Self-Cleaning Mortar Façades with Addition of Anatase and Rutile Titanium Dioxide for Cool Façades
Abstract
:1. Introduction
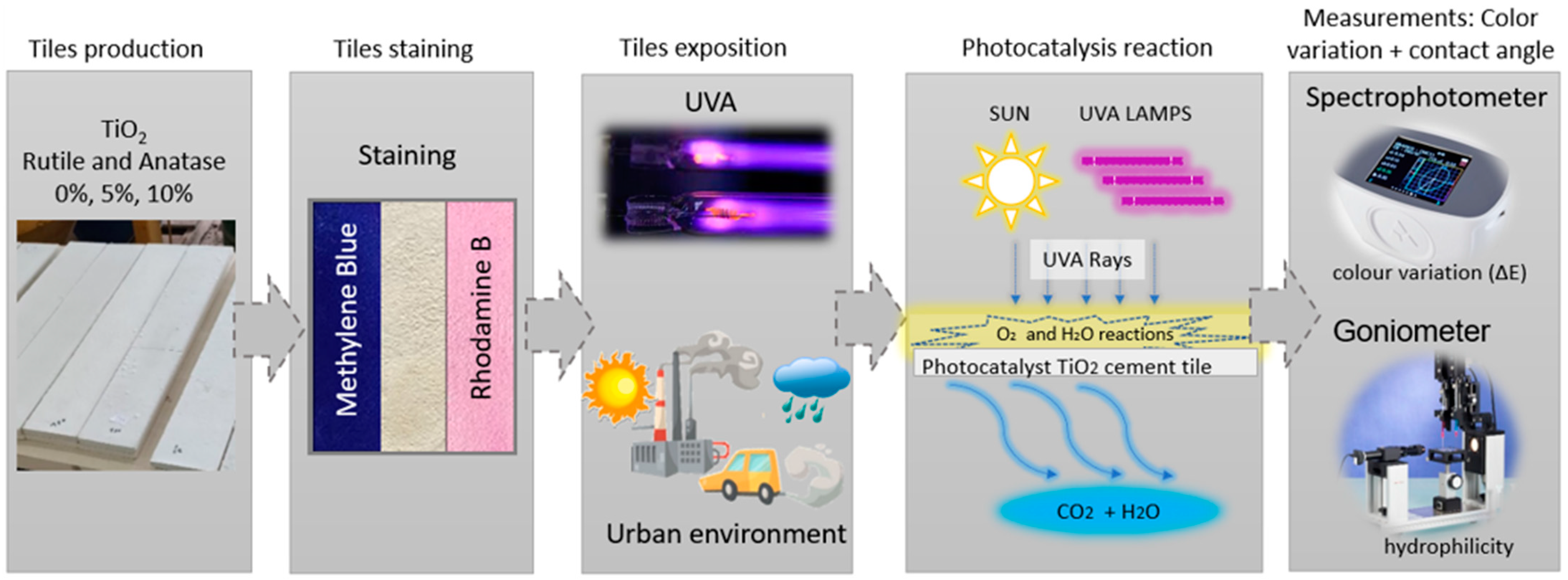
2. Materials and Methods
2.1. Materials
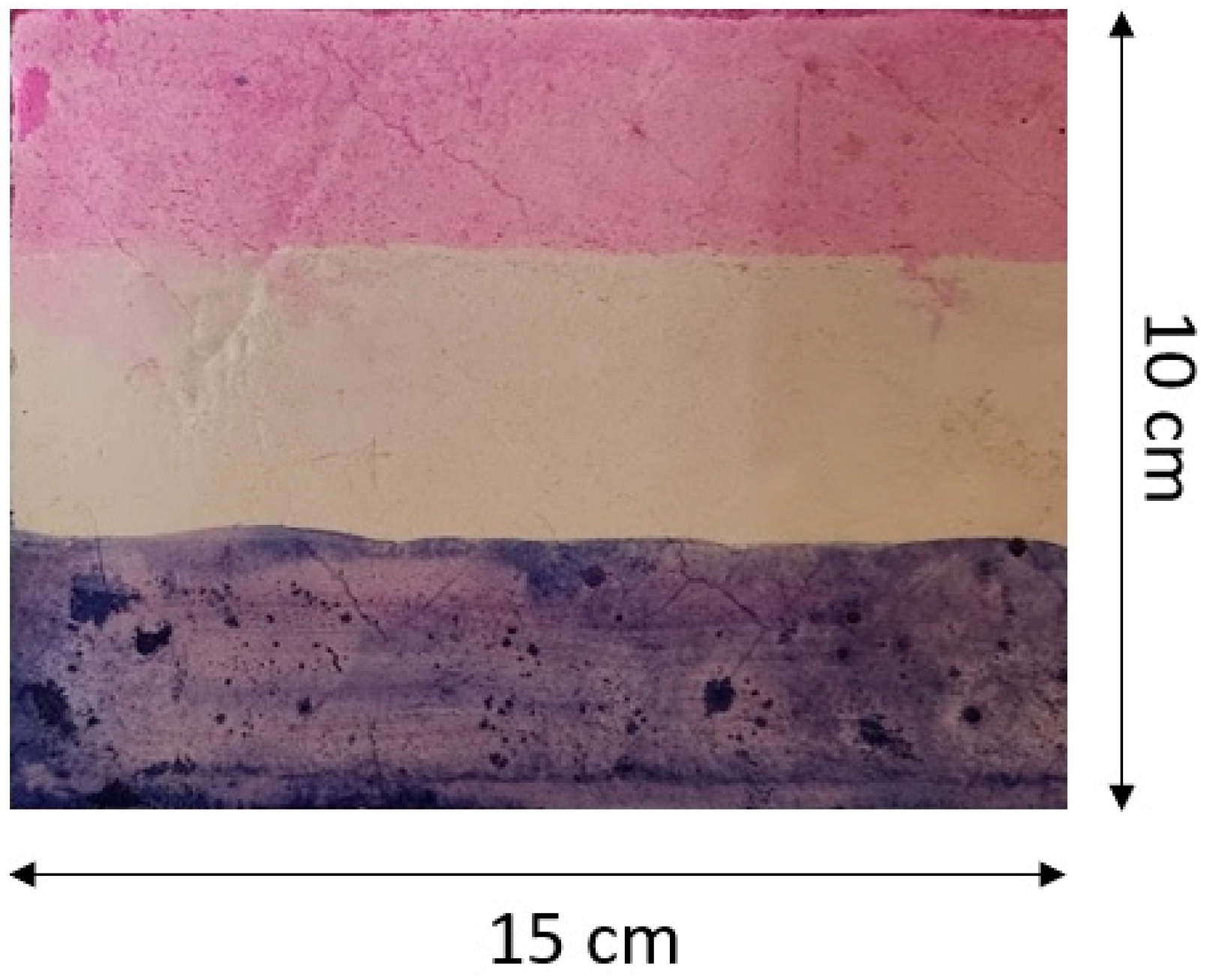
2.2. Methodology of Tests Performed
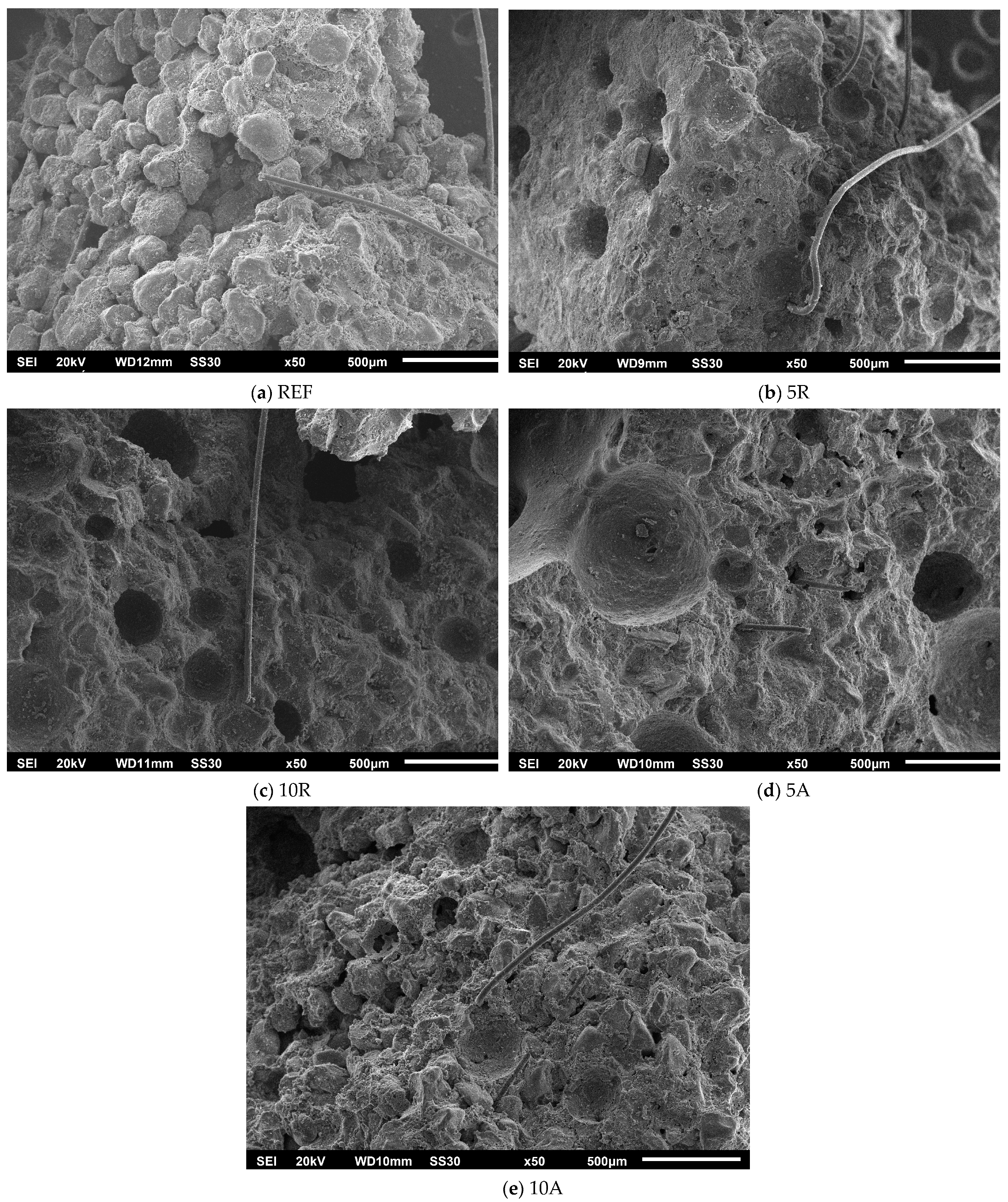
3. Results
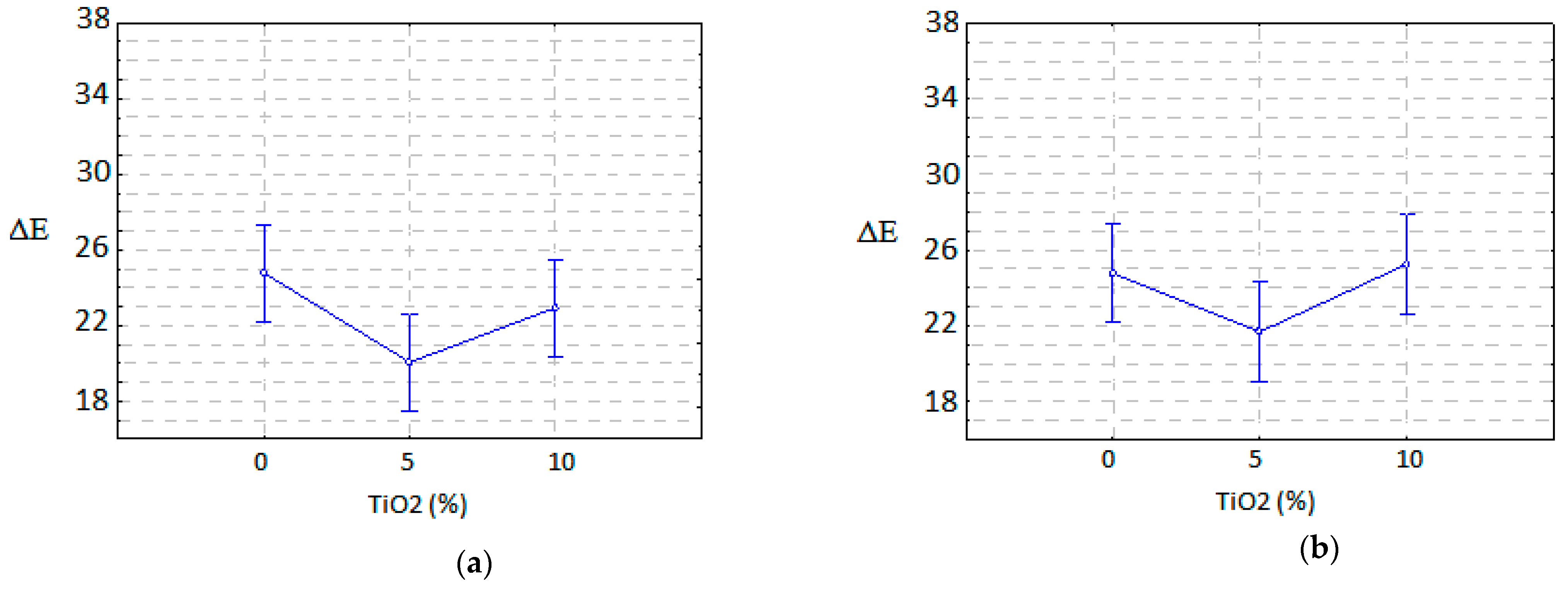
3.1. Photocatalysis Analysis
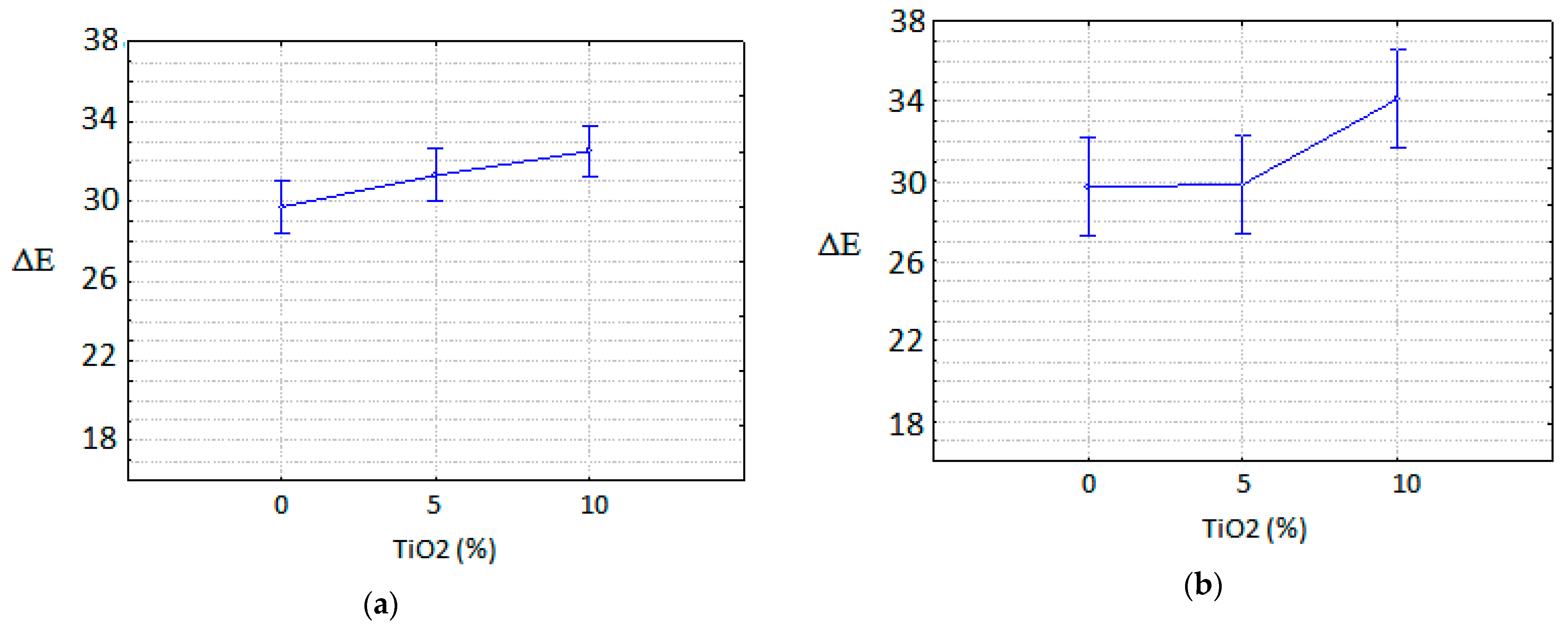
3.1.1. Cement Tiles Exposed to the Urban Environment
3.1.2. Cement Tiles Exposed to a UVA Chamber
3.1.3. Evaluation of the Superficial Tension through Contact Angle
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| UVA Cure | |||||||
|---|---|---|---|---|---|---|---|
| Sample | CP | ΔE Point 1 | ΔE Point 2 | ΔE Point 3 | Average ΔE | ||
| Reference | UVA | REF | CP1 | −1.20 | −0.25 | −0.66 | 1.39 |
| UVA | REF | CP2 | −3.63 | 0.31 | 0.34 | 3.66 | |
| UVA | 5R | CP1 | −1.43 | −0.97 | −0.45 | 1.79 | |
