Absorption-Enhanced Methanol Steam Reforming for Low-Temperature Hydrogen Production with Carbon Capture †
Abstract
:1. Introduction
2. Method Description and Experiments
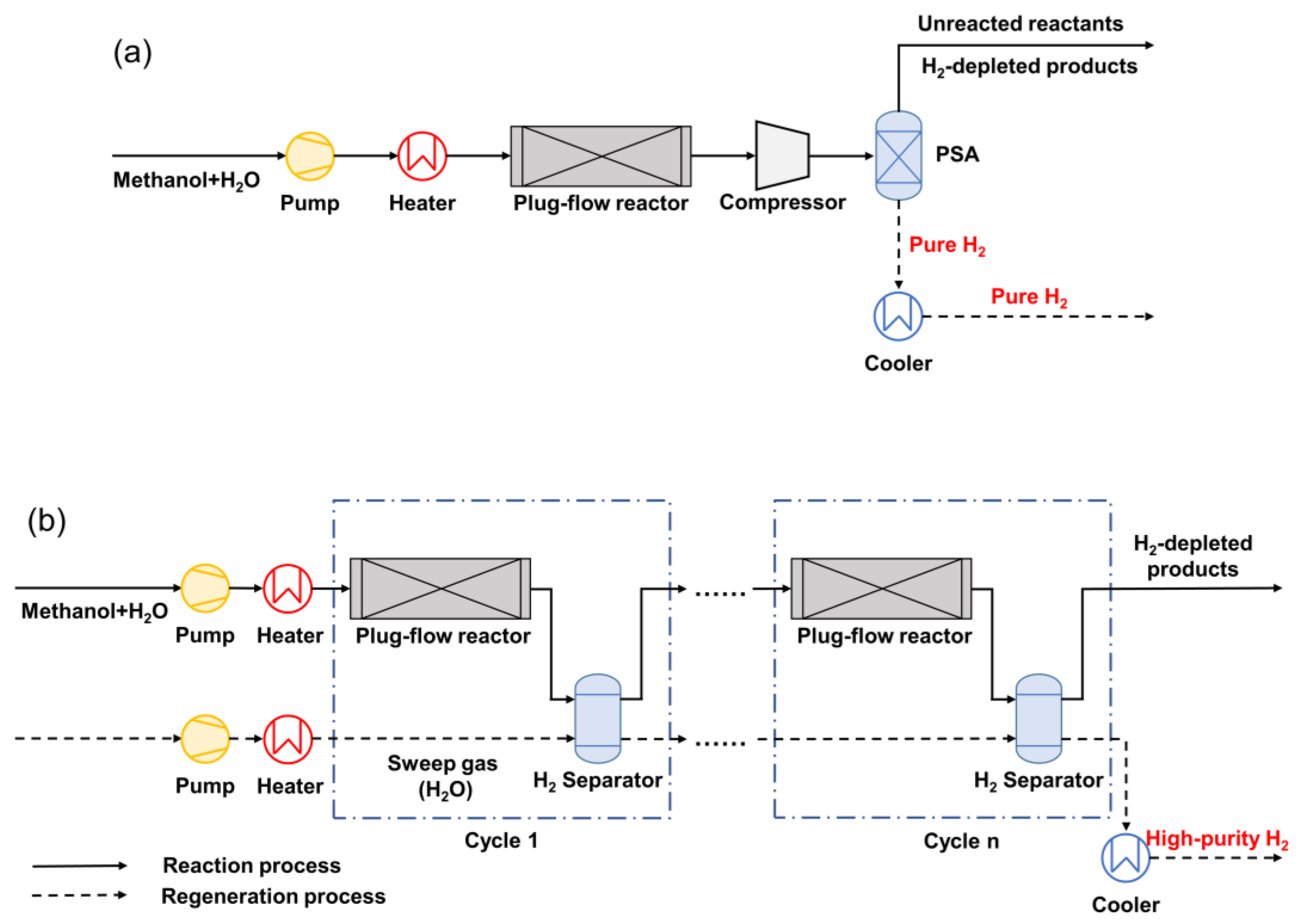
2.1. Method Description
2.2. Thermodynamic Analysis Method
2.3. Materials
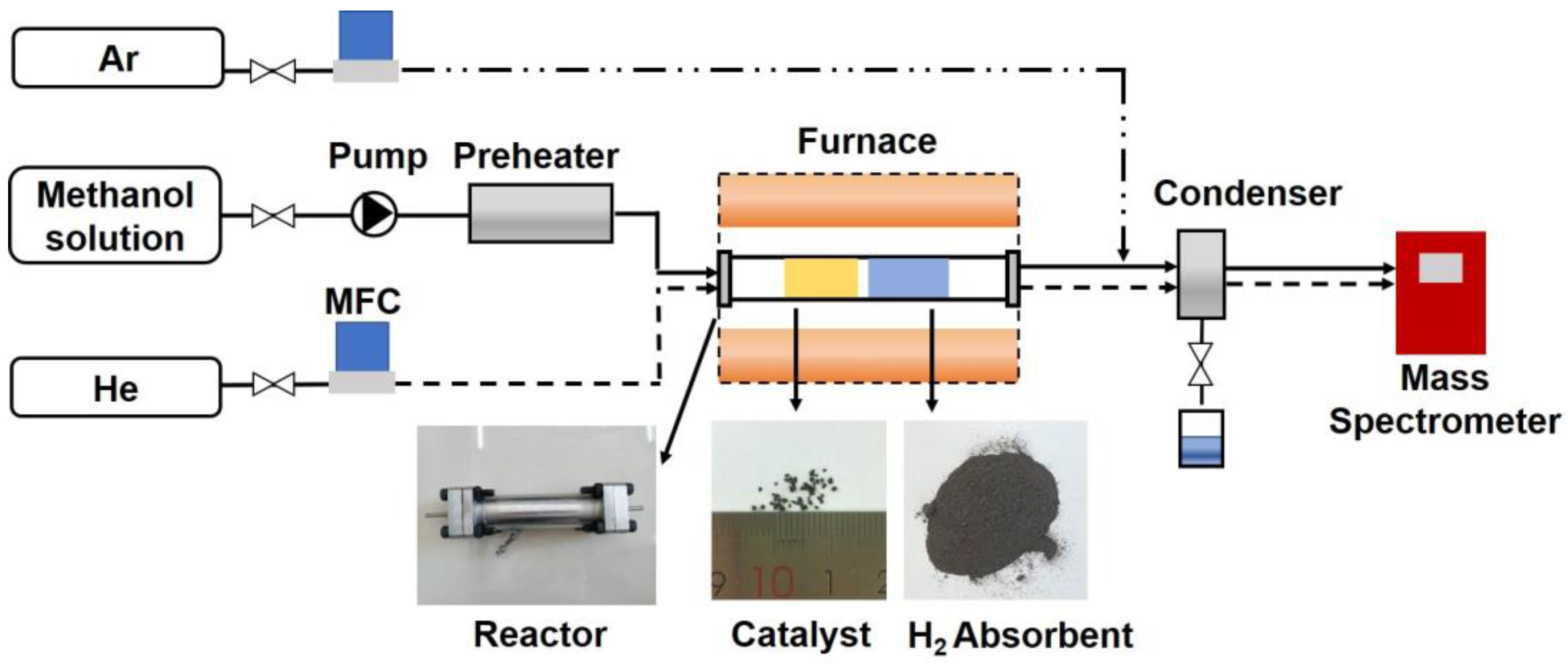
2.4. MSR and AE-MSR Experiments
3. Process Simulation
4. Results and Discussions
4.1. Thermodynamic Analysis
4.2. Experimental Results
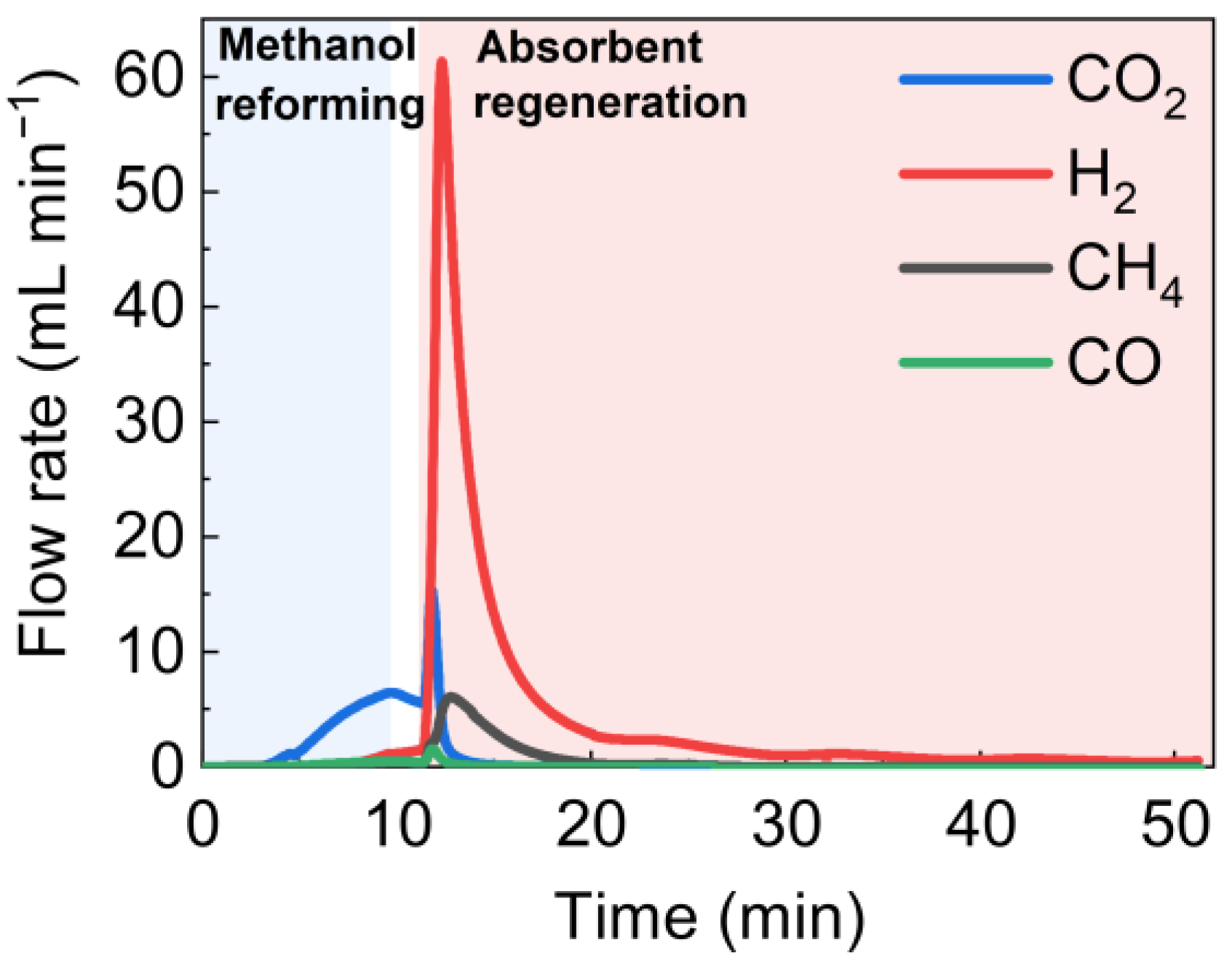
4.2.1. Single-Cycle Experiments
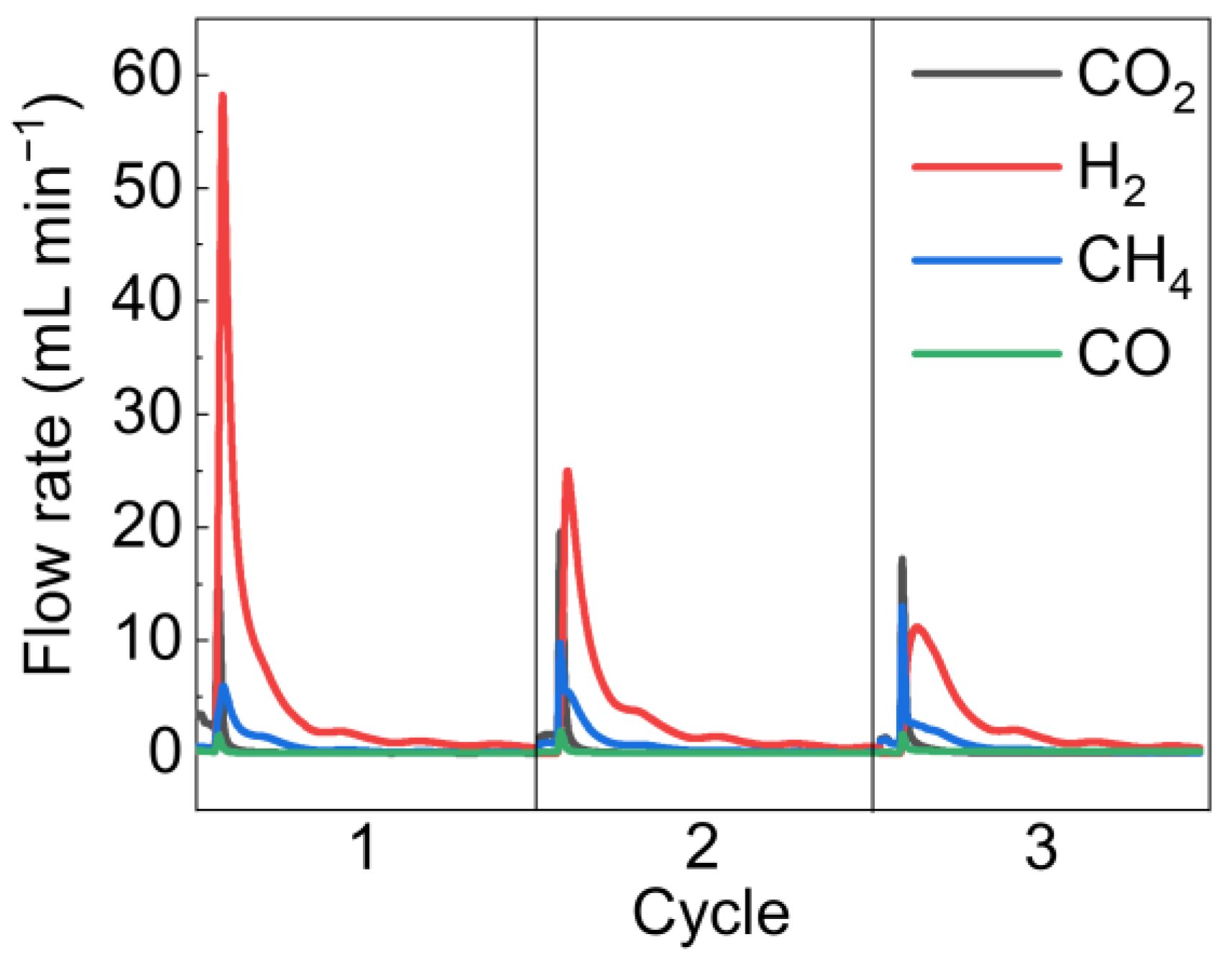
4.2.2. Multi-Cycle Experiments
4.3. Simulation Results
4.3.1. Model Validation
4.3.2. Effect of the Number of Cycles
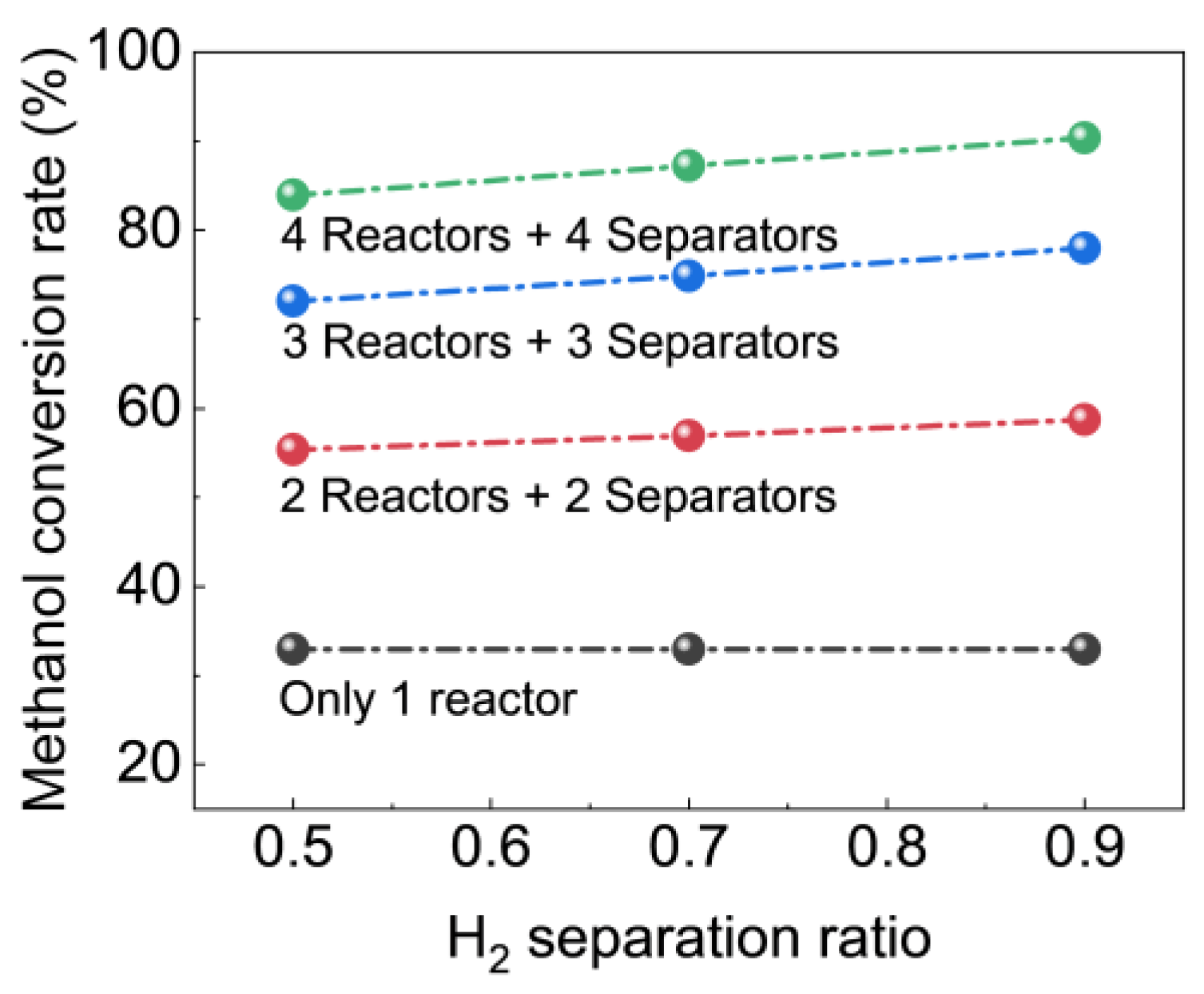
4.3.3. Effect of the H2 Separation Ratio
4.4. Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lewis, N.S. A prospective on energy and environmental science. Energy Environ. Sci. 2019, 12, 16–18. [Google Scholar] [CrossRef]
- Rosen, M.A.; Koohi-Fayegh, S. The prospects for hydrogen as an energy carrier: An overview of hydrogen energy and hydrogen energy systems. Energy Ecol. Environ. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Faye, O.; Szpunar, J.; Eduok, U. A critical review on the current technologies for the generation, storage, and transportation of hydrogen. Int. J. Hydrogen Energy 2022, 47, 13771–13802. [Google Scholar] [CrossRef]
