Composting of Municipal Sewage Sludge and Lignocellulosic Waste: Nitrogen Transformations and Humic Substances Molecular Weight
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioreactor and Windrow
2.2. Sewage Sludge, Lignocellulosic Materials and Feedstock Characteristics
2.3. Analytical Methods
3. Results and Discussion
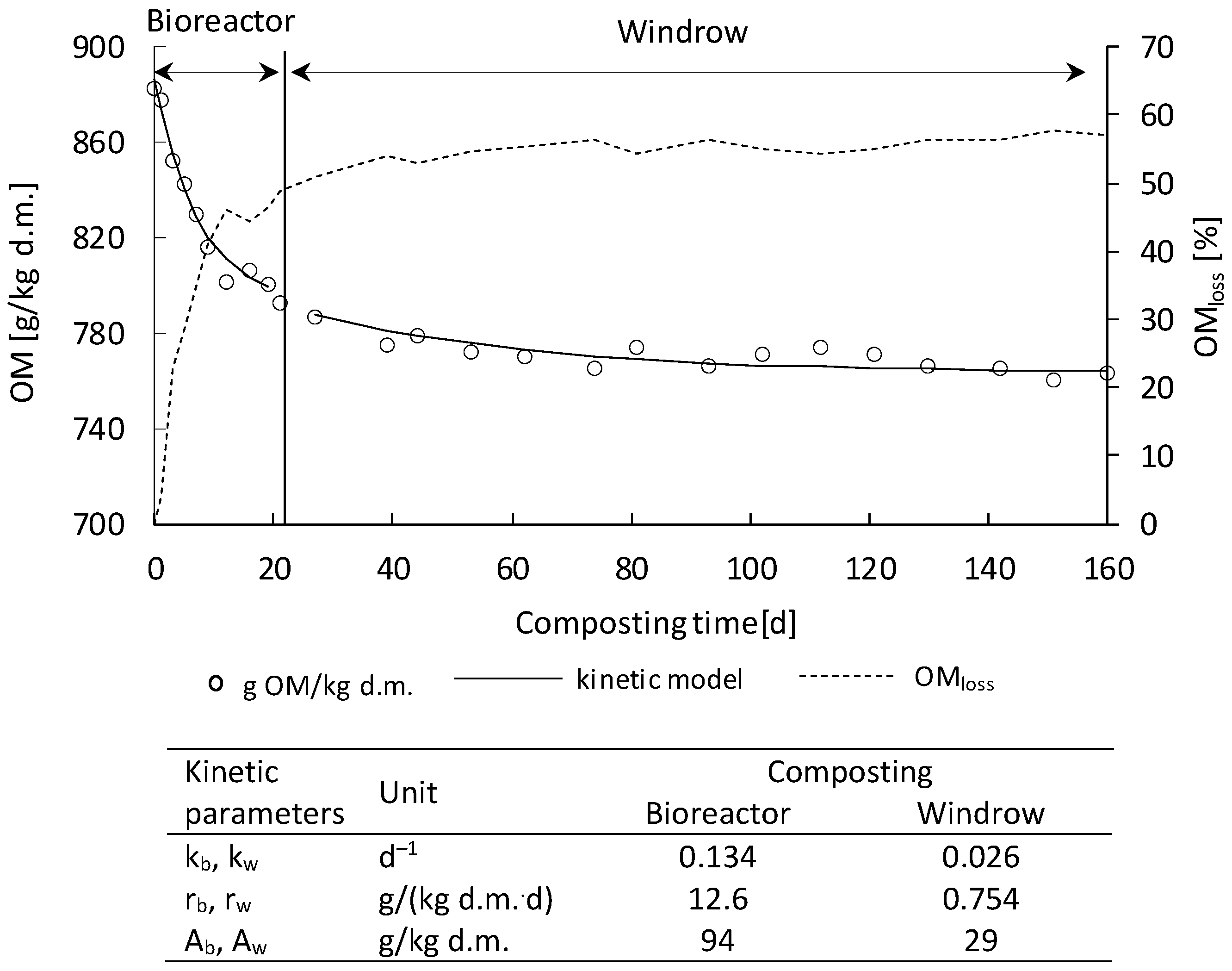
3.1. Temperature Profiles and Organics Removal
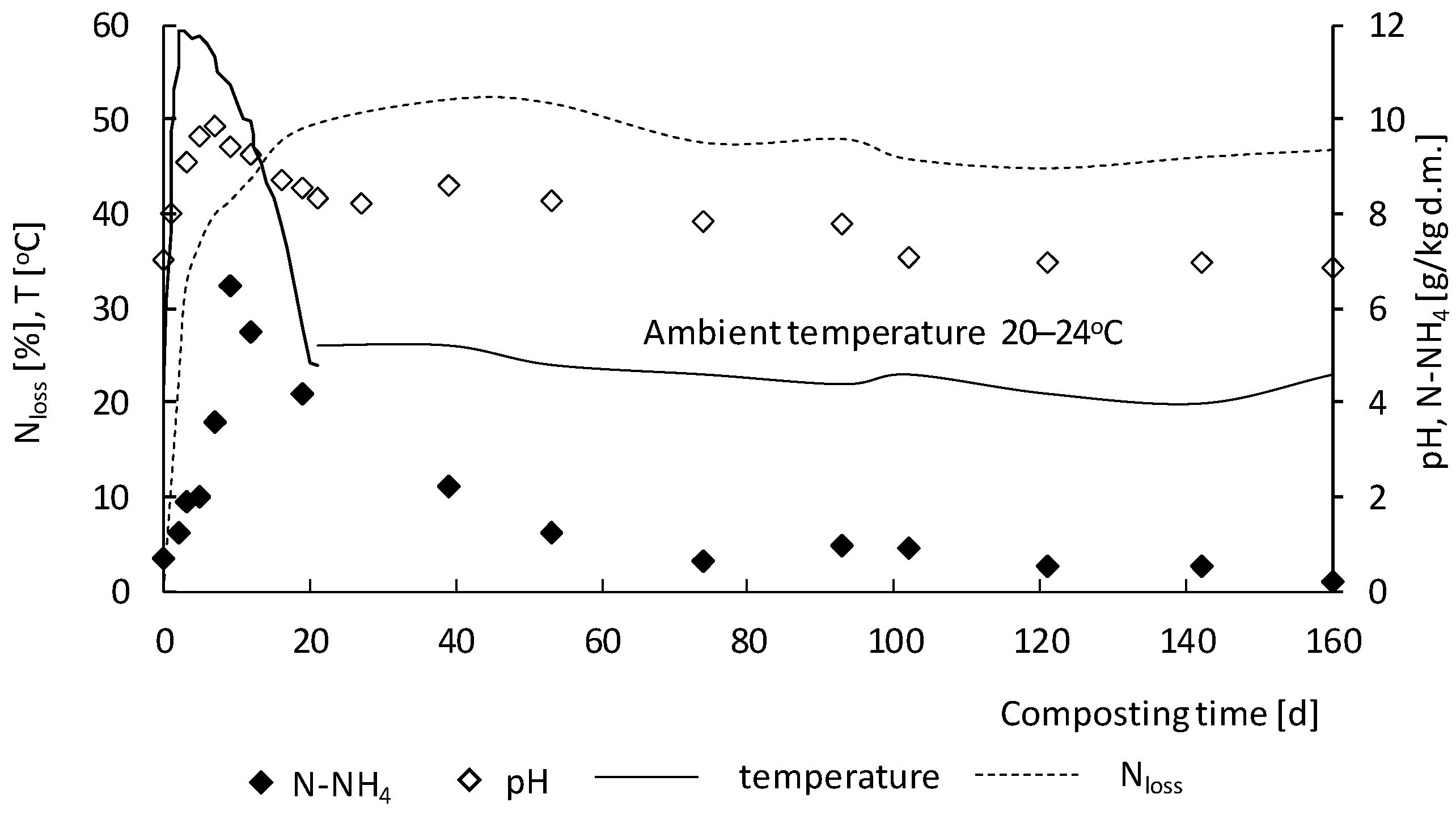
3.2. Nitrogen Transformations
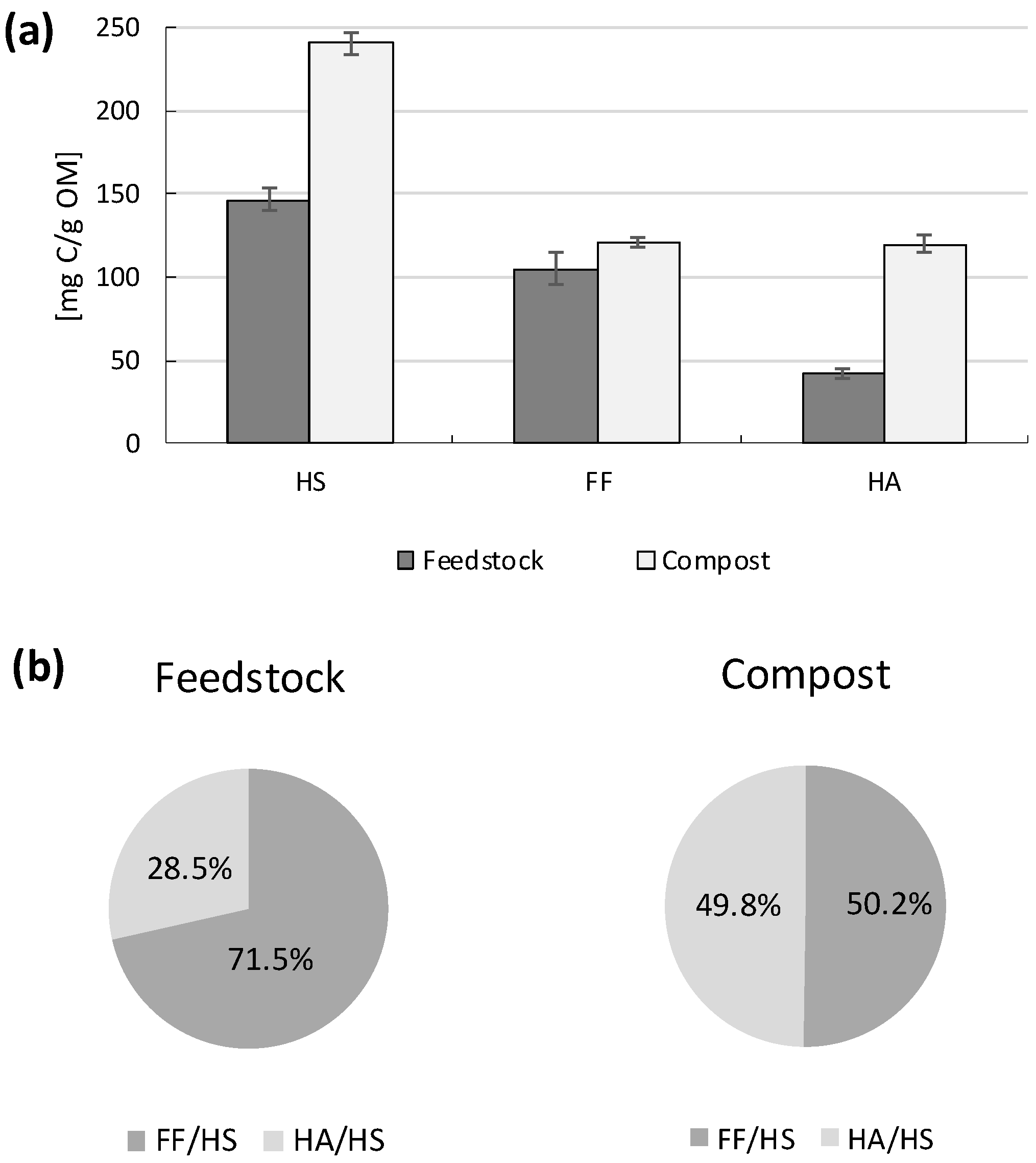
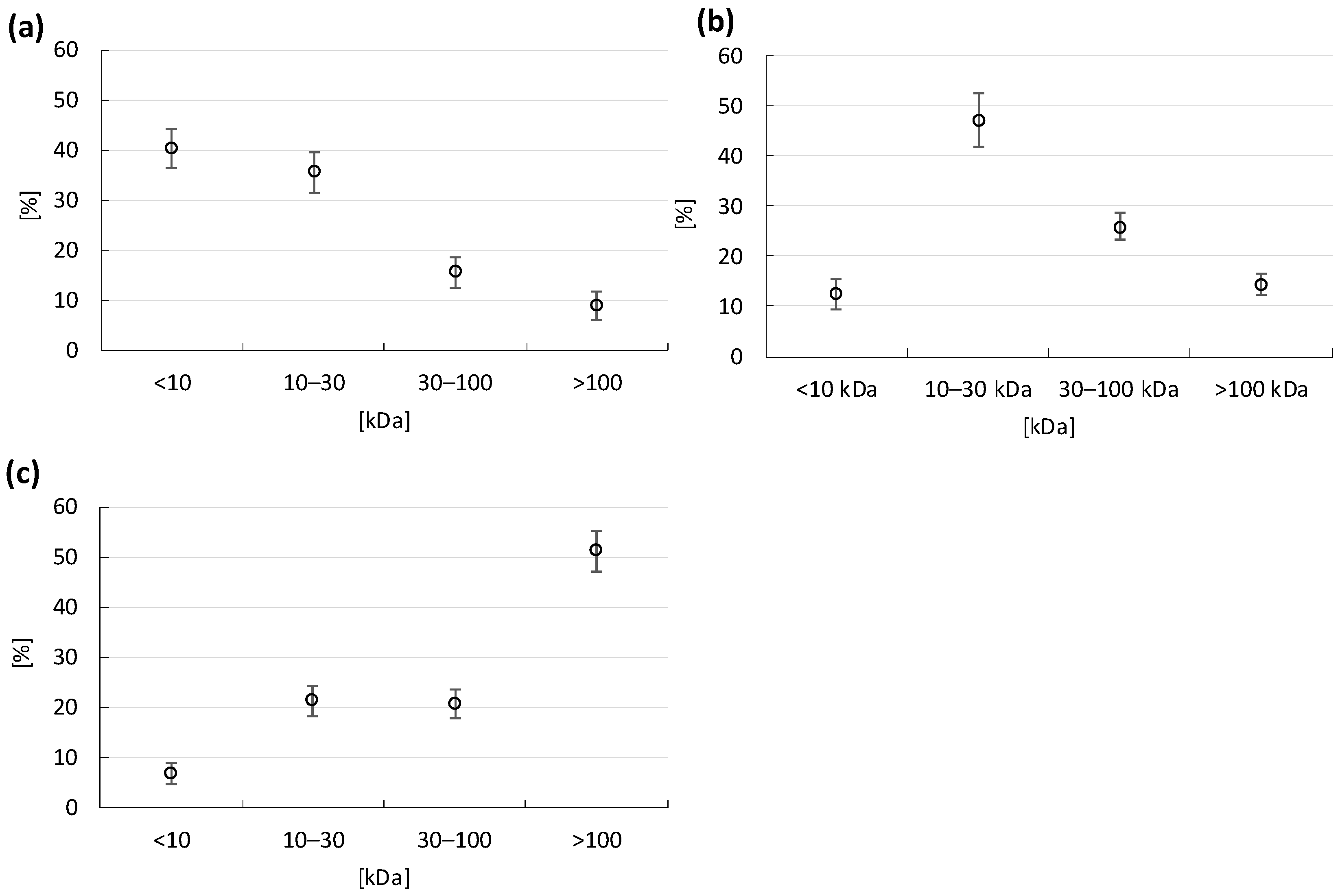
3.3. Humic Substances Concentration and Molecular Weight of HA, FA and DOM
3.4. Compost Quality
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smidt, E.; Tintner, J.; Meissl, K.; Binner, E. Influence of input materials and composting operation on humification of organic matter. In Dynamic Soil, Dynamic Plant; GSB: Lethbridge, AB, Canada, 2008; pp. 50–59. [Google Scholar]