| UVA | 5R | CP2 | −0.85 | −0.25 | 0.61 | 1.08 | |
| UVA | 10R | CP1 | −0.63 | 0.27 | 0.88 | 1.12 | |
| UVA | 10R | CP2 | −0.10 | 0.93 | 2.86 | 3.01 | |
| UVA | 5A | CP1 | −1.31 | −0.24 | 0.04 | 1.34 | |
| UVA | 5A | CP2 | −2.49 | 0.47 | 0.79 | 2.66 | |
| UVA | 10A | CP1 | 0.04 | −0.35 | 0.11 | 0.37 | |
| UVA | 10A | CP2 | 1.40 | −0.46 | 0.13 | 1.48 | |
| Methylene blue | UVA | REF | CP1 | 0.46 | −3.11 | 3.75 | 4.89 |
| UVA | REF | CP2 | 5.75 | −14.16 | 8.92 | 17.70 | |
| UVA | 5R | CP1 | 0.35 | −1.92 | 4.75 | 5.14 | |
| UVA | 5R | CP2 | −1.24 | −1.42 | 6.98 | 7.23 | |
| UVA | 10R | CP1 | 1.43 | −3.98 | 5.30 | 6.78 | |
| UVA | 10R | CP2 | 5.53 | −7.73 | 10.05 | 13.83 | |
| UVA | 5A | CP1 | 0.90 | −2.12 | 4.57 | 5.11 | |
| UVA | 5A | CP2 | 2.62 | −4.07 | 7.97 | 9.32 | |
| UVA | 10A | CP1 | 1.69 | −3.07 | 6.73 | 7.59 | |
| UVA | 10A | CP2 | 9.40 | −4.26 | 10.77 | 14.91 | |
| Rhodamine B | UVA | REF | CP1 | 2.75 | −5.59 | 5.81 | 8.52 |
| UVA | REF | CP2 | 1.08 | −3.03 | 7.07 | 7.77 | |