- Garcia, G.; Arriola, E.; Chen, W.-H.; De Luna, M.D. A comprehensive review of hydrogen production from methanol thermochemical conversion for sustainability. Energy 2021, 217, 119384. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, Z.-Q. Hydrogen generation from methanol reforming for fuel cell applications: A review. J. Cent. South Univ. 2020, 27, 1074–1103. [Google Scholar] [CrossRef]
- Kaftan, A.; Kusche, M.; Laurin, M.; Wasserscheid, P.; Libuda, J. KOH-promoted Pt/Al2O3 catalysts for water gas shift and methanol steam reforming: An operando DRIFTS-MS study. Appl. Catal. B Environ. 2017, 201, 169–181. [Google Scholar] [CrossRef]
- Shih, C.F.; Zhang, T.; Li, J.; Bai, C. Powering the Future with Liquid Sunshine. Joule 2018, 2, 1925–1949. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Tian, Z.; Zhao, Y.; Hao, Y. Experimental investigation of steam reforming of methanol over La2CuO4/CuZnAl-oxides nanocatalysts. Appl. Energy 2019, 254, 113022. [Google Scholar] [CrossRef]
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol-steam reforming on Cu/ZnO/Al2O3 catalysts. Part 2. A comprehensive kinetic model. Appl. Catal. A Gen. 1999, 179, 31–49. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Zeng, C.; Liu, Q.; Hu, H.; Huang, Q.; Chen, X. Preparation of CuZnZrAl catalysts by coprecipitation-ammonia method for methanol steam reforming and the effect of promoters Y and Ce. Mol. Catal. 2023, 537, 112887. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Wang, J.-H.; Shen, C.-C.; Yeh, C.-T. Promotion of a copper–zinc catalyst with rare earth for the steam reforming of methanol at low temperatures. J. Catal. 2011, 279, 241–245. [Google Scholar] [CrossRef]
