wen, Z.; Liu, Q. Self-Assembly of Mesoporous Nanotubes Assembled from Interwoven Ultrathin Birnessite-Type MnO2 Nanosheets for Asymmetric Supercapacitors. Sci. Rep. 2014, 4, 3878. [Google Scholar] [CrossRef]
- Sun, Y.-A.; Chen, L.-T.; Hsu, S.-Y.; Hu, C.-C.; Tsai, D.-H. Silver Nanoparticles-Decorating Manganese Oxide Hybrid Nanostructures for Supercapacitor Applications. Langmuir 2019, 35, 14203–14212. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, B.; Wu, C.-W.; Chen, I.-W.P.; Chang, H.-T.; Lin, C.-H.; Huang, C.-C. Carbon Dot-Mediated Synthesis of Manganese Oxide Decorated Graphene Nanosheets for Supercapacitor Application. ACS Sustain. Chem. Eng. 2016, 4, 3008–3016. [Google Scholar] [CrossRef]
- Ashraf, I.; Abbas, Q.; Huang, Y.; Hassan, N.U.; Albaqami, M.D.; Tighezza, A.M.; Eldin, S.M.; Javed, M.S.; Ahmad, A.; Luque, R. V-Mn-O Aerogel Composite-Based High-Energy Zn-Ion Hybrid Supercapacitor. J. Energy Storage 2023, 60, 106601. [Google Scholar] [CrossRef]
- Iamprasertkun, P.; Krittayavathananon, A.; Seubsai, A.; Chanlek, N.; Kidkhunthod, P.; Sangthong, W.; Maensiri, S.; Yimnirun, R.; Nilmoung, S.; Pannopard, P.; et al. Charge Storage Mechanisms of Manganese Oxide Nanosheets and N-Doped Reduced Graphene Oxide Aerogel for High-Performance Asymmetric Supercapacitors. Sci. Rep. 2016, 6, 37560. [Google Scholar] [CrossRef] [PubMed]
- Suktha, P.; Chiochan, P.; Krittayavathananon, A.; Sarawutanukul, S.; Sethuraman, S.; Sawangphruk, M. In Situ Mass Change and Gas Analysis of 3D Manganese Oxide/Graphene Aerogel for Supercapacitors. RSC Adv. 2019, 9, 28569–28575. [Google Scholar] [CrossRef] [PubMed]
- Boynuegri, T.A.; Gürü, M. Catalytic Dehydrogenation of Calcium Borohydride by Using Hydrogel Catalyst. Int. J. Hydrogen Energy 2017, 42, 17869–17873. [Google Scholar] [CrossRef]
- Lakra, R.; Kumar, R.; Sahoo, P.K.; Sharma, D.; Thatoi, D.; Soam, A. Facile Synthesis of Cobalt Oxide and Graphene Nanosheets Nanocomposite for Aqueous Supercapacitor Application. Carbon Trends 2022, 7, 100144. [Google Scholar] [CrossRef]
- Ramkumar, R.; Dhakal, G.; Shim, J.-J.; Kim, W.K. NiO/Ni Nanowafer Aerogel Electrodes for High Performance Supercapacitors. Nanomaterials 2022, 12, 3813. [Google Scholar] [CrossRef]