| UVA | 5R | CP1 | 6.60 | −21.52 | 11.06 | 25.08 | |
| UVA | 5R | CP2 | 6.06 | −19.24 | 11.72 | 23.33 | |
| UVA | 10R | CP1 | 6.34 | −17.12 | 9.03 | 20.37 | |
| UVA | 10R | CP2 | 5.39 | −15.91 | 10.48 | 19.80 | |
| UVA | 5A | CP1 | 5.09 | −18.53 | 6.94 | 20.43 | |
| UVA | 5A | CP2 | 4.03 | −17.71 | 7.78 | 19.76 | |
| UVA | 10A | CP1 | 4.47 | −17.65 | 8.14 | 19.95 | |
| UVA | 10A | CP2 | 7.99 | −18.43 | 6.78 | 21.20 | |
| Curing in an Urban Environment | ||||||
|---|---|---|---|---|---|---|
| Sample | CP | ΔE Point 1 | ΔE Point 2 | ΔE Point 3 | Average ΔE | |
| Reference | REF | CP1 | 1.03 | 1.27 | 1.45 | 1.25 |
| REF | CP2 | 0.70 | 0.81 | 1.58 | 1.03 | |
| REF | CP3 | 2.41 | 2.06 | 2.15 | 2.21 | |
| 5R | CP1 | 0.56 | 0.33 | 0.50 | 0.46 | |
| 5R | CP2 | 1.41 | 0.57 | 0.48 | 0.82 | |
| 5R | CP3 | 0.76 | 0.48 | 0.95 | 0.73 | |
| 10R | CP1 | 1.98 | 3.97 | 4.08 | 3.34 | |
| 10R | CP2 | 1.46 | 3.72 | 2.07 | 2.42 | |