- Real, D.; Dumanyan, I.; Hotz, N. Renewable hydrogen production by solar-powered methanol reforming. Int. J. Hydrogen Energy 2016, 41, 11914–11924. [Google Scholar] [CrossRef]
- Thattarathody, R.; Artoul, M.; Digilov, R.M.; Sheintuch, M. Pressure, Diffusion, and S/M Ratio Effects in Methanol Steam Reforming Kinetics. Ind. Eng. Chem. Res. 2018, 57, 3175–3186. [Google Scholar] [CrossRef]
- Amphlet, J.C.; Crebe, K.A.M.; Davis, J.M.; Mann, R.F.; Peppley, B.A.; Stokes, D.M. Hydrogen Production by Steam Reforming of Methanol for Polymer Electrolyte Fuel Cells. Int. J. Hydrogen Energy 1994, 19, 131–137. [Google Scholar] [CrossRef]
- Kang, J.; Song, Y.; Kim, T.; Kim, S. Recent trends in the development of reactor systems for hydrogen production via methanol steam reforming. Int. J. Hydrogen Energy 2022, 47, 3587–3610. [Google Scholar] [CrossRef]
- Hosseini Abbandanak, M.; Taghizadeh, M.; Fallah, N. High-purity hydrogen production by sorption-enhanced methanol steam reforming over a combination of Cu–Zn–CeO2–ZrO2/MCM-41 catalyst and (Li–Na–K) NO3·MgO adsorbent. Int. J. Hydrogen Energy 2021, 46, 7099–7112. [Google Scholar] [CrossRef]
- Li, H.; Tian, H.; Chen, S.; Sun, Z.; Liu, T.; Liu, R.; Assabumrungrat, S.; Saupsor, J.; Mu, R.; Pei, C.; et al. Sorption enhanced steam reforming of methanol for high-purity hydrogen production over Cu-MgO/Al2O3 bifunctional catalysts. Appl. Catal. B Environ. 2020, 276, 119052. [Google Scholar] [CrossRef]