- Stebounova, L.V.; Gonzalez-Pech, N.I.; Peters, T.M.; Grassian, V.H. Physicochemical Properties of Air Discharge-Generated Manganese Oxide Nanoparticles: Comparison to Welding Fumes. Environ. Sci. Nano 2018, 5, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Douk, A.S.; Farsadrooh, M.; Damanigol, F.; Moghaddam, A.A.; Saravani, H.; Noroozifar, M. Porous Three-Dimensional Network of Pd–Cu Aerogel toward Formic Acid Oxidation. RSC Adv. 2018, 8, 23539–23545. [Google Scholar] [CrossRef] [PubMed]
- Amirtharaj, S.N.; Mariappan, M. Rapid and Controllable Synthesis of Mn2O3 Nanorods via a Sonochemical Method for Supercapacitor Electrode Application. Appl. Phys. A 2021, 127, 607. [Google Scholar] [CrossRef]
- Sun, X.; Hao, Z.; Nan, H.; Xu, J.; Tian, H. Silver Decorated Lanthanum Calcium Manganate for Electrochemical Supercapacitor. Mater. Res. Express 2021, 8, 075502. [Google Scholar] [CrossRef]
- Mahamad Yusoff, N.F.; Idris, N.H.; Md Din, M.F.; Majid, S.R.; Harun, N.A.; Noerochim, L. Coupling of Mn2O3 with Heteroatom-Doped Reduced Graphene Oxide Aerogels with Improved Electrochemical Performances for Sodium-Ion Batteries. Nanomaterials 2023, 13, 732. [Google Scholar] [CrossRef] [PubMed]
- Pronin, I.A.; Averin, I.A.; Karmanov, A.A.; Yakushova, N.D.; Komolov, A.S.; Lazneva, E.F.; Sychev, M.M.; Moshnikov, V.A.; Korotcenkov, G. Control over the Surface Properties of Zinc Oxide Powders via Combining Mechanical, Electron Beam, and Thermal Processing. Nanomaterials 2022, 12, 1924. [Google Scholar] [CrossRef] [PubMed]
- Komolov, A.S.; Lazneva, E.F.; Gerasimova, N.B.; Panina, Y.A.; Sobolev, V.S.; Koroleva, A.V.; Pshenichnyuk, S.A.; Asfandiarov, N.L.; Modelli, A.; Handke, B.; et al. Conduction Band Electronic States of Ultrathin Layers of Thiophene/Phenylene Co-Oligomers on an Oxidized Silicon Surface. J. Electron Spectrosc. Relat. Phenom. 2019, 235, 40–45. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA; Weinheim, Germany, 2001; ISBN 978-0-471-04372-0. [Google Scholar]
- Luo, S.; Shang, J.; Xu, Y.; Cheng, H.; Zhang, L.; Tang, Y. Stabilizing NiS2 on Conductive Component via Electrostatic Self-assembly and Covalent Bond Strategy for Promoting Sodium Storage. Adv. Funct. Mater. 2024, 2403166. [Google Scholar] [CrossRef]
