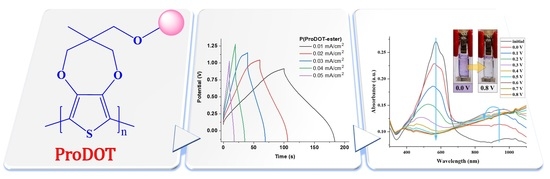
ProDOT-Based Polymers: From Energy Storage to Smart Window Applications
Abstract
:1. Introduction
2. Experimental
2.1. Starting Materials
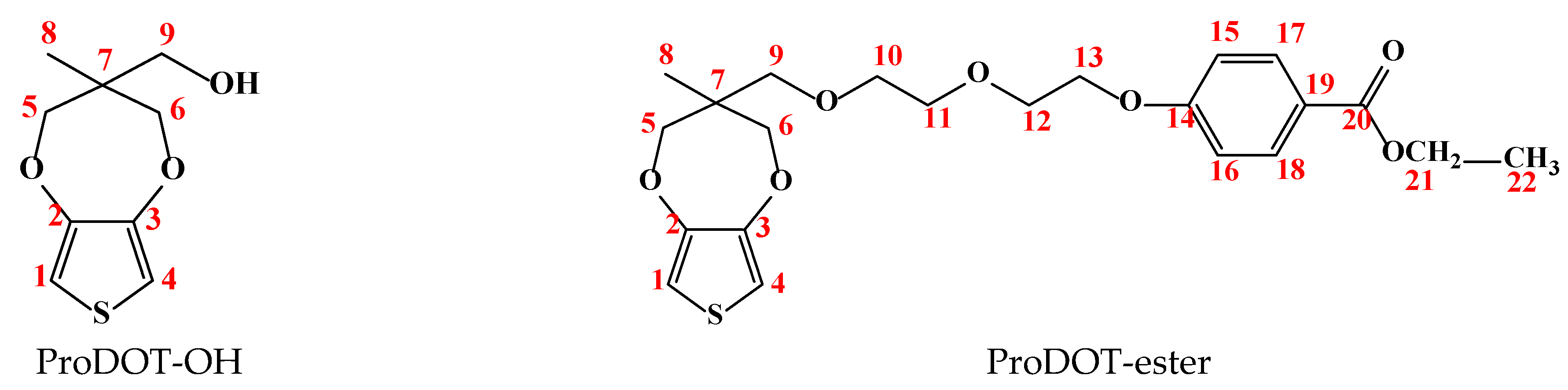
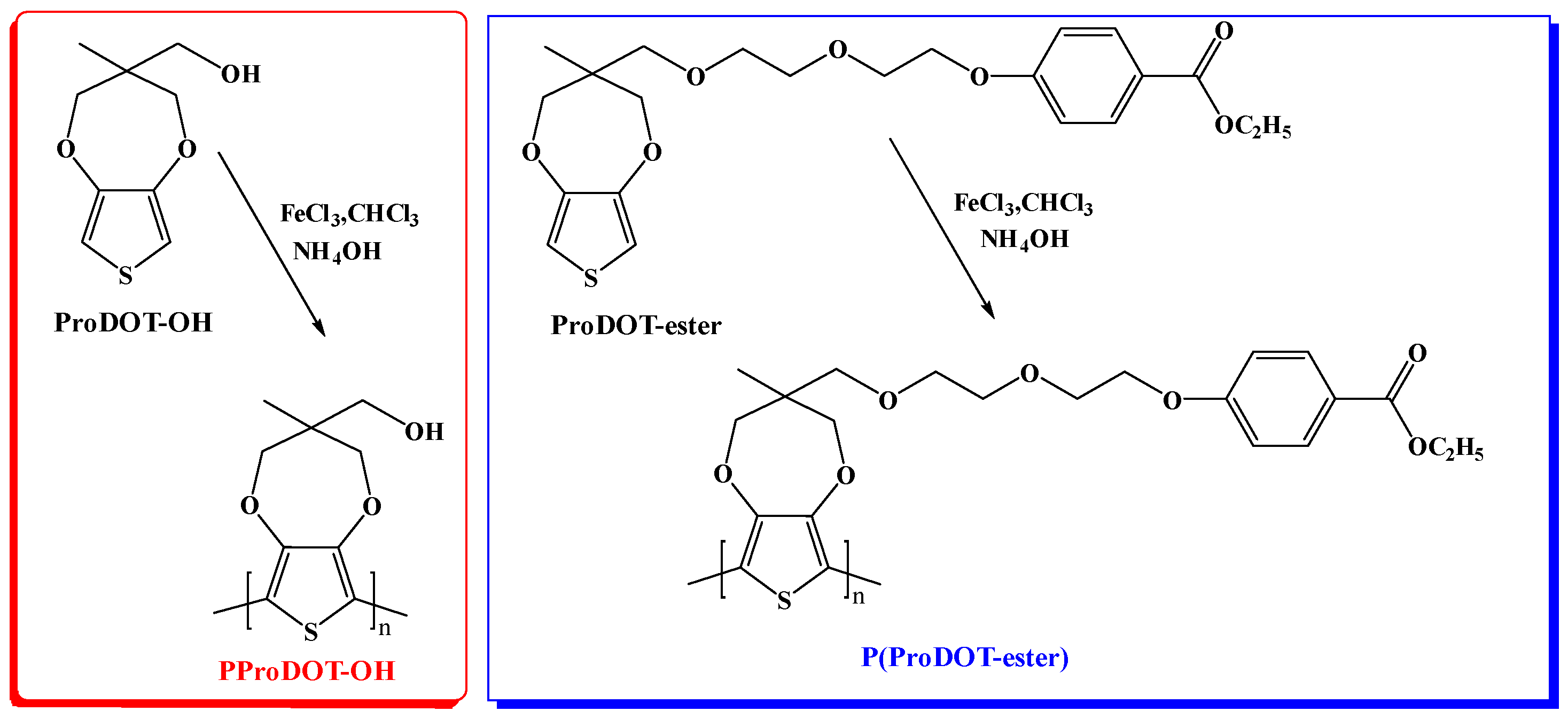
2.2. Synthesis of Monomers
2.3. Synthesis of Polymers
2.4. Preparation of Polymer Films
2.5. Measurements
3. Results and Discussions
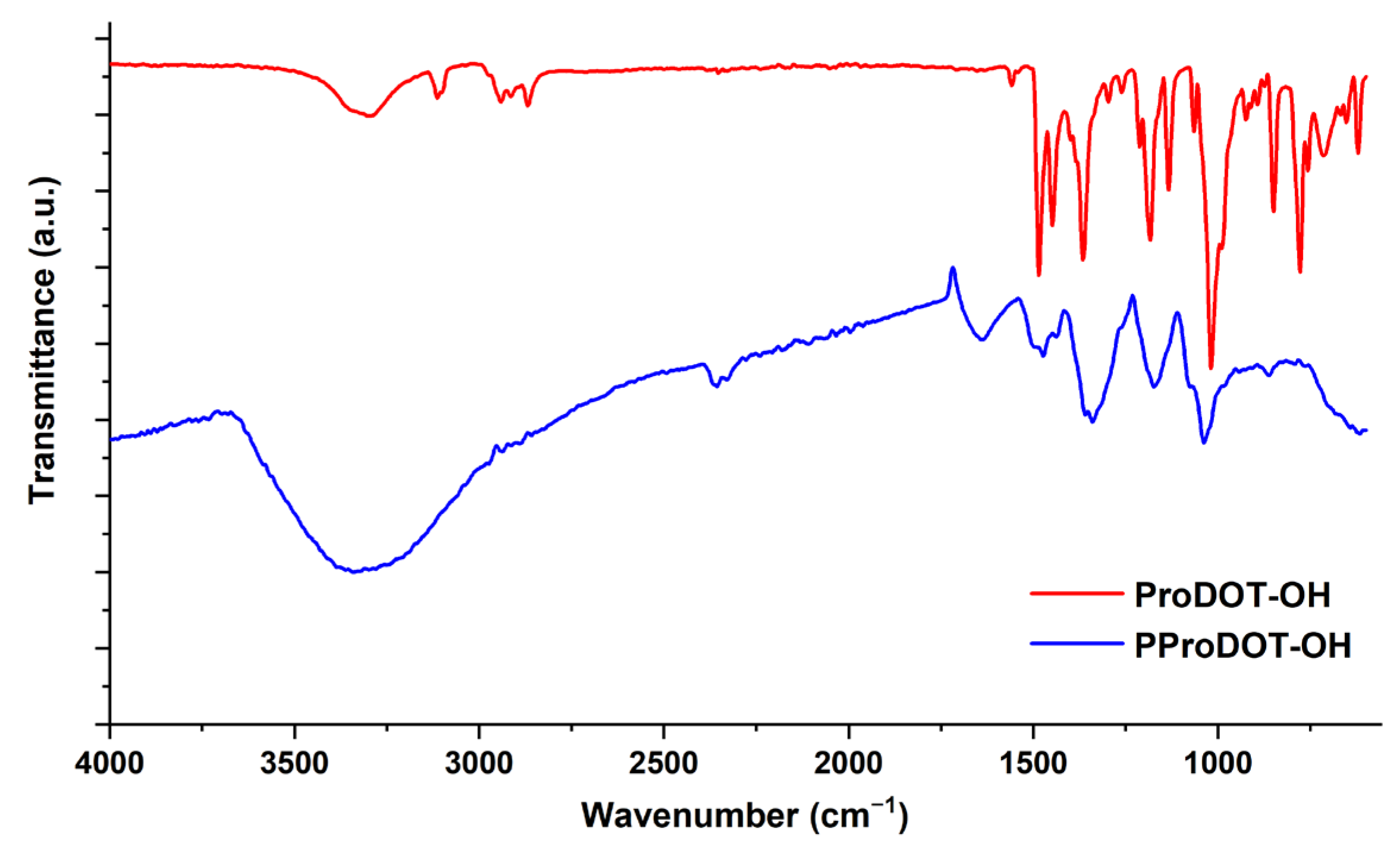
3.1. Structural Characterization
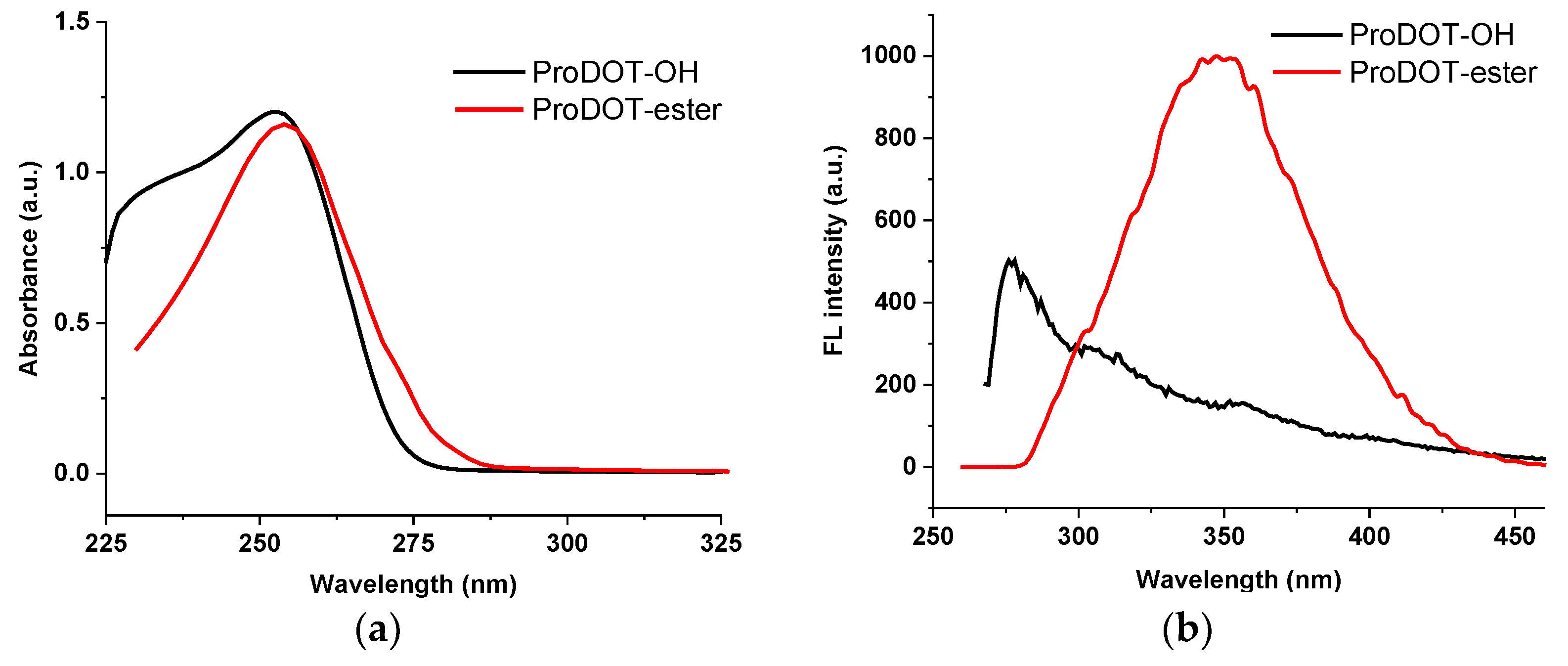
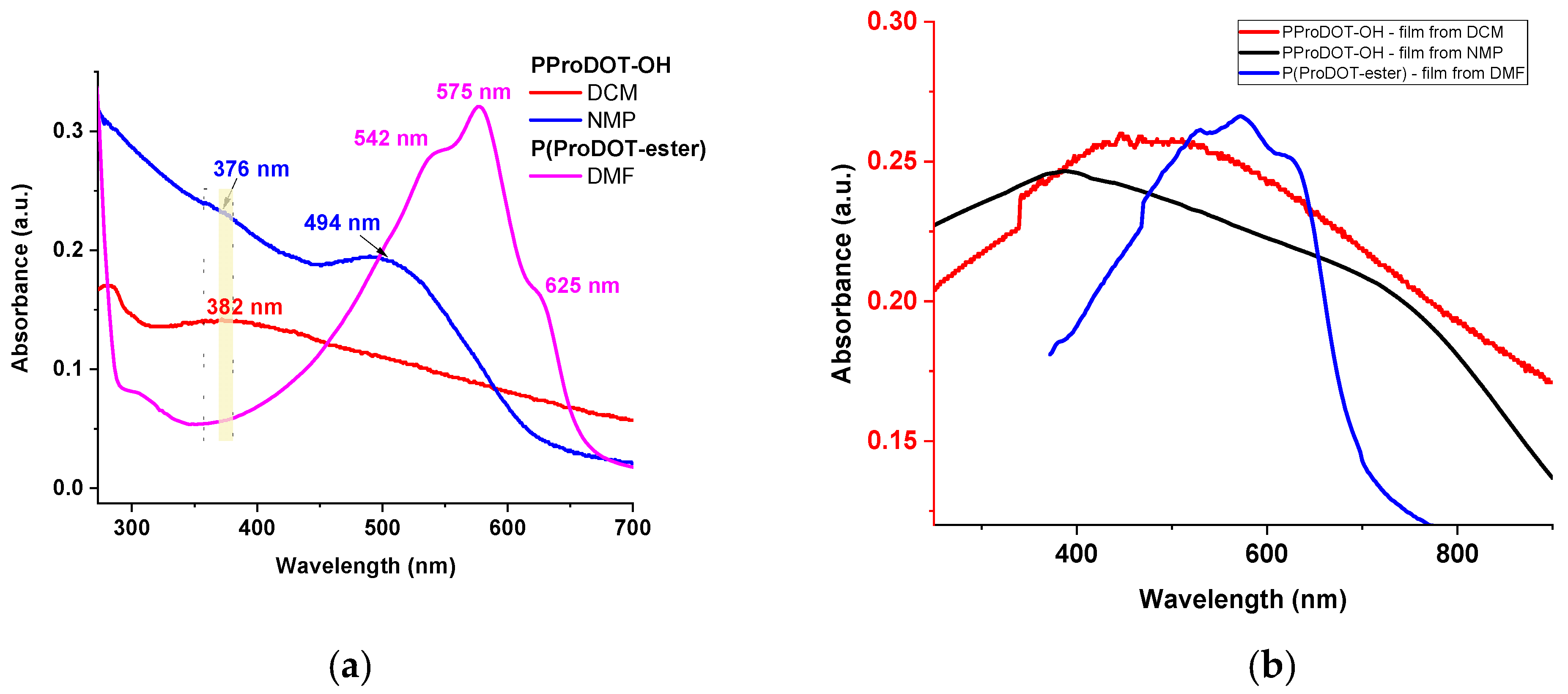
3.2. Optical Properties
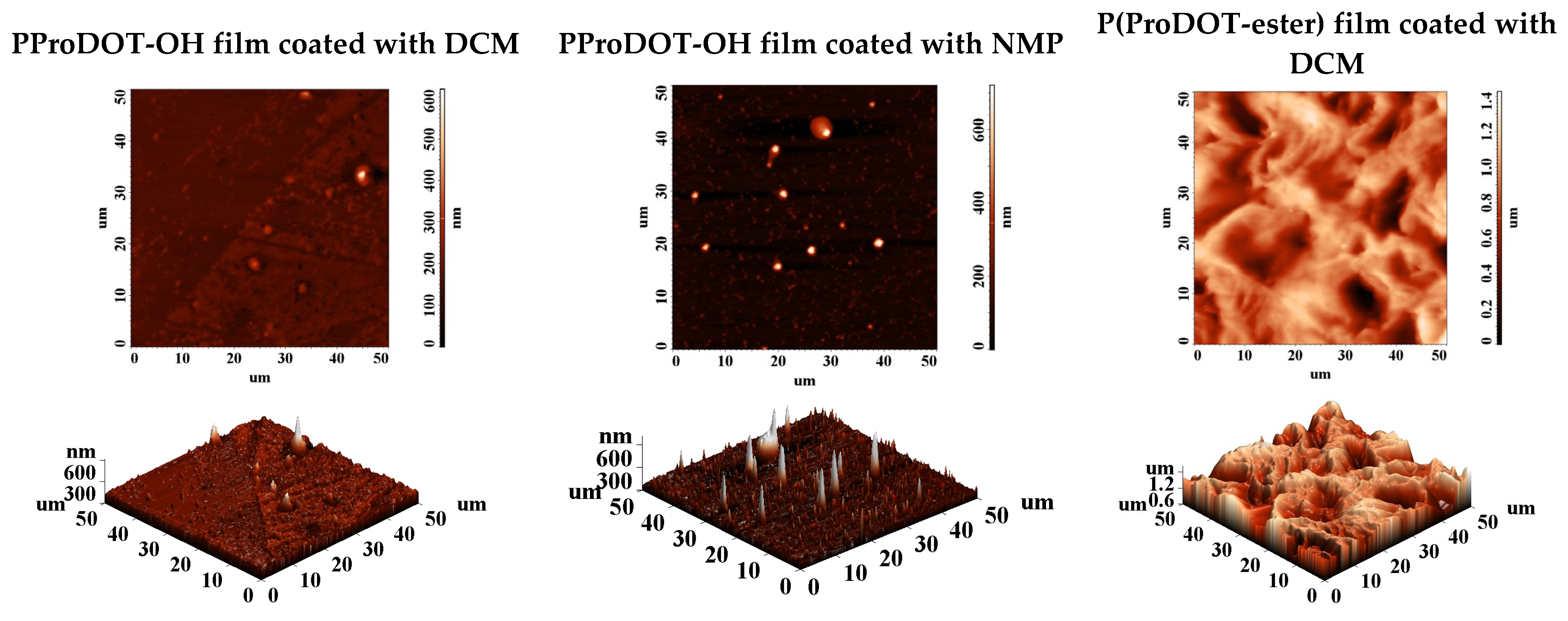
3.3. Films Morphology
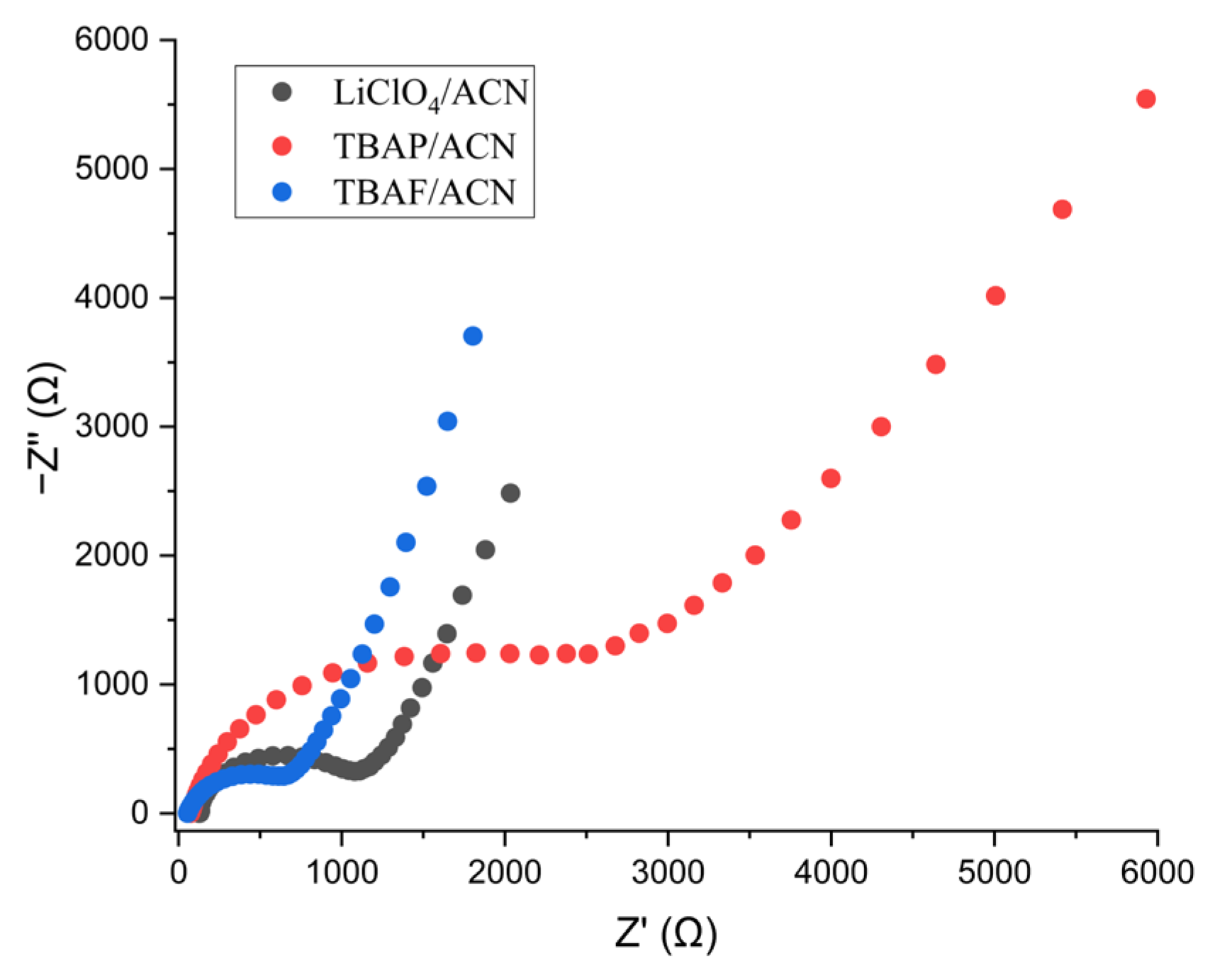
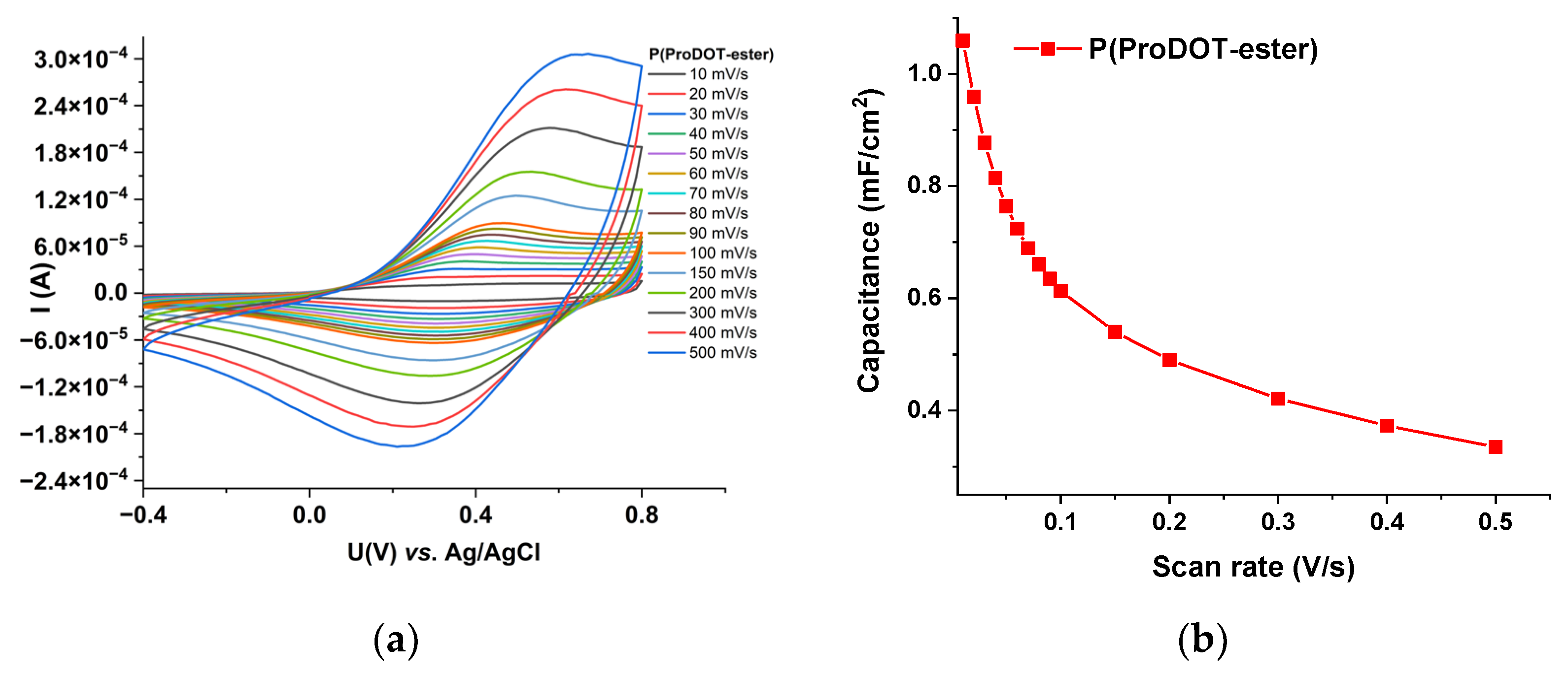
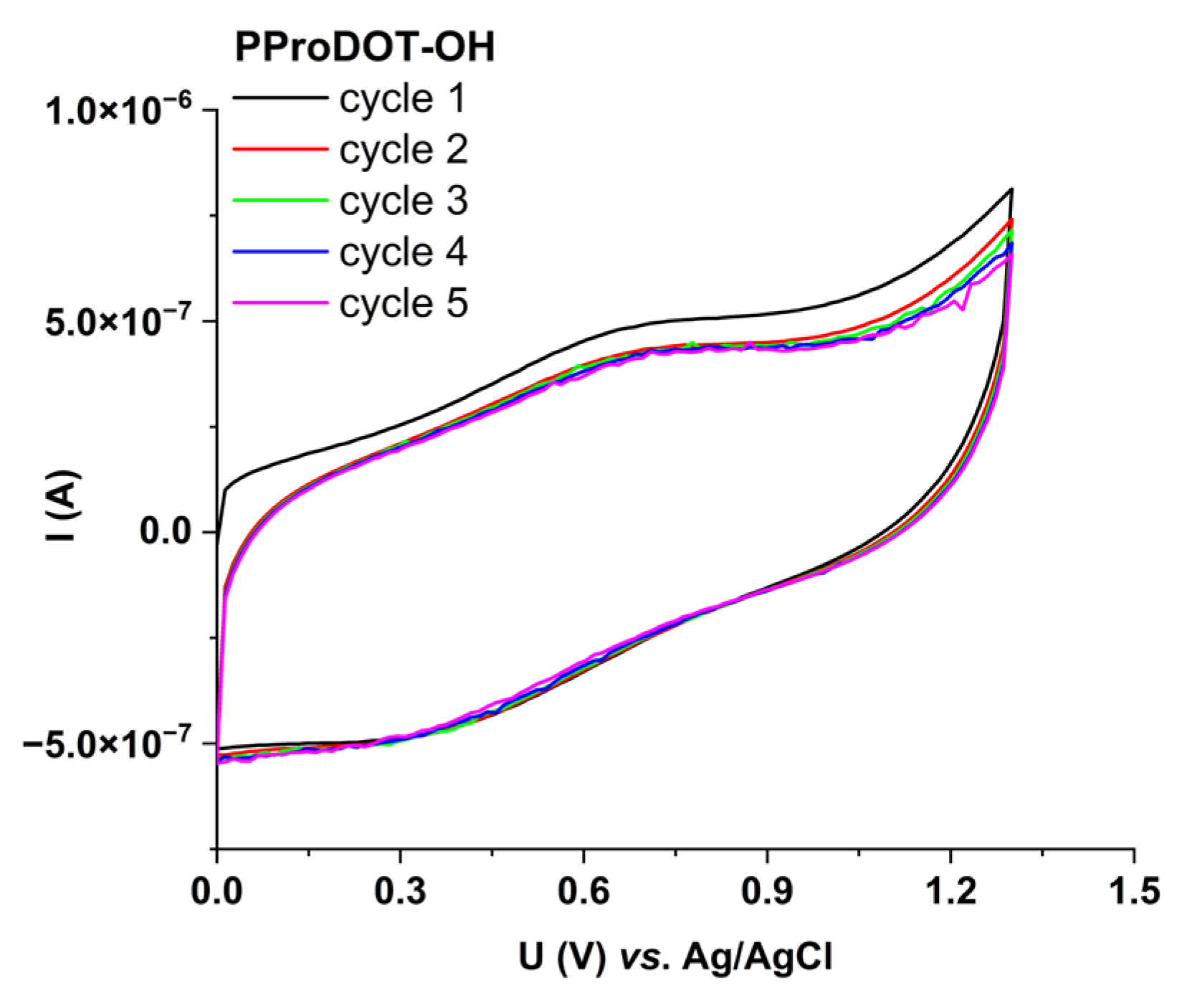
3.4. Energy Storage Capability
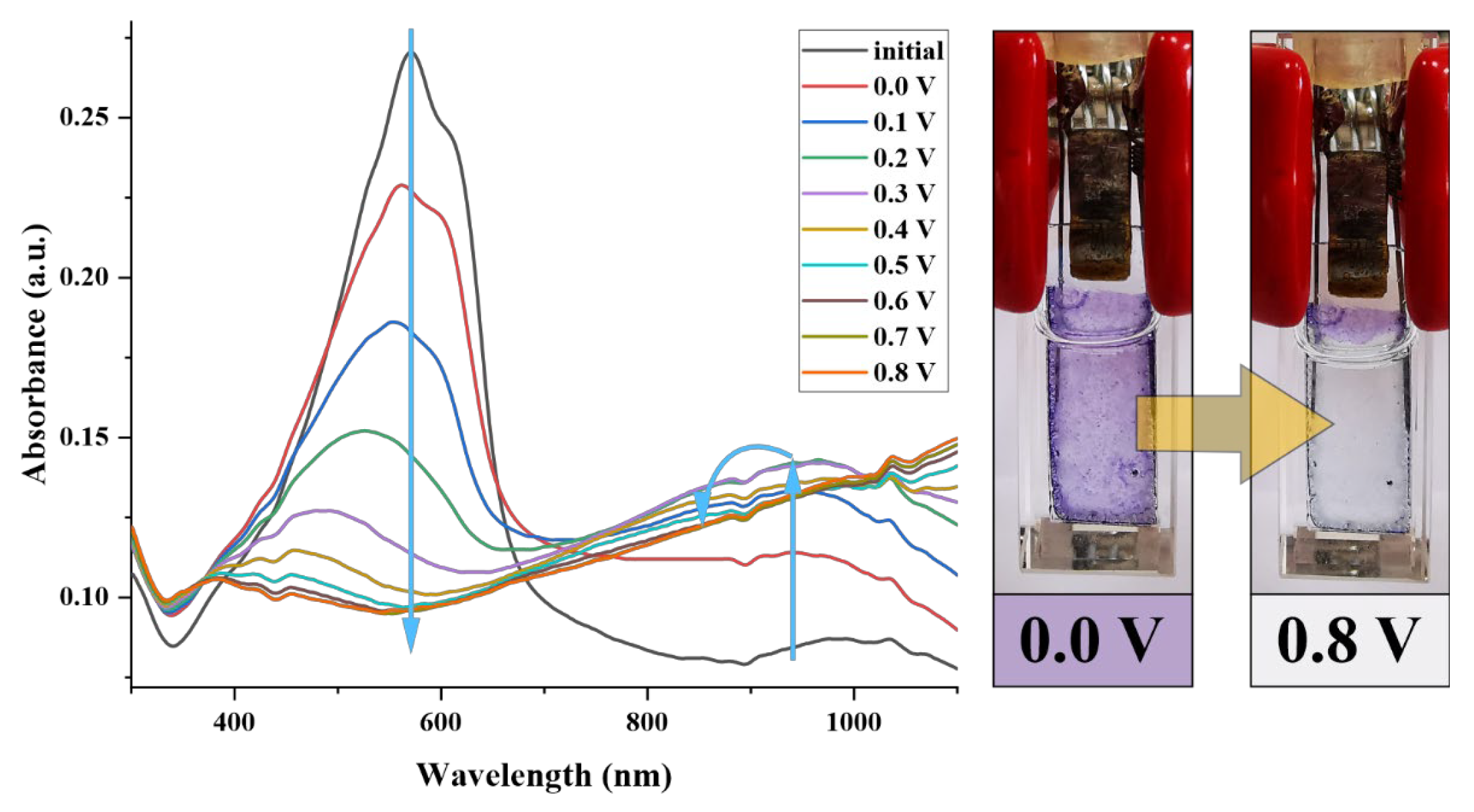
3.5. Electrochromic Evaluation of P(ProDOT-Ester)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Groenendaal, L.; Zotti, G.; Aubert, P.; Waybright, S.M.; Reynolds, J.R. Electrochemistry of Poly(3,4-alkylenedioxythiophene) Derivatives. Adv. Mater. 2003, 15, 855–879. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Raja, P.P.; Shivaprakash, N.C.; Sindhu, S. Fabrication of fast switching electrochromic window based on poly(3,4-(2,2-dimethylpropylenedioxy)thiophene) thin film. J. Mater. Sci. Mater. Electron. 2016, 27, 6035–6042. [Google Scholar] [CrossRef]
- Sikder, B.K. A step towards the processability of insoluble or partially soluble functional and structural variants of polymers based on 3,4-alkylenedioxythiophene. RSC Adv. 2016, 6, 107776–107784. [Google Scholar] [CrossRef]
- Kausar, A. Overview on conducting polymer in energy storage and energy conversion system. J. Macromol. Sci. Part A Pure Appl. Chem. 2017, 54, 640–653. [Google Scholar] [CrossRef]
- Raza, S.; Li, X.; Soyekwo, F.; Liao, D.; Xiang, Y.; Liu, C. A comprehensive overview of common conducting polymer-based nanocomposites; Recent advances in design and applications. Eur. Polym. J. 2021, 160, 110773. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar]
- Tran, V.V.; Lee, S.; Lee, D.; Le, T.H. Recent Developments and Implementations of Conductive Polymer-Based Flexible Devices in Sensing Applications. Polymers 2022, 14, 3730. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From Fundamental Perspectives to Applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Hebert, D.D.; Naley, M.A.; Cunningham, C.C.; Sharp, D.J.; Murphy, E.E.; Stanton, V.; Irvin, J.A. Enabling Conducting Polymer Applications: Methods for Achieving High Molecular Weight in Chemical Oxidative Polymerization in Alkyl- and Ether- Substituted Thiophenes. Materials 2021, 14, 6146. [Google Scholar] [CrossRef]
- Karazehir, T.; Sarac, B.; Gilsing, H.D.; Eckert, J.; Sarac, A.S. Oligoether Ester-Functionalized ProDOT Copolymers on Si/Monolayer Graphene as Capacitive Thin Film Electrodes. J. Electrochem. Soc. 2020, 167, 070543. [Google Scholar] [CrossRef]
- Guler, F.G.; Gilsing, H.D.; Schulz, B.; Sarac, A.S. Impedance and Morphology of Hydroxy- and Chloro-Functionalized Poly(3,4-propylenedioxythiophene) Nanostructures. J. Nanosci. Nanotechnol. 2012, 12, 7869–7878. [Google Scholar] [CrossRef]
- Mishra, S.P.; Sahoo, R.; Ambade, A.V.; Contractor, A.Q.; Kumar, A. Synthesis and characterization of functionalized 3,4-propylenedioxythiophene and its derivatives. J. Mater. Chem. 2004, 14, 1896–1900. [Google Scholar] [CrossRef]
- Wei, B.; Ouyang, L.; Liu, J.; Martin, D.C. Post-polymerization functionalization of poly(3,4-propylenedioxythiophene) (PProDOT) via thiol–ene “click” chemistry. J. Mater. Chem. B 2015, 3, 5028–5034. [Google Scholar] [CrossRef]