- Stroosnijder, L. Land use planning for mitigation desertification in Europe. In Desertification in Europe: Mitigation Strategies, Land-Use Planning; Alghero: Sardinia, Italy, 2000; pp. 155–183. [Google Scholar]
- Miricioiu, M.G.; Zaharioiu, A.; Oancea, S.; Bucura, F.; Raboaca, M.S.; Filote, C.; Ionete, R.E.; Niculescu, V.C.; Constantinescu, M. Sewage sludge derived materials for CO2 adsorption. Appl. Sci. 2021, 11, 7139. [Google Scholar] [CrossRef]
- Maji, D.; Misra, P.; Singh, S.; Kalra, A. Humic acid rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of Pisum sativum. Appl. Soil Ecol. 2017, 110, 97–108. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.; Wu, X.; Cui, R.; Li, G.; Zheng, F.; Tan, D. Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: A three-year experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef] [Green Version]
- Annabi, M.; Houot, S.; Francou, C.; Poitrnaud, M.; LeBissonnais, Y. Soil aggregate stability improvement with urban compost of different maturities. Soil. Sci. Soc. Am. 2007, 71, 413–423. [Google Scholar] [CrossRef]
- Zhou, L.; Monreal, C.M.; Xu, S.; McLaughlin, N.B.; Zhang, H.; Hao, G. Effect of bentonite-humic acid application on the improvement of soil structure and maize yield in a sandy soil of a semi-arid region. Geoderma 2019, 338, 269–280. [Google Scholar] [CrossRef]
- Yang, F.; Tang, C.; Antonietti, M. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021, 50, 6221–6239. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gil, J.C.; Ceppi, S.; Valesca, M.; Polo, A.; Sensei, N. Long-term effects of amendment with municipal solid waste compost on the elemental and acid functional group composition and pH-buffer capacity of soil humic acid. Geoderma 2004, 121, 135–142. [Google Scholar] [CrossRef]
- Huang, M.; Zhu, Y.; Li, Z.; Huang, B.; Luo, N.; Liu, C.; Zeng, G. Compost as a soil amendment to remediate heavy metal-contaminated agricultural soil: Mechanisms, efficacy, problems, and strategies. Wat. Air Soil Poll. 2016, 227, 359. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Francioso, O.; Tugnoli, V.; Nardi, S. The auxin-like activity of humic substances is related to membrane interactions in carrot cell cultures. J. Chem. Ecol. 2007, 33, 115–129. [Google Scholar] [CrossRef]
- Ouni, Y.; Ghnaya, T.; Montemurro, F.; Abdellya, C.; Lakhdara, A. The role of humic substances in mitigating the harmful effects of soil salinity and improve plant productivity. Int. J. Plant Prod. 2014, 8, 353–374. [Google Scholar]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. 2016, 62, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the role of humic acids on crop performance and soil health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Yang, F.; Antonietti, M. The sleeping giant: A polymer view on humic matter in synthesis and applications. Prog. Polym. Sci. 2020, 100, 101182. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Yang, Z.; Kappler, A.; Jiang, J. Reducing capacities and distribution of redox-active functional groups in low molecular weight fractions of humic acids. Environ. Sci. Technol. 2016, 50, 12105–12113. [Google Scholar] [CrossRef]
- Sayara, T.; Sánchez, A. Gaseous emissions from the composting process: Controlling parameters and strategies of mitigation. Processes 2021, 9, 1844. [Google Scholar] [CrossRef]
- Cassity-Duffey, K.; Cabrera, M.; Gaskin, J.; Franklin, D.; Kissel, D.; Saha, U. Nitrogen mineralization from organic materials and fertilizers: Predicting N release. Soil Sci. Soc. Am. J. 2020, 84, 522–533. [Google Scholar] [CrossRef]
- Lazicki, P.; Geisseler, D.; Lloyd, M. Nitrogen mineralization from organic amendments is variable but predictable. J. Environ. Qual. 2020, 49, 483–495. [Google Scholar] [CrossRef]
- Regulation of the Minister of the Environment of 6 February 2015 on the Municipal Sewage Sludge (Journal of Law 2015, Item. 257); Minister of the Environment: Warsaw, Poland, 2015.