- Zhu, C.; Hao, Y.; Wu, H.; Chen, M.; Quan, B.; Liu, S.; Hu, X.; Liu, S.; Ji, Q.; Lu, X.; et al. Self-Assembly of Binderless MXene Aerogel for Multiple-Scenario and Responsive Phase Change Composites with Ultrahigh Thermal Energy Storage Density and Exceptional Electromagnetic Interference Shielding. Nano-Micro Lett. 2024, 16, 57. [Google Scholar] [CrossRef]


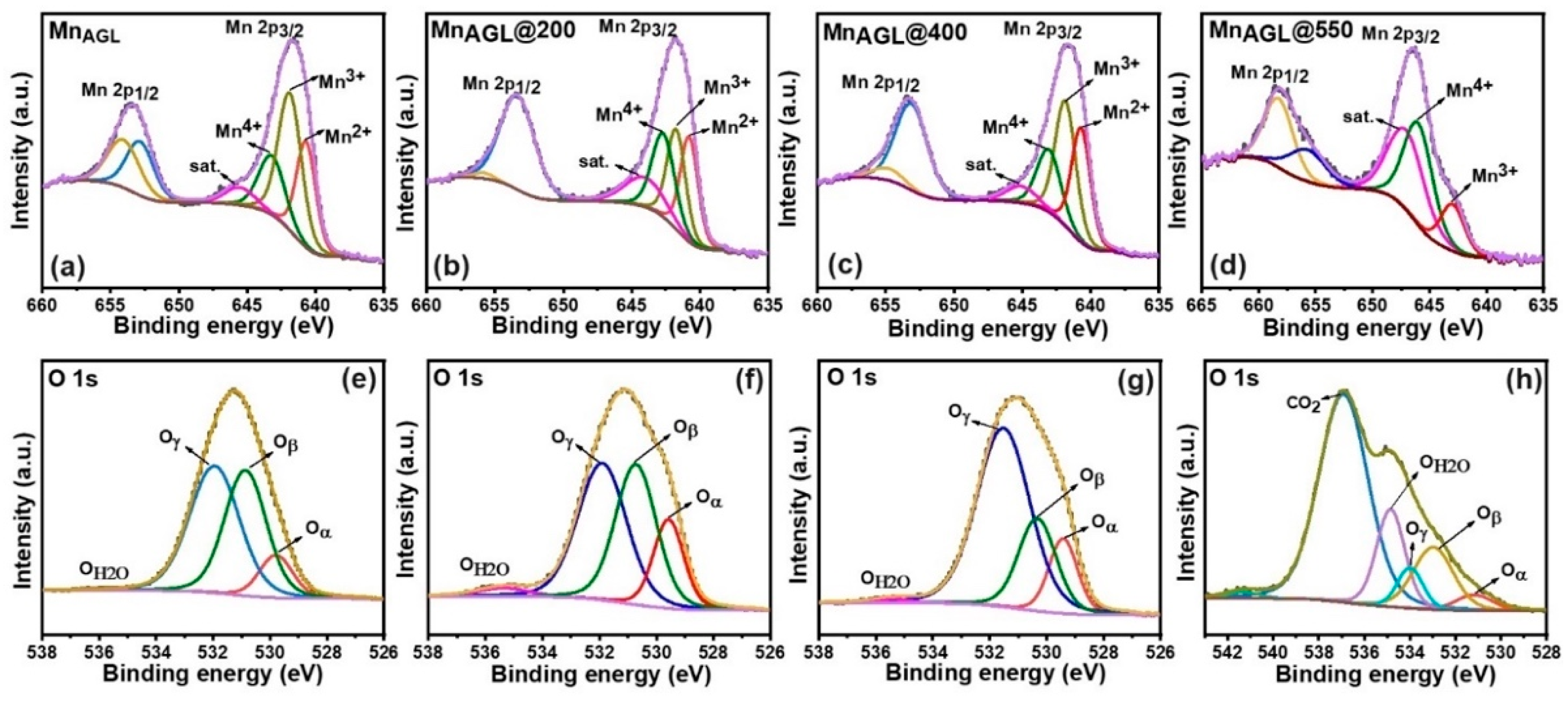

| Deconvoluted Mn 2p3/2 | MnAGL | MnAGL@200 | MnAGL@400 | MnAGL@550 |
|---|---|---|---|---|
| 2+ | 0.32 | 0.32 | 0.33 | - |
| 3+ | 0.38 | 0.37 | 0.34 | 0.44 |
| 4+ | 0.30 | 0.30 | 0.33 | 0.56 |
| Electrode | Electrolyte | Csp (F/g@1 A/g) | ED (Wh/kg) | PD (W/kg) | Ref. |
|---|---|---|---|---|---|
| MnO2/CA | 6M KOH | 170 | 8 | 3000 | [16] |
| Mn2O3/n-CA/CN | 1M Na2SO4 | 275 | 23 | 600 | [17] |
| CNF/MnOx | 1M Na2SO4 | 269 (5 mV/s) | 37 | 2750 | [18] |
| MnO2/rGO | 0.5M Na2SO4 | 63 | 18 | 400 | [19] |
| Lignin/Mn-CA | 6M KOH | 186 | - | - | [20] |
| Mn2O3/MnO2 | 1M Na2SO4 | 225 | 27 | 90 | [6] |
| Mn2O3 | 1M Na2SO4 | 209 | 29 | 250 | [21] |
| MnO2 NT | 1M KOH | 243 | - | - | [22] |
| CTAB-Ag-MnOx | Na2SO4/NaHCO3 | 145 | - | - | [23] |
| MnOx-CDGs | 1M Na2SO4 | 280 | 19 | 15,630 | [24] |
| V-Mn-O//Zn ion | 6M KOH/0.2M(CH3COO)2 | 333 | 150 | 900 | [25] |
| MnO2/N-rGO | 0.5M Na2SO4 | 467 | 40 | 39 | [26] |
| MnOx/rGOae | 1M Na2SO4 | 240 | 11 | 286 | [27] |
| MnAGL@400 | 3M KOH | 230.8 | 9.8 | 16,523 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramkumar, R.; Suganthi, S.; Milton, A.; Park, J.; Shim, J.-J.; Oh, T.H.; Kim, W.K. MnO/Mn2O3 Aerogels as Effective Materials for Supercapacitor Applications. Energies 2024, 17, 2258. https://doi.org/10.3390/en17102258
Ramkumar R, Suganthi S, Milton A, Park J, Shim J-J, Oh TH, Kim WK. MnO/Mn2O3 Aerogels as Effective Materials for Supercapacitor Applications. Energies. 2024; 17(10):2258. https://doi.org/10.3390/en17102258
Chicago/Turabian StyleRamkumar, Ramya, Sanjeevamuthu Suganthi, Ahamed Milton, Jungbin Park, Jae-Jin Shim, Tae Hwan Oh, and Woo Kyoung Kim. 2024. "MnO/Mn2O3 Aerogels as Effective Materials for Supercapacitor Applications" Energies 17, no. 10: 2258. https://doi.org/10.3390/en17102258






