Reverse Water Gas Shift versus Carbon Dioxide Electro-Reduction: The Reaction Pathway Responsible for Carbon Monoxide Production in Solid Oxide Co-Electrolysis Cells
Abstract
:1. Introduction
2. Theoretical Formulation and Experimental Comparison
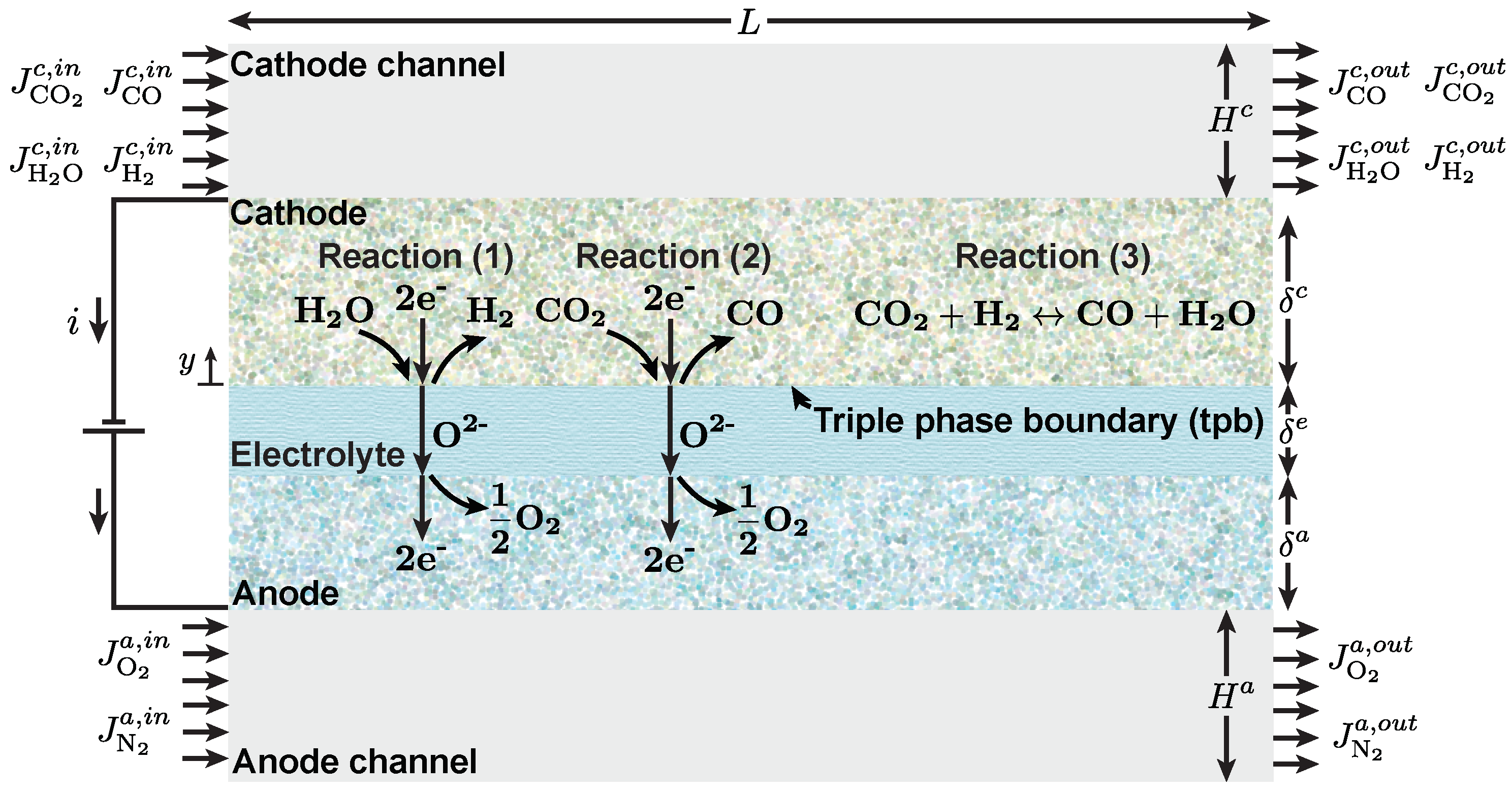
2.1. Carbon Monoxide Reaction Pathways
2.2. Thermodynamics of Solid Oxide Co-Electrolysis
2.3. Electrochemical Kinetics and Mass Transport
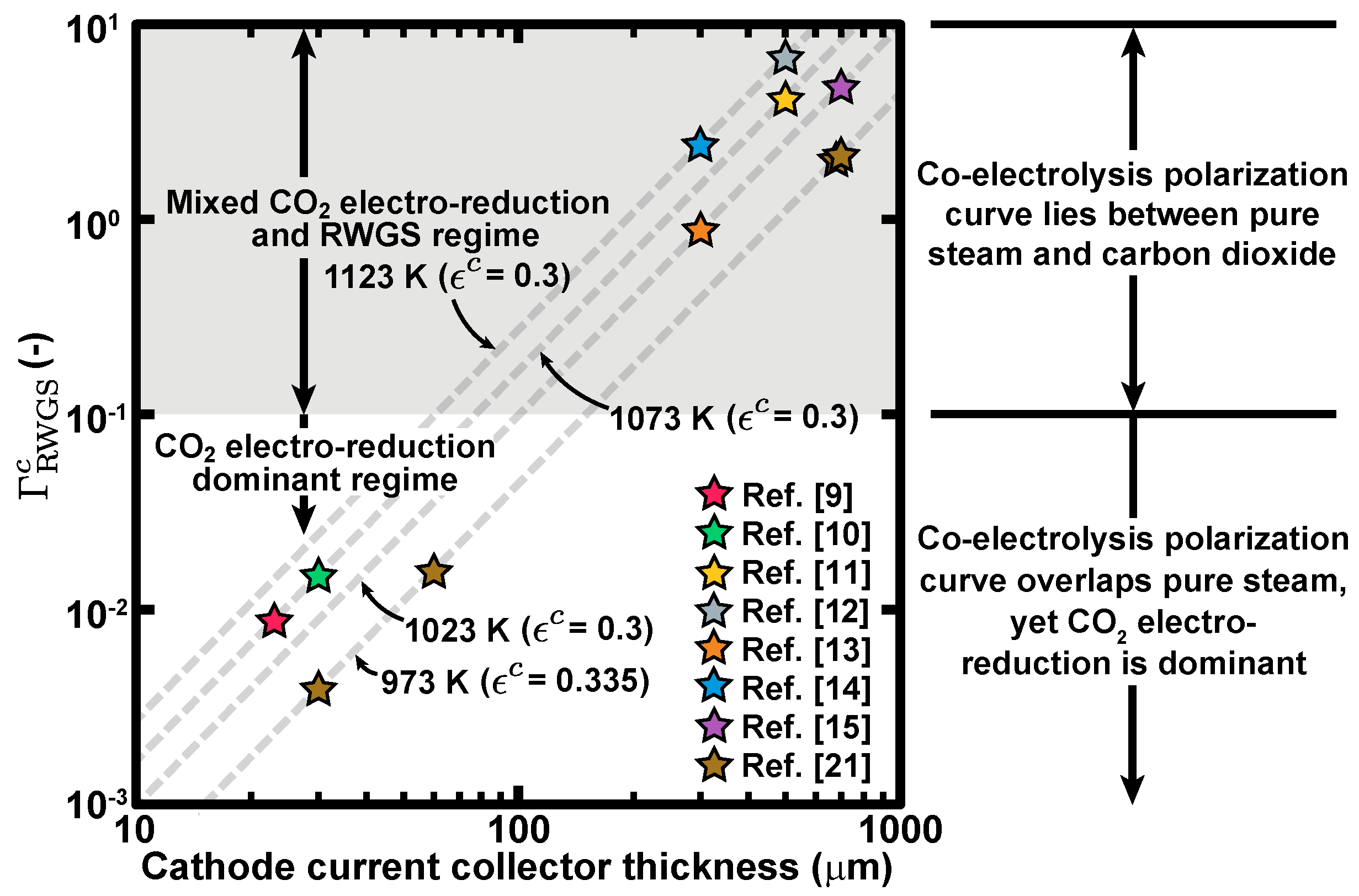
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, F.; Wang, T.; Li, J.; Wei, T.; Ye, Z.; Dong, D.; Chen, B.; Ling, Y.; Shao, Z. Elevated-temperature bio-ethanol-assisted water electrolysis for efficient hydrogen production. Chem. Eng. J. 2022, 434, 134699. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tong, X.; Hauch, A.; Sun, X.; Yang, Z.; Peng, S.; Chen, M. Study of solid oxide electrolysis cells operated in potentiostatic mode: Effect of operating temperature on durability. Chem. Eng. J. 2021, 417, 129260. [Google Scholar] [CrossRef]
- Kamkeng, A.D.; Wang, M. Long-term performance prediction of solid oxide electrolysis cell (SOEC) for CO2/H2O co-electrolysis considering structural degradation through modelling and simulation. Chem. Eng. J. 2022, 429, 132158. [Google Scholar] [CrossRef]
- Yu, S.B.; Lee, S.H.; Mehran, M.T.; Hong, J.E.; Lee, J.W.; Lee, S.B.; Park, S.J.; Song, R.H.; Shim, J.H.; Shul, Y.G.; et al. Syngas production in high performing tubular solid oxide cells by using high-temperature H2O/CO2 co-electrolysis. Chem. Eng. J. 2018, 335, 41–51. [Google Scholar] [CrossRef]
- Technology Roadmap: Energy and GHG Reductions in the Chemical Industry via Catalytic Processes. Available online: https://icca-chem.org/wp-content/uploads/2020/05/Technology-Roadmap.pdf (accessed on 31 October 2022).
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Wang, G.; Bao, X. Co-electrolysis of CO2 and H2O in high-temperature solid oxide electrolysis cells: Recent advance in cathodes. J. Energy Chem. 2017, 26, 839–853. [Google Scholar] [CrossRef] [Green Version]
