Pyrolysis of Biomass Wastes into Carbon Materials
Abstract
:1. Introduction
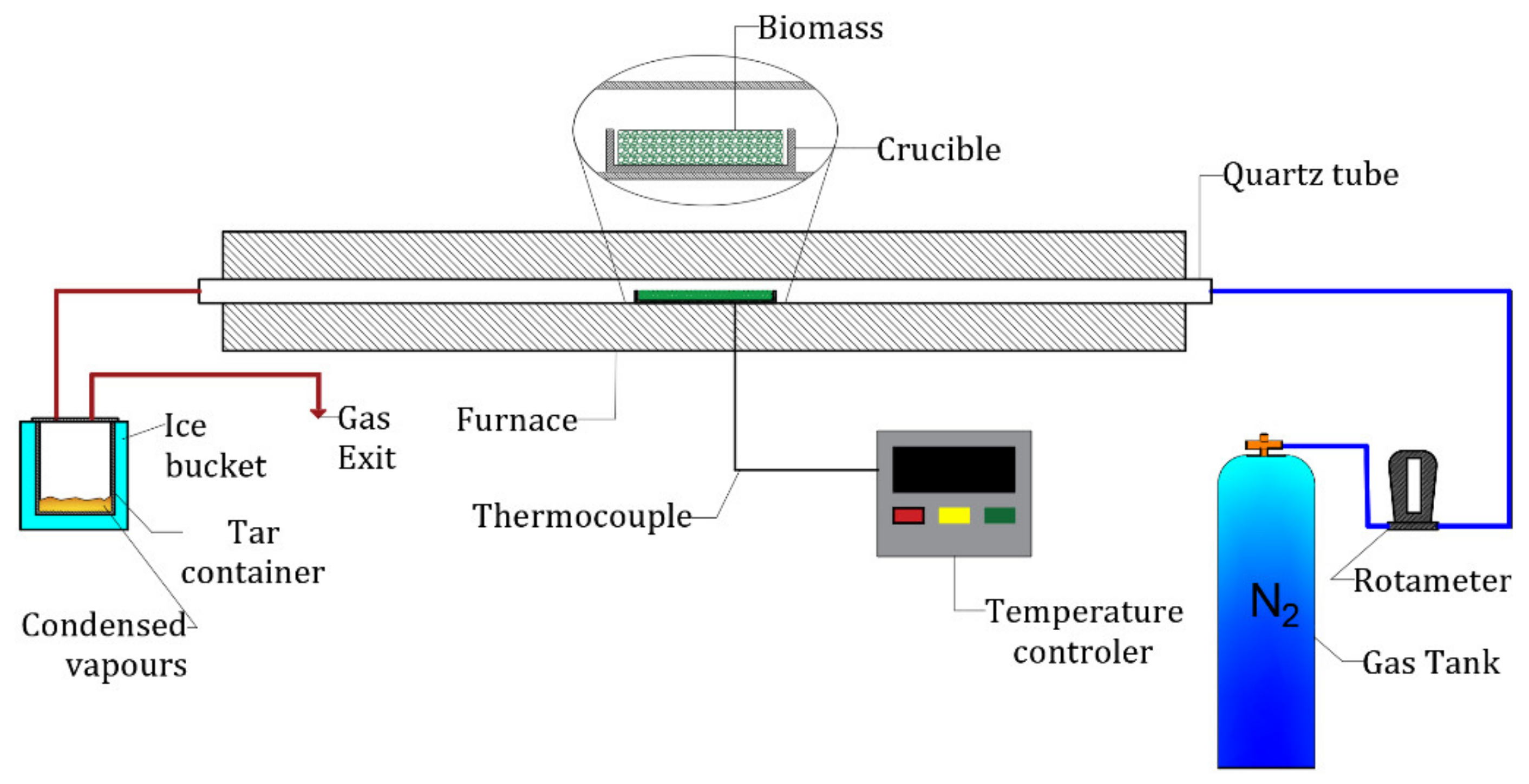
2. Materials and Methods
3. Results and Discussion
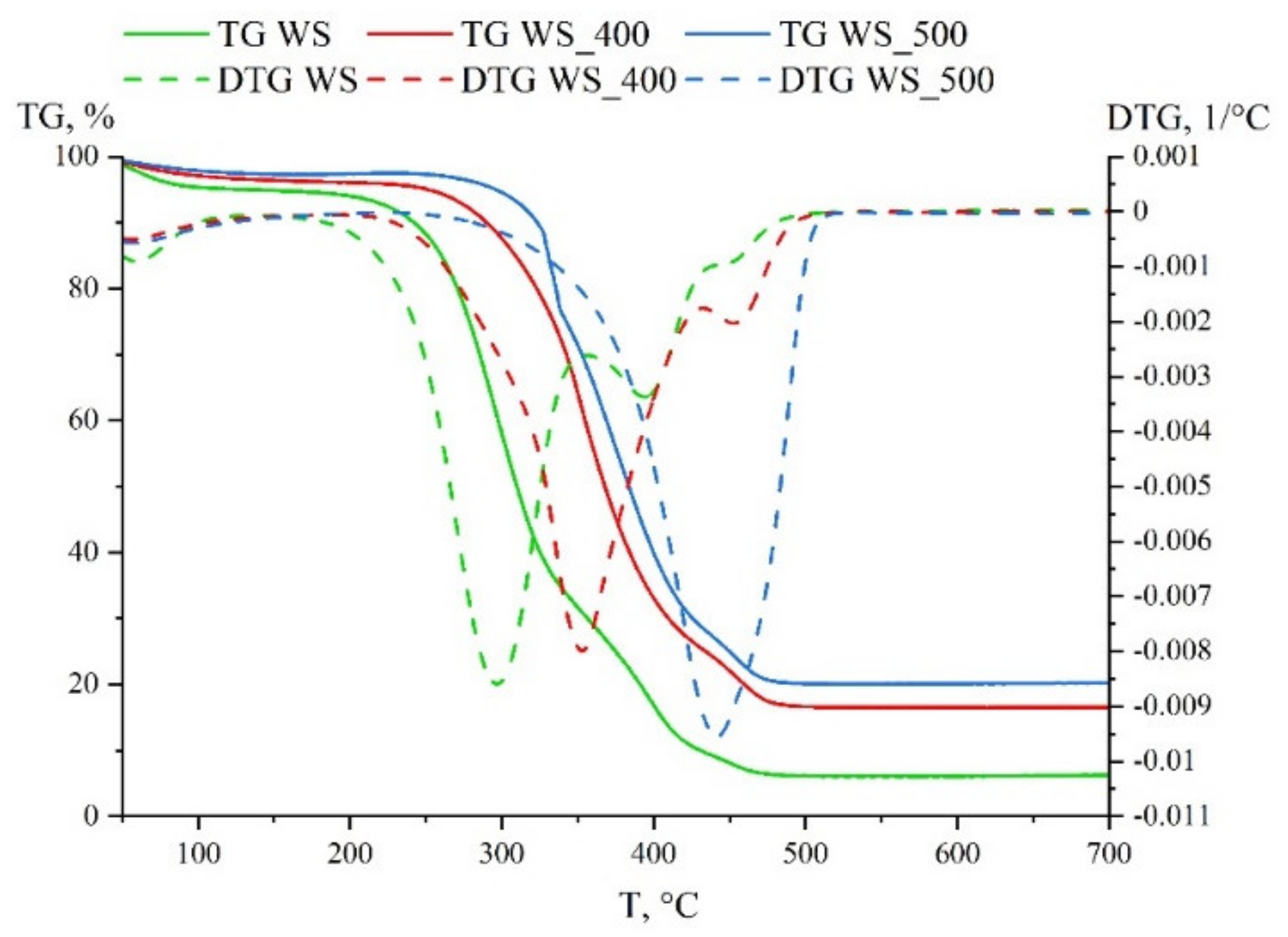
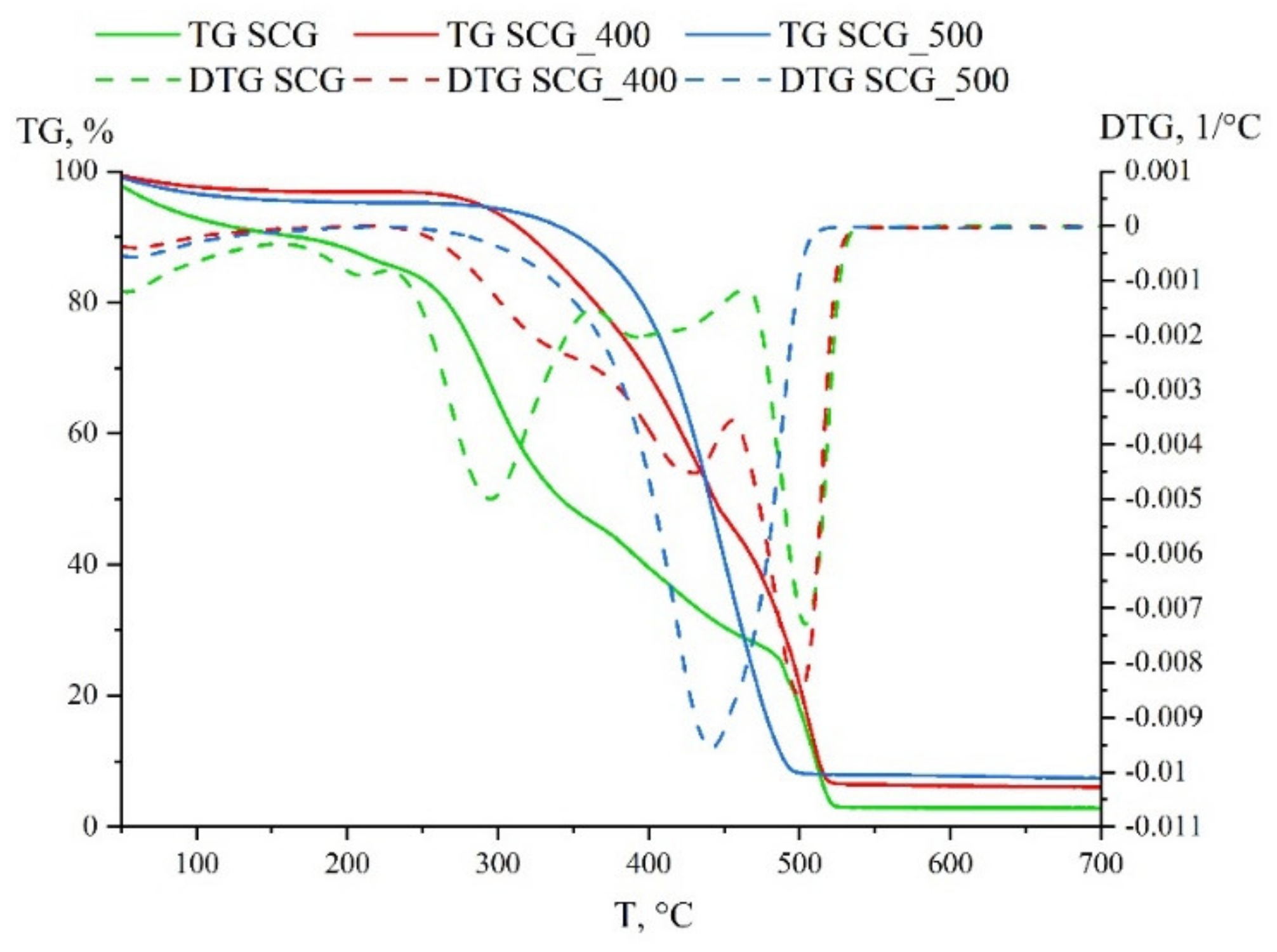
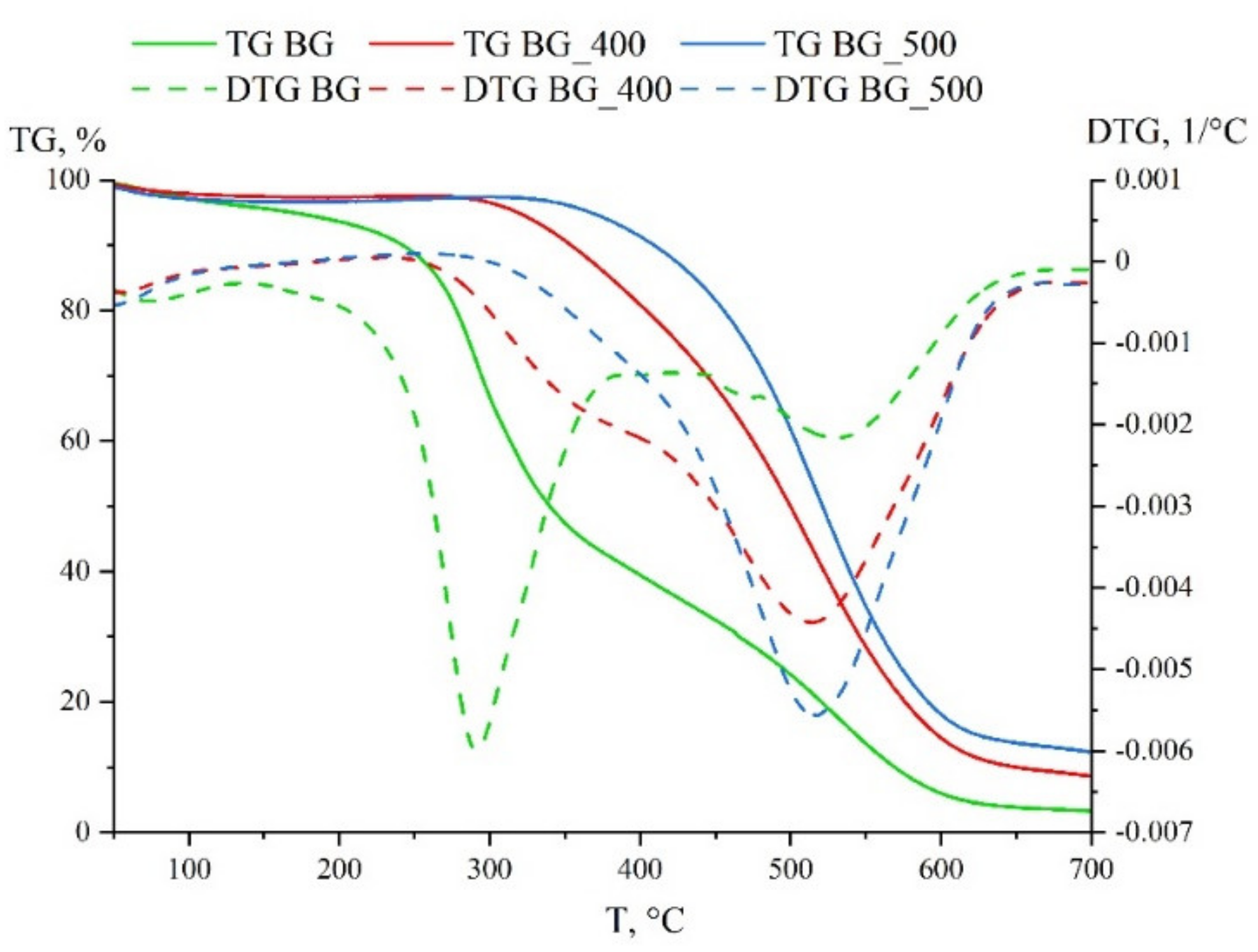
3.1. Solid Fuel Properties
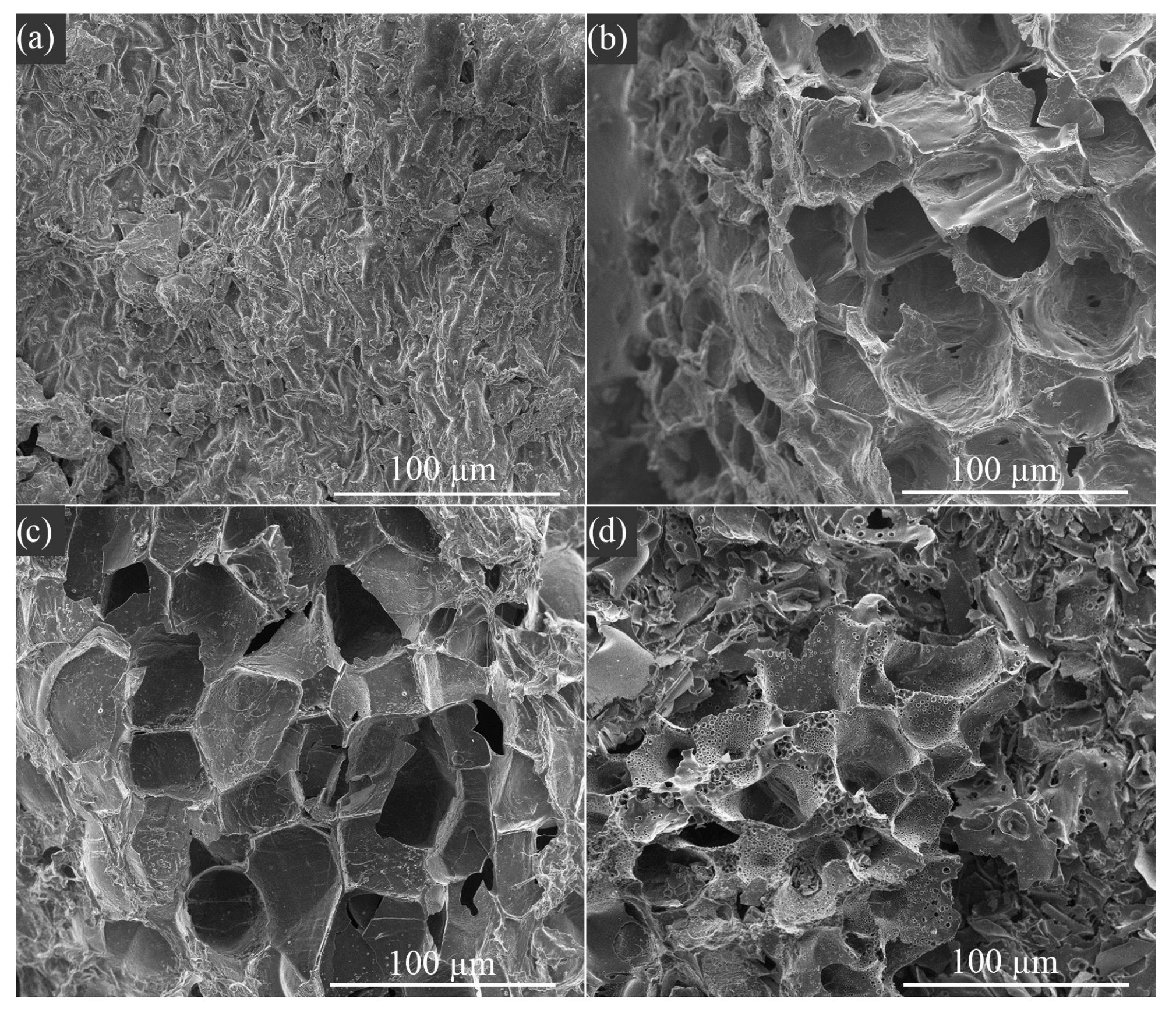
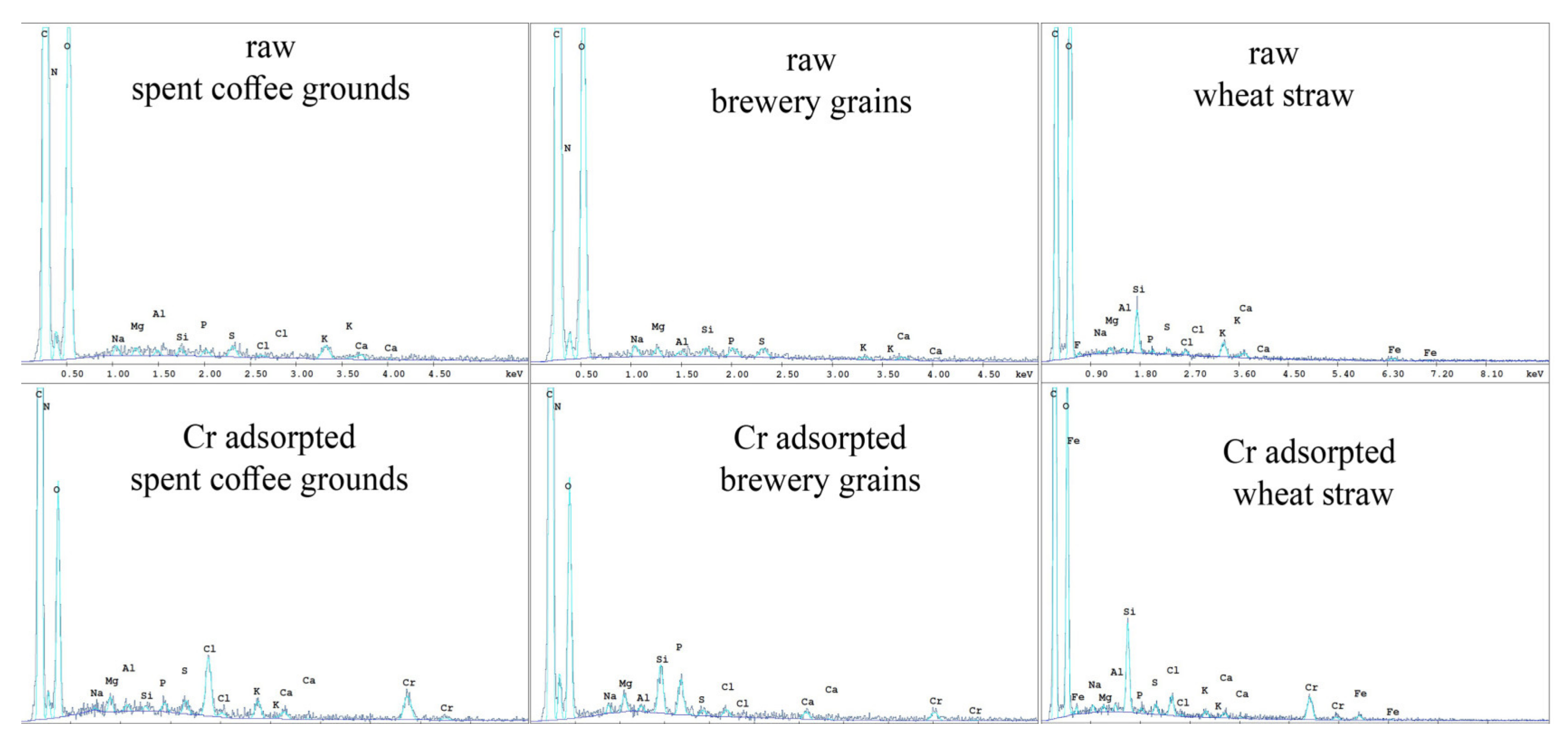
3.2. Adsorbent Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Leaders Summit on Climate. Available online: https://www.state.gov/leaders-summit-on-climate/ (accessed on 18 January 2022).