- Damaceanu, M.D.; Gilsing, H.D.; Schulz, B.; Arvinte, A.; Bruma, M. An easily functionalizable oligo(oxyethylene)- and ester-substituted poly(3,4-propylenedioxythiophene) derivative exhibiting alkali metal ion response. RSC Adv. 2014, 4, 52467–52475. [Google Scholar] [CrossRef]
- Argun, A.A.; Aubert, P.H.; Thompson, B.C.; Schwendeman, I.; Gaupp, C.L.; Hwang, J.; Pinto, N.J.; Tanner, D.B.; MacDiarmid, A.G.; Reynolds, J.R. Multicolored Electrochromism in Polymers: Structures and Devices. Chem. Mater. 2004, 16, 4401–4412. [Google Scholar] [CrossRef]
- Christiansen, D.T.; Ohtani, S.; Chujo, Y.; Tomlinson, A.L.; Reynolds, J.R. All Donor Electrochromic Polymers Tunable across the Visible Spectrum via Random Copolymerization. Chem. Mater. 2019, 31, 6841–6849. [Google Scholar] [CrossRef]
- Perera, K.; Yi, Z.; You, L.; Ke, Z.; Mei, J. Conjugated electrochromic polymers with amide-containing side chains enabling aqueous electrolyte compatibility. Polym. Chem. 2020, 11, 508–516. [Google Scholar] [CrossRef]