- Stoots, C.; O’Brien, J.; Hartvigsen, J. Results of recent high temperature coelectrolysis studies at the Idaho National Laboratory. Int. J. Hydrogen Energy 2009, 34, 4208–4215. [Google Scholar] [CrossRef] [Green Version]
- Kim-Lohsoontorn, P.; Bae, J. Electrochemical performance of solid oxide electrolysis cell electrodes under high-temperature coelectrolysis of steam and carbon dioxide. J. Power Sources 2011, 196, 7161–7168. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Y.; Zhu, J.; Han, M.; Sun, K.; Sun, Z. Investigation on the reaction mechanism of solid oxide co-electrolysis with different inlet mixtures based on the comparison of CO2 electrolysis and H2O electrolysis. Energy Convers. Manag. 2023, 277, 116621. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Y.; Yu, B.; Wang, J.; Chen, J. Electrochemical characterization and mechanism analysis of high temperature Co-electrolysis of CO2 and H2O in a solid oxide electrolysis cell. Int. J. Hydrogen Energy 2017, 42, 29911–29920. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Knibbe, R.; Mogensen, M. Co-Electrolysis of Steam and Carbon Dioxide in Solid Oxide Cells. J. Electrochem. Soc. 2012, 159, F482. [Google Scholar] [CrossRef] [Green Version]
- Graves, C.; Ebbesen, S.D.; Mogensen, M. Co-electrolysis of CO2 and H2O in solid oxide cells: Performance and durability. Solid State Ion. 2011, 192, 398–403. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Shi, Y.; Cai, N. Performance and methane production characteristics of H2O–CO2 co-electrolysis in solid oxide electrolysis cells. Int. J. Hydrogen Energy 2013, 38, 11104–11109. [Google Scholar] [CrossRef]
- Aicart, J.; Laurencin, J.; Petitjean, M.; Dessemond, L. Experimental Validation of Two-Dimensional H2O and CO2 Co-Electrolysis Modeling. Fuel Cells 2014, 14, 430–447. [Google Scholar] [CrossRef]