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Nimmanterdwong, P.; Chalermsinsuwan, B.; Piumsomboon, P. Prediction of lignocellulosic biomass structural components from ultimate/proximate analysis. Energy 2021, 222, 119945. [Google Scholar] [CrossRef]
- Roman, K.; Roman, M.; Szadkowska, D.; Szadkowski, J.; Grzegorzewska, E. Evaluation of physical and chemical parameters according to energetic willow (Salix viminalis L.) cultivation. Energies 2021, 14, 2968. [Google Scholar] [CrossRef]
- Szwaja, S.; Magdziarz, A.; Zajemska, M.; Poskart, A. A torrefaction of Sida hermaphrodita to improve fuel properties. Advanced analysis of torrefied products. Energ. Renew. 2019, 141, 894–902. [Google Scholar] [CrossRef]
- Czerwińska, K.; Śliz, M.; Wilk, M. Hydrothermal carbonization process: Fundamentals, main parameter characteristics and possible applications including an effective method of SARS-CoV-2 mitigation in sewage sludge: A review. Renew. Sustain. Energy Rev. 2022, 154, 111873. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Evangelopoulos, P.; Sieradzka, M.; Zajemska, M.; Magdziarz, A. Pyrolysis of agriculture waste biomass towards gas fuel and high-quality char production-experimental and numerical investigations. Fuel 2021, 296, 120611. [Google Scholar] [CrossRef]