- Savagian, L.R.; Österholm, A.M.; Ponder, J.F.; Barth, K.J.; Rivnay, J.; Reynolds, J.R. Balancing Charge Storage and Mobility in an Oligo(Ether) Functionalized Dioxythiophene Copolymer for Organic- and Aqueous-Based Electrochemical Devices and Transistors. Adv. Mater. 2018, 30, 1804647. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, W.; Mo, D.; Zhang, K.; Wang, Z.; Jiang, F.; Hu, D.; Dong, L.; Xu, J. Electrochemical preparation of poly(2,3-dihydrothieno[3,4-b][1,4]dioxin-2-yl)methanol)/carbon fiber core/shell structure composite and its high capacitance performance. J. Electroanal. Chem. 2015, 743, 53–59. [Google Scholar] [CrossRef]
- Ersozoglu, M.G.; Gilsing, H.D.; Gencturk, A.; Sarac, A.S. A Novel Dioxythiophene Based Conducting Polymer as Electrode Material for Supercapacitor Application. Int. J. Electrochem. Sci. 2019, 14, 9504–9519. [Google Scholar] [CrossRef]
- Niu, J.; Chen, S.; Zhang, W.; Zhang, W.; Chai, K.; Ye, G.; Li, D.; Zhou, W.; Duan, X.; Xu, J. Supercapacitor properties of nanowire poly((3,4-dihydro-2 H -thieno[3,4- b ][1,4]dioxepin-3-yl)methanol) free-supporting films. Electrochim. Acta 2018, 283, 488–496. [Google Scholar] [CrossRef]