- Lytkina, A.A.; Orekhova, N.V.; Ermilova, M.M.; Petriev, I.S.; Baryshev, M.G.; Yaroslavtsev, A.B. Ru Rh based catalysts for hydrogen production via methanol steam reforming in conventional and membrane reactors. Int. J. Hydrogen Energy 2019, 44, 13310–13322. [Google Scholar] [CrossRef]
- Basile, A.; Parmaliana, A.; Tosti, S.; Iulianelli, A.; Gallucci, F.; Espro, C.; Spooren, J. Hydrogen production by methanol steam reforming carried out in membrane reactor on Cu/Zn/Mg-based catalyst. Catal. Today 2008, 137, 17–22. [Google Scholar] [CrossRef]
- Gallucci, F.; Basile, A.; Tosti, S.; Iulianelli, A.; Drioli, E. Methanol and ethanol steam reforming in membrane reactors: An experimental study. Int. J. Hydrogen Energy 2007, 32, 1201–1210. [Google Scholar] [CrossRef]
- Basile, A.; Tosti, S.; Capannelli, G.; Vitulli, G.; Iulianelli, A.; Gallucci, F.; Drioli, E. Co-current and counter-current modes for methanol steam reforming membrane reactor: Experimental study. Catal. Today 2006, 118, 237–245. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Harasi, J.N.; Amiri, T.Y.; Basile, A.; Iulianelli, A. Methanol steam reforming for hydrogen generation: A comparative modeling study between silica and Pd-based membrane reactors by CFD method. Fuel Process. Technol. 2020, 199, 106273. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, D.; Zhou, J.; Wu, Y.; Zhao, D.; Wang, T.; Qiu, J. Process Intensification of the Hydrogen Production Reaction Using a Carbon Membrane Reactor: Kinetics Analysis. Energy Technol. 