| 10R | CP3 | 1.34 | 2.67 | 3.40 | 2.47 | |
| 5A | CP1 | 9.19 | 0.86 | 5.47 | 5.17 | |
| 5A | CP2 | 0.44 | 2.13 | 5.18 | 2.58 | |
| 5A | CP3 | 0.47 | 0.17 | 2.87 | 1.17 | |
| 10A | CP1 | 2.12 | 1.94 | 1.90 | 1.99 | |
| 10A | CP2 | 1.87 | 2.68 | 1.24 | 1.93 | |
| 10A | CP3 | 1.22 | 2.65 | 1.82 | 1.89 | |
| Methylene blue | REF | CP1 | 54.34 | 61.47 | 56.15 | 57.32 |
| REF | CP2 | 53.24 | 64.70 | 56.18 | 58.04 | |
| REF | CP3 | 58.46 | 61.60 | 55.40 | 58.49 | |
| 5R | CP1 | 63.38 | 65.48 | 60.25 | 63.04 | |
| 5R | CP2 | 51.52 | 62.43 | 64.04 | 59.33 | |
| 5R | CP3 | 66.25 | 58.67 | 65.55 | 63.49 | |
| 10R | CP1 | 58.22 | 57.57 | 72.16 | 62.65 | |
| 10R | CP2 | 59.16 | 59.15 | 69.68 | 62.66 | |
| 10R | CP3 | 65.36 | 55.69 | 63.62 | 61.56 | |
| 5A | CP1 | 50.06 | 61.75 | 56.34 | 56.05 | |
| 5A | CP2 | 53.22 | 59.03 | 60.30 | 57.52 | |
| 5A | CP3 | 58.74 | 46.23 | 64.63 | 56.53 | |
| 10A | CP1 | 64.79 | 67.99 | 67.44 | 66.74 | |
| 10A | CP2 | 67.05 | 67.80 | 69.36 | 68.07 | |