- PN−EN 12880:2004; Characteristics of Sewage Sludge, Determination of Dry Residue and Water Content. Polish Committee for Standardization: Warsaw, Poland, 2004.
- PN-EN 5664:2002; Water Quality. Determination of Ammonium Nitrogen. Distillation Method with Titration. Polish Committee for Standardization: Warsaw, Poland, 2002.
- PN-EN 26777:1999; Determination of Nitrite Nitrogen by Colorimetric Method with Sulfanilic Acid and 1-Naphthylamine. Polish Committee for Standardization: Warsaw, Poland, 1999.
- PN-C-04576/08:1982; Tests for the Content of Nitrogen Compounds. Determination of Nitrate Nitrogen by Colorimetric Method with Sodium Salicylate. Polish Committee for Standardization: Warsaw, Poland, 1982.
- Kulikowska, D.; Klimiuk, E. Organic matter transformations and kinetics during sewage sludge composting in a two-stage system. Bioresour. Technol. 2011, 102, 10951–10958. [Google Scholar] [CrossRef]
- PN-EN 15169:2011; Characterization of Waste. Determination of Loss on Ignition in Waste, Sludge and Sediments. Polish Committee for Standardization: Warsaw, Poland, 2011.
- PN-Z-15011-3; Municipal Waste Compost. Determination of pH, Organic Matter, Organic Carbon, Nitrogen, Phosphorus and Potassium. Polish Committee for Standardization: Warsaw, Poland, 2001.
- Kulikowska, D.; Gusiatin, Z.M. Sewage sludge composting in a two-stage system: Carbon and nitrogen transformations and potential ecological risk assessment. Waste Manag. 2015, 38, 312–320. [Google Scholar] [CrossRef]
- Kulikowska, D. Kinetics of organic matter removal and humification progress during sewage sludge composting. Waste Manag. 2016, 49, 196–203. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 6579-1:2017-04; A Method for the Detection, Enumeration and Serotyping of Salmonella. Polish Committee for Standardization: Warsaw, Poland, 2017.