2017, 5, 1990–1997. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Aghaeinejad-Meybodi, A.; Vaezi, M.J.; Gholizadeh, A.; Abdi, M.A.; Babaluo, A.A.; Haghighi, M.; Basile, A. Hydrogen production via silica membrane reactor during the methanol steam reforming process: Experimental study. RSC Adv. 2015, 5, 95823–95832. [Google Scholar] [CrossRef]
- Sun, C.; Zheng, X.; Bai, B. Hydrogen purification using nanoporous graphene membranes and its economic analysis. Chem. Eng. Sci. 2019, 208, 115141. [Google Scholar] [CrossRef]
- Amandusson, H.; Ekedahl, L.G.; Dannetun, H. The effect of CO and O2 on hydrogen permeation through a palladium membrane. Appl. Surf. Sci. 2000, 153, 259–267. [Google Scholar] [CrossRef]
- Woeste, A.-L.; Balcerzak, M.; Urbanczyk, R.; Felderhoff, M. Mg-Based System for H-2 Sorption from CH4/H2 Gas Mixture. Energy Technol. 2021, 9, 2001079. [Google Scholar] [CrossRef]
- Moon, D.-K.; Lee, D.-G.; Lee, C.-H. H2 pressure swing adsorption for high pressure syngas from an integrated gasification combined cycle with a carbon capture process. Appl. Energy 2016, 183, 760–774. [Google Scholar] [CrossRef]
- Joubert, J.-M.; Paul-Boncour, V.; Cuevas, F.; Zhang, J.; Latroche, M. LaNi5 related AB5 compounds: Structure, properties and applications. J. Alloys Compd. 2021, 862, 158163. [Google Scholar] [CrossRef]
- Borzone, E.M.; Baruj, A.; Meyer, G.O. Design and operation of a hydrogen purification prototype based on metallic hydrides. J. Alloys Compd. 2017, 695, 2190–2198. [Google Scholar] [CrossRef]