- Luo, Y.; Shi, Y.; Li, W.; Cai, N. Comprehensive modeling of tubular solid oxide electrolysis cell for co-electrolysis of steam and carbon dioxide. Energy 2014, 70, 420–434. [Google Scholar] [CrossRef]
- Ni, M. 2D thermal modeling of a solid oxide electrolyzer cell (SOEC) for syngas production by H2O/CO2 co-electrolysis. Int. J. Hydrogen Energy 2012, 37, 6389–6399. [Google Scholar] [CrossRef] [Green Version]
- Ni, M. An electrochemical model for syngas production by co-electrolysis of H2O and CO2. J. Power Sources 2012, 202, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Shi, Y.; Luo, Y.; Cai, N. Elementary reaction modeling of solid oxide electrolysis cells: Main zones for heterogeneous chemical/electrochemical reactions. J. Power Sources 2015, 273, 1–13. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Luo, Y.; Cai, N. Elementary reaction modeling of CO2/H2O co-electrolysis cell considering effects of cathode thickness. J. Power Sources 2013, 243, 118–130. [Google Scholar] [CrossRef]
- Xu, H.; Chen, B.; Irvine, J.; Ni, M. Modeling of CH4-assisted SOEC for H2O/CO2 co-electrolysis. Int. J. Hydrogen Energy 2016, 41, 21839–21849. [Google Scholar] [CrossRef] [Green Version]
- Menon, V.; Fu, Q.; Janardhanan, V.M.; Deutschmann, O. A model-based understanding of solid-oxide electrolysis cells (SOECs) for syngas production by H2O/CO2 co-electrolysis. J. Power Sources 2015, 274, 768–781. [Google Scholar] [CrossRef]
- Beale, S.B.; Andersson, M.; Boigues-Muñoz, C.; Frandsen, H.L.; Lin, Z.; McPhail, S.J.; Ni, M.; Sundén, B.; Weber, A.; Weber, A.Z. Continuum scale modelling and complementary experimentation of solid oxide cells. Prog. Energy Combust. Sci. 2021, 85, 100902. [Google Scholar] [CrossRef]
- Ni, M. Modeling of a solid oxide electrolysis cell for carbon dioxide electrolysis. Chem. Eng. J. 2010, 164, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Haberman, B.; Young, J. Three-dimensional simulation of chemically reacting gas flows in the porous support structure of an integrated-planar solid oxide fuel cell. Int. J. Heat Mass Transf. 2004, 47, 3617–3629. [Google Scholar] [CrossRef]