- Sarac, A.S.; Gilsing, H.D.; Gencturk, A.; Schulz, B. Electrochemically polymerized 2,2-dimethyl-3,4-propylenedioxythiophene on carbon fiber for microsupercapacitor. Prog. Org. Coat. 2007, 60, 281–286. [Google Scholar] [CrossRef]
- Kim, J.; Rémond, M.; Kim, D.; Jang, H.; Kim, E. Electrochromic Conjugated Polymers for Multifunctional Smart Windows with Integrative Functionalities. Adv. Mater. Technol. 2020, 5, 1900890. [Google Scholar] [CrossRef]
- Tong, Z.; Tian, Y.; Zhang, H.; Li, X.; Ji, J.; Qu, H.; Li, N.; Zhao, J.; Li, Y. Recent advances in multifunctional electrochromic energy storage devices and photoelectrochromic devices. Sci. China Chem. 2017, 60, 13–37. [Google Scholar] [CrossRef]
- Kros, A.; Nolte, R.J.M.; Sommerdijk, N.A.J.M. Poly(3,4-ethylenedioxythiophene)-Based Copolymers for Biosensor Applications. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 738–747. [Google Scholar] [CrossRef]
- Reeves, B.D.; Unur, E.; Ananthakrishnan, N.; Reynolds, J.R. Defunctionalization of Ester-Substituted Electrochromic Dioxythiophene Polymers. Macromolecules 2007, 40, 5344–5352. [Google Scholar] [CrossRef]
- Shi, P.; Amb, C.M.; Dyer, A.L.; Reynolds, J.R. Fast Switching Water Processable Electrochromic Polymers. ACS Appl. Mater. Interfaces 2012, 4, 6512–6521. [Google Scholar] [CrossRef]