- Miura, S.; Fujisawa, A.; Tomekawa, S.; Taniguchi, Y.; Hanada, N.; Ishida, M. A hydrogen purification and storage system using CO adsorbent and metal hydride. J. Alloys Compd. 2013, 580, S414–S417. [Google Scholar] [CrossRef]
- Hao, P.; Wang, X.; Li, S.; Zhang, H.; Khalkhali, M.; Shi, Y.; Cai, N. Warm hydrogen direct adsorptive separation and purification with highly CO/H2S-tolerant rare earth alloys. Appl. Energy Combust. Sci. 2020, 1–4, 100004. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Hao, Y. Absorption-enhanced methanol steam reforming for low-temperature hydrogen production with carbon capture. In Proceedings of the 8th Applied Energy Symposium-CUE2022, Matsue, Japan, 24–27 November 2022. [Google Scholar]
- Wu, X.; Wu, S. Production of high-purity hydrogen by sorption-enhanced steam reforming process of methanol. J. Energy Chem. 2015, 24, 315–321. [Google Scholar] [CrossRef]
- Katiyar, N.; Kumar, S.; Kumar, S. Thermodynamic Analysis for Quantifying Fuel Cell Grade H2 Production by Methanol Steam Reforming. Chem. Eng. Technol. 2013, 36, 581–590. [Google Scholar] [CrossRef]
- Ewing, M.B.; Lilley, T.H.; Olofsson, G.M.; Ratzsch, M.T.; Somsen, G. Standard quantities in chemical thermodynamics. Fugacities, activities and equilibrium constants for pure and mixed phases. Pure Appl. Chem. 1994, 66, 533–552. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Abdollahzadeh, M.; Boaventura, M.; Mendes, A. H2 production with low carbon content via MSR in packed bed membrane reactors for high-temperature polymeric electrolyte membrane fuel cell. Appl. Energy 2017, 188, 409–419. [Google Scholar] [CrossRef]