- Roman, K.; Barwicki, J.; Hryniewicz, M.; Szadkowska, D.; Szadkowski, J. Production of electricity and heat from biomass wastes using a converted aircraft turbine AI-20. Processes 2021, 9, 364. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Qu, Y.; Mao, L. Characteristics of hydrogen-rich gas production of biomass gasification with porous ceramic reforming. Int. J. Hydrogen Energy 2012, 37, 9610–9618. [Google Scholar] [CrossRef]
- Sobek, S.; Werle, S. Solar pyrolysis of waste biomass: A comparative study of products distribution, in situ heating behavior, and application of model-free kinetic predictions. Fuel 2021, 292, 120365. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, M.; Gao, N.; Duan, Y.; Li, J. Experimental study on pyrolysis/gasification of biomass and plastics for H2 production under new dual-support catalyst. Chem. Eng. J. 2020, 396, 125260. [Google Scholar] [CrossRef]
- Fernandez, E.; Santamaria, L.; Artetxe, M.; Amutio, M.; Arregi, A.; Lopez, G.; Bilbao, J.; Olazar, M. Conditioning the volatile stream from biomass fast pyrolysis for the attenuation of steam reforming catalyst deactivation. Fuel 2022, 312, 122910. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum. Renew. Sustain. Energy Rev. 2020, 119, 109546. [Google Scholar] [CrossRef]
- Poskart, A.; Skrzyniarz, M.; Sajdak, M.; Zajemska, M.; Skibiński, A. Management of lignocellulosic waste towards energy recovery by pyrolysis in the framework of circular economy strategy. Energies 2021, 14, 5864. [Google Scholar] [CrossRef]
- Flanders Investment & Trade in Poznań, Renewable Energy in Poland. 2019. Available online: https://www.flandersinvestmentandtrade.com/export/sites/trade/files/market_studies/2019-Poland-Renewable_Energy.pdf (accessed on 18 January 2022).