| 10A | CP3 | 58.68 | 64.35 | 69.34 | 64.12 | |
| Rhodamine B | REF | CP1 | 47.37 | 54.79 | 47.74 | 49.97 |
| REF | CP2 | 45.28 | 52.71 | 47.41 | 48.46 | |
| REF | CP3 | 44.29 | 47.81 | 45.27 | 45.79 | |
| 5R | CP1 | 34.10 | 34.02 | 38.39 | 35.50 | |
| 5R | CP2 | 39.12 | 35.51 | 38.01 | 37.55 | |
| 5R | CP3 | 41.90 | 44.50 | 49.58 | 45.33 | |
| 10R | CP1 | 41.12 | 39.54 | 45.92 | 42.19 | |
| 10R | CP2 | 45.21 | 46.82 | 42.91 | 44.98 | |
| 10R | CP3 | 42.32 | 41.32 | 43.02 | 42.22 | |
| 5A | CP1 | 37.08 | 43.23 | 40.35 | 40.22 | |
| 5A | CP2 | 37.39 | 39.24 | 38.25 | 38.29 | |
| 5A | CP3 | 44.94 | 43.57 | 39.52 | 42.68 | |
| 10A | CP1 | 50.17 | 43.83 | 44.16 | 46.06 | |
| 10A | CP2 | 52.16 | 51.92 | 50.29 | 51.46 | |
| 10A | CP3 | 51.93 | 45.94 | 46.81 | 48.23 | |
References
- UN (United Nations). Sustainable Cities and Human Settlements. Available online: https://sdgs.un.org/topics/sustainable-cities-and-human-settlements (accessed on 20 December 2022).