- Kulikowska, D.; Bernat, K.; Zaborowska, M.; Zielińska, M. Municipal sewage sludge composting in the two-stage system: The role of different bulking agents and amendments. Energies 2022, 15, 5014. [Google Scholar] [CrossRef]
- Paredes, C.; Roig, A.; Bernal, M.P.; Sánchez-Monedero, M.A.; Cegarra, J. Evolution of organic matter and nitrogen during co-composting of olive mill wastewater with solid organic wastes. Biol. Fert. Soils 2000, 32, 222–227. [Google Scholar] [CrossRef]
- Hernandez, T.; Masciandaro, G.; Moreno, J.I.; Garcia, C. Changes in organic matter composition during composting of two digested sewage sludges. Waste Manag. 2006, 26, 1370–1376. [Google Scholar] [CrossRef]
- Ponsa, S.; Pagans, E.; Sánchez, A. Composting of dewatered wastewater sludge with various ratios of pruning waste used as a bulking agent and monitored by respirometer. Biosyst. Eng. 2009, 102, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Kulcu, R.; Yaldiz, O. Composting of goat manure and wheat straw using pine cones as a bulking agent. Biores. Technol. 2007, 98, 2700–2704. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Impraim, R.; Coates, T.; Flesch, T.; Trouve, R.; van Grinsven, H.; Cao, Y.; Hill, J.; Chen, D. Lignite effects on NH3, N2O, CO2 and CH4 emissions during composting of manure. J. Environ. Manag. 2020, 271, 110960. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, Q.; Zhang, Z.; Li, G.; Luo, W.; Zhang, D. Use of additive and pretreatment to control odors in municipal kitchen waste during aerobic composting. J. Environ. Sci. 2015, 37, 83–90. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A. Evaluation of two different aeration systems for composting two-phase olive mill wastes. Process Biochem. 2006, 41, 616–623. [Google Scholar] [CrossRef]
- Yang, X.; Liu, E.; Zhu, X.; Wang, H.; Liu, H.; Dong, W. Impact of composting methods on nitrogen retention and losses during dairy manure composting. Int. J. Environ. Res. Public Health 2019, 16, 3324. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Wang, X.; Liu, L.; Velthof, G.L.; Misselbrook, T.; Bai, Z.; Ma, L. 2020. Acidification of manure reduces gaseous emissions and nutrient losses from subsequent composting process. J. Environ. Manag. 2020, 264, 110454. [Google Scholar] [CrossRef]
- He, Y.; Inamori, Y.; Mizuochi, M.; Kong, H.; Iwami, N.; Sun, T. Nitrous oxide emissions from aerated composting of organic waste. Environ. Sci. Technol. 2001, 35, 2347–2351. [Google Scholar] [CrossRef]
- Wu, J.; Shangguan, H.; Fu, T.; Chen, J.; Tang, J.; Zeng, R.-J.; Ye, W.; Zhou, S. 2021. Alternating magnetic field mitigates N2O emission during the aerobic composting of chicken manure. J. Hazard. Mater. 2021, 406, 124329. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within composting: A review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef]
- Cáceres, R.; Coromina, N.; Malińska, K.; Martínez-Farré, F.X.; López, M.; Soliva, M.; Marfà, M. Nitrification during extended co-composting of extreme mixtures of green waste and solid fraction of cattle slurry to obtain growing media. Waste Manag. 2016, 58, 118–125. [Google Scholar] [CrossRef] [PubMed]
- López-Cano, I.; Roig, A.; Cayuela, M.; Alburquerque, J.; Sánchez-Monedero, M. Biochar improves N cycling during composting of olive mill wastes and sheep manure. Waste Manag. 2016, 49, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Y.; Wang, W.; Wei, S.; Jing, Z.; Lin, X. N2O emissions and nitrogen transformation during windrow composting of dairy manure. J. Environ. Manag. 2015, 160, 121–127. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Suzuki, K.; Osada, T.; Kuroda, K.; Hanajima, D.; Yasuda, T.; Haga, K. Reduction of nitrous oxide emission from pig manure composting by addition of nitrite-oxidizing bacteria. Environ. Sci. Technol. 2006, 40, 6787–6791. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Inubushi, K. Effect of nitrite accumulation on nitrous oxide emission and total nitrogen loss during swine manure composting. J. Soil Sci. Plant Nutr. 2009, 55, 428–434. [Google Scholar] [CrossRef]