- Grzes, G.; Wolski, K.; Uchacz, T.; Bała, J.; Louis, B.; Scheblykin, I.G.; Zapotoczny, S. Ladder-like Polymer Brushes Containing Conjugated Poly(Propylenedioxythiophene) Chains. Int. J. Mol. Sci. 2022, 23, 5886. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chen, H.W.; Lee, K.M.; Hu, C.W.; Ho, K.C. A dye-sensitized photo-supercapacitor based on PProDOT-Et2 thick films. J. Power Source 2010, 195, 6232–6238. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.H.; Mao, Y.W.; Yang, Y.J.; Yang, W.Y.; Li, S.B. Electrochemical performance of graphene-polyethylenedioxythiophene nanocomposites. Mater. Sci. Eng. B-Adv. 2013, 178, 1152–1157. [Google Scholar] [CrossRef]
- Manjakkal, L.; Navaraj, W.T.; Núñez, C.G.; Dahiya, R. Graphene–graphite polyurethane composite based high-energy density flexible supercapacitors. Adv. Sci. 2019, 6, 1802251. [Google Scholar] [CrossRef]
- Niaz, N.A.; Shakoor, A.; Imran, M.; Khalid, N.R.; Hussain, F.; Kanwal, H.; Maqsood, M.; Afzal, S. Enhanced electrochemical performance of MoS2/PPy nanocomposite as electrodes material for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2020, 31, 11336–11344. [Google Scholar] [CrossRef]