- Li, S.; Song, H.; Hu, J.; Yang, H.; Zou, J.; Zhu, Y.; Tang, Z.; Chen, H. CO2 gasification of straw biomass and its correlation with the feedstock characteristics. Fuel 2021, 297, 120780. [Google Scholar] [CrossRef]
- Proszak-Miasik, D.; Jarecki, W.; Nowak, K. Selected parameters of oat straw as an alternative energy raw material. Energies 2022, 15, 331. [Google Scholar] [CrossRef]
- Atabani, A.E.; Ala’a, H.; Kumar, G.; Saratale, G.D.; Aslam, M.; Khan, H.A.; Said, Z.; Mahmoud, E. Valorization of spent coffee grounds into biofuels and value-added products: Pathway towards integrated bio-refinery. Fuel 2019, 254, 115640. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gao, W.; Ok, Y.S.; Chen, W.-H.; Goh, B.H.H.; Chong, C.T. Solid biofuel production from spent coffee ground wastes: Process optimisation, characterisation and kinetic studies. Fuel 2021, 292, 120309. [Google Scholar] [CrossRef]
- Espuelas, S.; Marcelino, S.; Echeverría, A.M.; del Castillo, J.M.; Seo, A. Low energy spent coffee grounds briquetting with organic binders for biomass fuel manufacturing. Fuel 2020, 278, 118310. [Google Scholar] [CrossRef]
- Seco, A.; Espuelas, S.; Marcelino, S.; Echeverría, A.M.; Prieto, E. Characterization of biomass briquettes from spent coffee grounds and xanthan gum using low pressure and temperature. Bioenergy Res. 2020, 13, 369–377. [Google Scholar] [CrossRef]
- Kibret, H.A.; Kuo, Y.L.; Ke, T.Y.; Tseng, Y.H. Gasification of spent coffee grounds in a semi-fluidized bed reactor using steam and CO2 gasification medium. J. Taiwan Inst. Chem. Eng. 2021, 119, 115–127. [Google Scholar] [CrossRef]
- Corchado-Lopo, C.; Martínez-Avila, O.; Marti, E.; Llimós, J.; Busquets, A.M.; Kucera, D.; Obruca, S.; Llenas, L.; Ponsá, S. Brewer’s spent grain as a no-cost substrate for polyhydroxyalkanoates production: Assessment of pretreatment strategies and different bacterial strains. N. Biotechnol. 2021, 62, 60–67. [Google Scholar] [CrossRef]
- Mishra, P.K.; Gregor, T.; Wimmer, R. Utilising brewer’s spent grain as a source of cellulose nanofibres following separation of protein-based biomass. BioResources 2017, 12, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Weiermuller, J.; Akermann, A.; Laudensack, W.; Chodorski, J.; Blank, L.M.; Uliber, R. Brewers’ spent grain as carbon source for itaconate production with engineered Ustilago maydis. Bioresour. Technol. 2021, 336, 125262. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Bilbao, A.; Vilches, P.; Angulo, I.; Luis, J.; Fité, B.; Paseiro-Losada, P.; Cruz, J.M. Brewery waste as a potential source of phenolic compounds: Optimisation of the extraction process and evaluation of antioxidant and antimicrobial activities. Food Chem. 2014, 145, 191–197. [Google Scholar] [CrossRef]
- Han, T.; Lu, X.; Sun, Y.; Jiang, J.; Yang, W.; Jönsson, P.G. Magnetic bio-activated carbon production from lignin via a streamlined process and its use in phosphate removal from aqueous solutions. Sci. Total Environ. 2020, 708, 135069. [Google Scholar] [CrossRef]