- Yi, Y.K. Building Facade Multi-Objective Optimization for Daylight and Aesthetical Perception. Build. Environ. 2019, 156, 178–190. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Dionísio, A. Self-Cleaning Property of Mortars with TiO2 Addition Using Real Diesel Exhaust Soot. J. Clean. Prod. 2017, 161, 850–859. [Google Scholar] [CrossRef]
- Dantas, S.R.A.; Vittorino, F.; Loh, K. Comparison of Reflectance to Solar Radiation between Mortars Treated with TiO2 and Painted Mortars after Three Years of Exposure. J. Build. Eng. 2022, 46, 103829. [Google Scholar] [CrossRef]
- Schreck, M.; Niederberger, M. Photocatalytic Gas Phase Reactions. Chem. Mater. 2019, 31, 597–618. [Google Scholar] [CrossRef]
- Sakar, M.; Mithun Prakash, R.; Trong-On, D. Insights into the Tio2-Based Photocatalytic Systems and Their Mechanisms. Catalysts 2019, 9, 680. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Krishnan, V. Perovskite Oxide Based Materials for Energy and Environment-Oriented Photocatalysis. ACS Catal. 2020, 10, 10253–10315. [Google Scholar] [CrossRef]
- Khan, S.; Park, J.S.; Ishihara, T. A Review of the Single-Step Flame Synthesis of Defective and Heterostructured TiO2 Nanoparticles for Photocatalytic Applications. Catalysts 2023, 13, 196. [Google Scholar] [CrossRef]
- Yang, L.; Hakki, A.; Zheng, L.; Jones, M.R.; Wang, F.; Macphee, D.E. Photocatalytic Concrete for NOx Abatement: Supported TiO2 Efficiencies and Impacts. Cem. Concr. Res. 2019, 116, 57–64. [Google Scholar] [CrossRef]
- Constantino, J.C.P.; Garcia, D.C.S.; Palhares, H.G.; Houmard, M.; Figueiredo, R.B. Development of Functional TiO2 Coatings Deposited on Cementitious Materials. Constr. Build. Mater. 2020, 250, 118732. [Google Scholar] [CrossRef]
- Poon, C.S.; Cheung, E. NO Removal Efficiency of Photocatalytic Paving Blocks Prepared with Recycled Materials. Constr. Build. Mater. 2007, 21, 1746–1753. [Google Scholar] [CrossRef]
- Ibrahim, A.; Phoohinkong, W.; Mekprasart, W.; Pecharapa, W. Anatase/Rutile TiO2 Composite Thin Films Prepared via Dip Coating Technique and Their Hydrophilicity, Stability and Photocatalytic Activity. Mater. Today Proc. 2018, 5, 10903–10909. [Google Scholar] [CrossRef]
- Croce, S.; D’Agnolo, E.; Caini, M.; Paparella, R. The Use of Cool Pavements for the Regeneration of Industrial Districts. Sustain. 2021, 13, 6322. [Google Scholar] [CrossRef]
- Mansouri, O.; Belarbi, R.; Bourbia, F. Albedo Effect of External Surfaces on the Energy Loads and Thermal Comfort in Buildings. Energy Procedia 2017, 139, 571–577. [Google Scholar] [CrossRef]
- Ramos, N.M.M.; Souza, A.R.; Maia, J.; Almeida, R.M.S.F. Colour Degradation of Façade Coatings - The Effect of Nanopigments Incorporation. E3S Web Conf. 2020, 172, 24004. [Google Scholar] [CrossRef]
- Yuxuan, Z.; Yunyun, Z.; Jianrong, Y.; Xiaoqiang, Z. Energy Saving Performance of Thermochromic Coatings with Different Colors for Buildings. Energy Build. 2020, 215, 109920. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Rodriguez-Garcia, J.R.; Castillo, A.; Castellote, M. Characteristics and Efficiency of Photocatalytic Cementitious Materials: Type of Binder, Roughness and Microstructure. Cem. Concr. Res. 2015, 71, 124–131. [Google Scholar] [CrossRef]
- Cárdenas, C.; Tobón, J.I.; García, C.; Vila, J. Functionalized Building Materials: Photocatalytic Abatement of NO x by Cement Pastes Blended with TiO2 Nanoparticles. Constr. Build. Mater. 2012, 36, 820–825. [Google Scholar] [CrossRef]
- Mohseni, E.; Khotbehsara, M.M.; Naseri, F.; Monazami, M.; Sarker, P. Polypropylene Fiber Reinforced Cement Mortars Containing Rice Husk Ash and Nano-Alumina. Constr. Build. Mater. 2016, 111, 429–439. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Del Curto, B.; Ormellese, M.; Pedeferri, M.P. Photocatalytic and Self-Cleaning Activity of Colored Mortars Containing TiO2. Constr. Build. Mater. 2013, 46, 167–174. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, C.; Li, Q.; Zhang, W.; Cao, L.; Ye, J. Preparation of Titanium Dioxide Nano Particle Modified Photocatalytic Self-Cleaning Concrete. J. Clean. Prod. 2015, 87, 762–765. [Google Scholar] [CrossRef]
- Infibra. Manual de Instalação—Placas Cimentícias NTF; Infibra: Leme, Brazil, 2021. [Google Scholar]
- Brasilit. Catálogo de Produtos Brasilit: Soluções Para Telhados e Construções Industrializadas; Brasilit: Brasilit, Brazil, 2018. [Google Scholar]
- Valcan. Ceramapanel Technical Information A1; Valcan: Bridgwater, UK, 2022; pp. 3–4. [Google Scholar]
- NBR 7215; Cimento Portland―Determinação Da Resistência à Compressão de Corpos de Prova Cilíndricos. ABNT: Rio de Janeiro, Brazil, 2019.