- Nielsen, A.S.; Peppley, B.A.; Burheim, O.S. Tuning transport mechanisms in fuel-assisted solid oxide electrolysis cells for enhanced performance and product selectivity: Thermodynamic and kinetic modeling. Chem. Eng. J. 2023, 452, 139079. [Google Scholar] [CrossRef]
- Nielsen, A.S.; Peppley, B.A.; Burheim, O.S. Controlling the contribution of transport mechanisms in solid oxide co-electrolysis cells to improve product selectivity and performance: A theoretical framework. Appl. Energy 2023, 344, 121301. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Yasuda, I. Electrochemical Oxidation of H2 and CO in a H2-H2O-CO-CO2 System at the Interface of a Ni-YSZ Cermet Electrode and YSZ Electrolyte. J. Electrochem. Soc. 2000, 147, 1630. [Google Scholar] [CrossRef]
- Sukeshini, A.M.; Habibzadeh, B.; Becker, B.P.; Stoltz, C.A.; Eichhorn, B.W.; Jackson, G.S. Electrochemical Oxidation of H2, CO, and CO/H2 Mixtures on Patterned Ni Anodes on YSZ Electrolytes. J. Electrochem. Soc. 2006, 153, A705. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, A.S.; Peppley, B.A.; Burheim, O.S. Reverse Water Gas Shift versus Carbon Dioxide Electro-Reduction: The Reaction Pathway Responsible for Carbon Monoxide Production in Solid Oxide Co-Electrolysis Cells. Energies 2023, 16, 5781. https://doi.org/10.3390/en16155781
Nielsen AS, Peppley BA, Burheim OS. Reverse Water Gas Shift versus Carbon Dioxide Electro-Reduction: The Reaction Pathway Responsible for Carbon Monoxide Production in Solid Oxide Co-Electrolysis Cells. Energies. 2023; 16(15):5781. https://doi.org/10.3390/en16155781
Chicago/Turabian StyleNielsen, Anders S., Brant A. Peppley, and Odne S. Burheim. 2023. "Reverse Water Gas Shift versus Carbon Dioxide Electro-Reduction: The Reaction Pathway Responsible for Carbon Monoxide Production in Solid Oxide Co-Electrolysis Cells" Energies 16, no. 15: 5781. https://doi.org/10.3390/en16155781