- Al-malah, K.I.M. Aspen Plus®: Chemical Engineering Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 15–23. [Google Scholar]
- Xu, X.; Shuai, K.; Xu, B. Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen. Catalysts 2017, 7, 183. [Google Scholar] [CrossRef]
- Herdem, M.S.; Sinaki, M.Y.; Farhad, S.; Hamdullahpur, F. An overview of the methanol reforming process: Comparison of fuels, catalysts, reformers, and systems. Int. J. Energy Res. 2019, 43, 5076–5105. [Google Scholar] [CrossRef]
- Zhong, D.; Ouyang, L.; Liu, J.; Wang, H.; Jia, Y.; Zhu, M. Metallic Ni nanocatalyst in situ formed from LaNi5H5 toward efficient CO2 methanation. Int. J. Hydrogen Energy 2019, 44, 29068–29074. [Google Scholar] [CrossRef]
- Ando, H.; Fujiwara, M.; Matsumura, Y.; Miyamura, H.; Souma, Y. Methanation of carbon dioxide over LaNi4X type catalysts. Energy Convers. Manag. 1995, 36, 653–656. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Xu, Z.; Li, M. Thermodynamic performance analysis of a fuel cell trigeneration system integrated with solar-assisted methanol reforming. Energy Convers. Manag. 2017, 150, 81–89. [Google Scholar] [CrossRef]
- Wang, H.; Yang, R.; Wang, B.; Wei, Z.; Kong, H.; Lu, X.; Jin, J. Thermodynamic performance of solar-driven methanol steam reforming system for carbon capture and high-purity hydrogen production. Appl. Therm. Eng. 2022, 209, 118280. [Google Scholar] [CrossRef]