- Butnaru, I.; Chiriac, A.P.; Constantin, C.P.; Damaceanu, M.D. Insights into MWCNTs/polyimide nanocomposites: From synthesis to application as free-standing flexible electrodes in low-cost micro-supercapacitors. Mater. Today Chem. 2022, 23, 100671. [Google Scholar] [CrossRef]
- Ni, D.; Chen, Y.; Song, H.; Liu, C.; Yang, X.; Cai, K. Free-standing and highly conductive PEDOT nanowire films for high-performance all-solid-state supercapacitors. J. Mater. Chem. A 2019, 7, 1323–1333. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiriac, A.-P.; Constantin, C.-P.; Damaceanu, M.-D. ProDOT-Based Polymers: From Energy Storage to Smart Window Applications. Energies 2023, 16, 3999. https://doi.org/10.3390/en16103999
Chiriac A-P, Constantin C-P, Damaceanu M-D. ProDOT-Based Polymers: From Energy Storage to Smart Window Applications. Energies. 2023; 16(10):3999. https://doi.org/10.3390/en16103999
Chicago/Turabian StyleChiriac, Adriana-Petronela, Catalin-Paul Constantin, and Mariana-Dana Damaceanu. 2023. "ProDOT-Based Polymers: From Energy Storage to Smart Window Applications" Energies 16, no. 10: 3999. https://doi.org/10.3390/en16103999