- Wang, C.; Lu, C.; Dong, D.; Deng, H.; Strong, P.J.; Wang, H.; Wu, W. Insight into the effects of biochar on manure composting: Evidence supporting the relationship between N2O emission and denitrifying community. Environ. Sci. Technol. 2013, 47, 7341–7349. [Google Scholar] [CrossRef]
- Myrold, D.D.; Bottomley, P.J. Nitrogen mineralization and immobilization. In Nitrogen in Agricultural Soils; Raun, W., Schepers, J.S., Eds.; ASA: Madison, WI, USA, 2008; pp. 157–172. [Google Scholar]
- Geisseler, D.; Smith, R.; Cahn, M.; Muramoto, C. Nitrogen mineralization from organic fertilizers and composts: Literature survey and model fitting. J. Environ. Qual. 2021, 50, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Kulikowska, D.; Sindrewicz, S. Effect of barley straw and coniferous bark on humification process during sewage sludge composting. Waste Manag. 2018, 79, 207–213. [Google Scholar] [CrossRef]
- Zhao, X.; Dang, Q.; Zhang, C.; Yang, T.; Gong, T.; Xi, B. Revisiting organic waste-source-dependent molecular-weight governing the characterization within humic acids liking to humic-reducing microorganisms in composting process. J. Hazard. Mater. 2023, 442, 130049. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, L.; Li, Y.; Zhao, B. Characterization of ultrafiltration-fractionated humic acid derived from chinese weathered coal by spectroscopic techniques. Agronomy 2021, 11, 2030. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Li, C. Characterization of humic acids and fulvic acids derived from sewage sludge. Asian J. Chem. 2013, 25, 10087–10091. [Google Scholar] [CrossRef]
- Regulation of the Minister of Agriculture and Rural Development of 18 June 2008 Regarding the Implementation of Certain Provisions of the Act on Fertilizers and Fertilization (Journal of Law 2008, No 119, Item 765); Minister of Agriculture and Rural Development: Warsaw, Poland, 2008.
- Borisova, D.V.; Kostadinova, G.S.; Petkov, G.S.; Dermendzhieva, D.M.; Beev, G.G. An assessment of two types of industrially produced municipal green waste compost by quality control indices. Appl. Sci. 2022, 12, 10668. [Google Scholar] [CrossRef]
- Yan, W.; Qu, J.; Qu, Y.; Yue, T.; Zhang, Q.; Yi, W.; Liu, X.; Sun, Y. Effect of biochar addition on mechanism of heavy metal migration and transformation in biogas residue aerobic compost. Fermentation 2022, 8, 523. [Google Scholar] [CrossRef]

| Characteristics | Unit | Concentrations |
|---|---|---|
| Moisture | % | 87.0 |
| Organic matter (OM) | % d.m. | 83.8 |
| Organic carbon (TOC) | % d.m. | 46.8 |
| Reaction | pH | 8.01 |
| N | % d.m. | 7.02 |
| P2O5 | g/kg d.m. | 27.6 |
| K2O | g/kg d.m. | 9.26 |
| MgO | g/kg d.m. | 4.60 |
| CaO | g/kg d.m. | 7.13 |
| Cd | mg/kg d.m. | 1.89 |
| Pb | 8.3 | |
| Ni | 9.4 | |
| Cr | 20.9 | |
| Cu | 87.0 | |
| Zn | 599.0 | |
| Hg | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikowska, D.; Bernat, K. Composting of Municipal Sewage Sludge and Lignocellulosic Waste: Nitrogen Transformations and Humic Substances Molecular Weight. Energies 2023, 16, 376. https://doi.org/10.3390/en16010376
Kulikowska D, Bernat K. Composting of Municipal Sewage Sludge and Lignocellulosic Waste: Nitrogen Transformations and Humic Substances Molecular Weight. Energies. 2023; 16(1):376. https://doi.org/10.3390/en16010376
Chicago/Turabian StyleKulikowska, Dorota, and Katarzyna Bernat. 2023. "Composting of Municipal Sewage Sludge and Lignocellulosic Waste: Nitrogen Transformations and Humic Substances Molecular Weight" Energies 16, no. 1: 376. https://doi.org/10.3390/en16010376