- Evangelisti, L.; De Lieto Vollaro, R.; Asdrubali, F. Latest advances on solar thermal collectors: A comprehensive review. Renew. Sustain. Energy Rev. 2019, 114, 109318. [Google Scholar] [CrossRef]
- Johnson, I.; Choate, W.T.; Davidson, A. Waste Heat Recovery: Technology and Opportunities in the U.S. Industry; BCS, Inc.: Laurel, MD, USA, 2008. [Google Scholar]
- Dat Vo, N.; Kang, J.-H.; Oh, M.; Lee, C.-H. Dynamic model and performance of an integrated sorption-enhanced steam methane reforming process with separators for the simultaneous blue H2 production and CO2 capture. Chem. Eng. J. 2021, 423, 130044. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, Y.; Yang, L.; Du, X. Kinetics for hydrogen production by methanol steam reforming in fluidized bed reactor. Sci. Bull. 2016, 61, 401–405. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yang, L.; Hao, Y. Absorption-Enhanced Methanol Steam Reforming for Low-Temperature Hydrogen Production with Carbon Capture. Energies 2023, 16, 7134. https://doi.org/10.3390/en16207134
Li X, Yang L, Hao Y. Absorption-Enhanced Methanol Steam Reforming for Low-Temperature Hydrogen Production with Carbon Capture. Energies. 2023; 16(20):7134. https://doi.org/10.3390/en16207134
Chicago/Turabian StyleLi, Xiao, Lingzhi Yang, and Yong Hao. 2023. "Absorption-Enhanced Methanol Steam Reforming for Low-Temperature Hydrogen Production with Carbon Capture" Energies 16, no. 20: 7134. https://doi.org/10.3390/en16207134