- NBR 13276; Argamassa Para Assentamento e Revestimento de Paredes e Tetos—Determinação Do Índice de Consistência. ABNT: Rio de Janeiro, Brazil, 2016.
- NBR 13278; Argamassa Para Assentamento e Revestimento de Paredes e Tetos—Determinação Da Densidade de Massa e Do Teor de Ar Incorporado. ABNT: Rio de Janeiro, Brazil, 2005.
- NBR 15498; Placa de Fibrocimento Sem Amianto—Requisitos e Métodos de Ensaio. ABNT: Rio de Janeiro, Brazil, 2021.
- UNI 11259; Photocatalysis—Determination of the Photocatalytic Activity of Hidraulic Binders—Rodammina Test Method. UNI: Milan, Italy, 2016.
- ASTM C1378-04(2014); Standard Test Method for Determination of Resistance to Staining. American National Standards Institute: West Conshohocken, PA, USA, 2014.
- Rosales, A.; Maury-Ramírez, A.; De Gutiérrez, R.M.; Guzmán, C.; Esquivel, K. SiO2@TiO2 Coating: Synthesis, Physical Characterization and Photocatalytic Evaluation. Coatings 2018, 8, 120. [Google Scholar] [CrossRef] [Green Version]




| Property | Results | NBR 16697-2018 Limits |
|---|---|---|
| Fire loss | ≤5% | ≤12% |
| Insoluble residue | ≤5% | ≤3.5% |
| Sulfate content | ≤4% | ≤4% |
| Chloride content | ≤0.1% | ≤0.1% |
| Set time | ≥45 min | ≥60 min |
| Expandability | ≤10 mm | ≤5 mm |
| Compressive strength—2 days | >37 MPa | >15 MPa |
| Compressive strength—28 days | ≥60 MPa | ≥40 MPa |
| Physical–Chemical Characteristics | Anatase | Rutile |
|---|---|---|
| Titanium dioxide concentration (%) | 99.10 | 96.3 |
| Evaporated material (%, 105 °C) | 0.20 | 0.26 |
| pH in aqueous solution | 7.7 | 7.3 |
| Oil absorption (g/100 g) | 24 | 19.2 |
| Fineness (% retained on a 45 μm sieve) | 0.03 | 0.05 |
| Cement Tile | Permeability (Qualitative) | Dimensional Variation (mm/m) | Water Absorption (%) | Flexural Tensile Strength (MPa) | ||
|---|---|---|---|---|---|---|
| X Axis | Y Axis | Not Saturated | Saturated | |||
| REF | Moisture | 2.49 | 2.90 | 7.17 | 4.27 | 4.06 |
| 5A | Not present | 2.02 | 1.94 | 6.08 | 4.15 | 4.04 |
| 10A | Not present | 2.12 | 2.90 | 6.11 | 3.91 | 3.27 |
| 5R | Not present | 3.02 | 2.86 | 6.67 | 4.25 | 4.12 |
| 10R | Not present | 2.84 | 3.19 | 6.52 | 3.42 | 3.16 |
| TiO2 | Variable | SS | DF | MS | Fcalc | p-Factor | Significative |
|---|---|---|---|---|---|---|---|
| Rutile | Staining | 15,714.69 | 1 | 15,714.69 | 7035.721 | 0.000000 | Yes |
| TiO2 (%) | 23.56 | 2 | 11.78 | 5.273 | 0.019635 | Yes | |
| Error | 31.27 | 14 | 2.23 | ||||
| Anatase | Staining | 15,234.43 | 1 | 15,234.43 | 1927.017 | 0.000000 | Yes |
| TiO2 (%) | 75.50 | 2 | 37.75 | 4.775 | 0.026243 | Yes | |
| Error | 110.68 | 14 | 7.91 |
| TiO2 | Variable | SS | DF | MS | Fcalc | p-Factor | Significative |
|---|---|---|---|---|---|---|---|
| Rutile | Staining | 7906.950 | 1 | 7906.950 | 922.301 | 0.000000 | Yes |
| TiO2 (%) | 67.882 | 2 | 33.941 | 3.959 | 0.043379 | Yes | |
| Error | 120.023 | 14 | 8.573 | ||||
| Anatase | Staining | 8534.28 | 1 | 8534.28 | 941.792 | 0.000000 | Yes |
| TiO2 (%) | 45.23 | 2 | 22.62 | 2.496 | 0.118295 | No | |
| Error | 126.86 | 14 | 9.06 |
| No Staining | Methylene Blue | Rhodamine B | |
|---|---|---|---|
| REF |  | 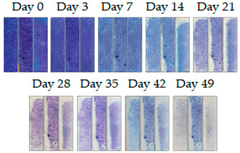 |  |
| 5A |  | 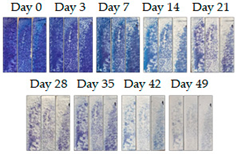 |  |
| 10A | 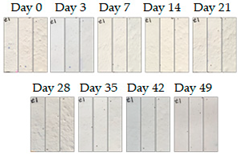 | 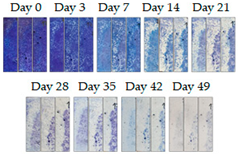 |  |
| 5R | 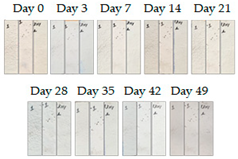 |  |  |
| 10R |  |  |  |
| TiO2 | Variable | SS | DGF | MS | F | p-Factor | Significative |
|---|---|---|---|---|---|---|---|
| Rutile | Staining | 157.8325 | 1 | 157.8325 | 10.24568 | 0.012596 | Yes |
| TiO2 (%) | 21.0350 | 2 | 10.5175 | 0.68274 | 0.532401 | No | |
| Error | 123.2384 | 8 | 15.4048 | ||||
| Anatase | Staining | 196.9920 | 1 | 196.9920 | 11.63158 | 0.009216 | Yes |
| TiO2 (%) | 10.8912 | 2 | 5.4456 | 0.32154 | 0.733982 | No | |
| Error | 135.4877 | 8 | 16.9360 |
| TiO2 | Variable | SS | DGF | MS | F | p-Factor | Significative |
|---|---|---|---|---|---|---|---|
| Rutile | Staining | 717.963 | 1 | 717.963 | 35.15020 | 0.000350 | Yes |
| TiO2 (%) | 122.690 | 2 | 61.345 | 3.00336 | 0.106418 | No | |
| Error | 163.405 | 8 | 20.426 | ||||
| Anatase | Staining | 626.8411 | 1 | 626.8411 | 40.55731 | 0.000216 | Yes |
| TiO2 (%) | 82.7245 | 2 | 41.3623 | 2.67618 | 0.128863 | No | |
| Error | 123.6455 | 8 | 15.4557 |
| REF | 5A | 10A | 5R | 10R | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 48 h | 0 h | 48 h | 0 h | 48 h | 0 h | 48 h | 0 h | 48 h | |
| No staining |  |  |  |  |  | |||||
| Methylene blue |  |  |  |  |  | |||||
| Rhodamine B |  | 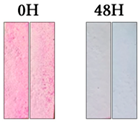 | 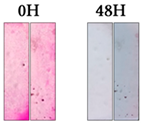 | 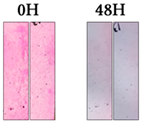 |  | |||||
| CP | Initial Reading (°) | Reading after Application of UVA Light (°) | ||
|---|---|---|---|---|
| Air Conditioned | Aged | Air Conditioned | Aged | |
| REF |  22.05 |  27.25 |  26.35 |  24.20 |
| 5R |  53.90 |  55.80 |  57.95 |  68.95 |
| 10R |  59.95 |  32.30 |  44.20 |  50.70 |
| 5A |  21.60 | 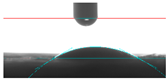 28.90 |  24.25 | 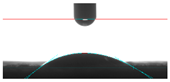 32.75 |
| 10A |  22.40 | 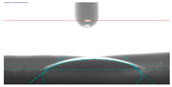 40.05 |  28.60 |  36.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qualharini, E.L.; Stolz, C.M.; Martini, M.; Polesello, E.; da Silva, C.R. Self-Cleaning Mortar Façades with Addition of Anatase and Rutile Titanium Dioxide for Cool Façades. Energies 2023, 16, 1874. https://doi.org/10.3390/en16041874
Qualharini EL, Stolz CM, Martini M, Polesello E, da Silva CR. Self-Cleaning Mortar Façades with Addition of Anatase and Rutile Titanium Dioxide for Cool Façades. Energies. 2023; 16(4):1874. https://doi.org/10.3390/en16041874
Chicago/Turabian StyleQualharini, Eduardo Linhares, Carina Mariane Stolz, Matheus Martini, Eduardo Polesello, and Clara Rocha da Silva. 2023. "Self-Cleaning Mortar Façades with Addition of Anatase and Rutile Titanium Dioxide for Cool Façades" Energies 16, no. 4: 1874. https://doi.org/10.3390/en16041874