- Kaźmierczak, B.; Molenda, J.; Swat, M. The Adsorption of chromium (III) ions from water solutions on biocarbons obtained from plant waste. Environ. Technol. Innov. 2021, 23, 101737. [Google Scholar] [CrossRef]
- Bahador, F.; Foroutan, R.; Esmaeili, H.; Ramavandi, B. Enhancement of the chromium removal behavior of moringa oleifera activated carbon by chitosan and iron oxide nanoparticles from water. Carbohydr. Polym. 2021, 251, 117085. [Google Scholar] [CrossRef] [PubMed]
- Vaiopoulou, E.; Gikas, P. Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere 2020, 254, 126876. [Google Scholar] [CrossRef] [PubMed]
- Payel, S.; Hashem, M.A.; Hasan, M.A. Recycling Biochar Derived from tannery liming sludge for chromium adsorption in static and dynamic conditions. Environ. Technol. Innov. 2021, 24, 102010. [Google Scholar] [CrossRef]
- Sieradzka, M.; Gao, N.; Quan, C.; Mlonka-Mędrala, A.; Magdziarz, A. Biomass thermochemical conversion via pyrolysis with integrated CO2 capture. Energies 2020, 13, 1050. [Google Scholar] [CrossRef] [Green Version]
- Parshetti, G.K.; Kent Hoekman, S.; Balasubramanian, R. Chemical, structural and combustion characteristics of carbonaceous products obtained by hydrothermal carbonization of palm empty fruit bunches. Bioresour. Technol. 2013, 135, 683–689. [Google Scholar] [CrossRef]
- Magdziarz, A.; Mlonka-Mędrala, A.; Sieradzka, M.; Aragon-Briceño, C.; Pożarlik, A.; Bramer, E.A.; Brem, G.; Niedzwiecki, Ł.; Pawlak-Kruczek, H. Multiphase analysis of hydrochars obtained by anaerobic digestion of municipal solid waste organic fraction. Renew. Energy 2021, 17, 108–118. [Google Scholar] [CrossRef]
- Mureddu, M.; Dessì, F.; Orsini, A.; Ferrara, F.; Pettinau, A. Air- and oxygen-blown characterization of coal and biomass by thermogravimetric analysis. Fuel 2018, 212, 626–637. [Google Scholar] [CrossRef]
- Cuong, D.V.; Matsagar, B.M.; Lee, M.; Hossain, M.S.A.; Yamauchi, Y.; Vithanage, M.; Sarkar, B.; Ok, Y.S.; Wu, K.C.-W.; Hou, C.-H. Critical review on biochar-based engineered hierarchical porous carbon for capacitive charge storage. Renew. Sustain. Energy Rev. 2021, 145, 111029. [Google Scholar] [CrossRef]
- Deng, Z.; Sun, S.; Li, H.; Pan, D.; Patil, R.R.; Guo, Z.; Seok, I. Modification of coconut shell-based activated carbon and purification of wastewater. Adv. Compos. Hybrid Mater. 2021, 4, 65–73. [Google Scholar] [CrossRef]
- Aboua, K.N.; Yobouet, K.Y.A.; Yao, K.B.; Goné, D.L.; Trokourey, A. Investigation of dye adsorption onto activated carbon from the shells of Macoré fruit. J. Environ. Manag. 2015, 156, 10–14. [Google Scholar] [CrossRef]
- Ding, S.; Liu, Y. Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel 2020, 260, 116382. [Google Scholar] [CrossRef]


| Sample | M | A | VM | C | H | N |
|---|---|---|---|---|---|---|
| WS | 5.8 | 6.8 | 76.0 | 43.23 | 5.97 | 0.97 |
| WS_400 | 18.2 | 30.6 | 62.58 | 3.95 | 1.64 | |
| WS_500 | 67.66 | 2.99 | 1.74 | |||
| SCG | 14.8 | 4.0 | 81.7 | 44.97 | 7.47 | 2.18 |
| SCG_400 | 8.6 | 38.4 | 73.32 | 5.17 | 4.17 | |
| SCG_500 | 76.78 | 3.38 | 4.25 | |||
| BG | 1.5 | 2.1 | 80.3 | 48.80 | 6.87 | 4.39 |
| BG_400 | 6.2 | 37.1 | 69.88 | 4.87 | 6.82 | |
| BG_500 | 70.86 | 3.26 | 6.80 |
| Sample | Di, wt. %/min3 | Df, wt. %/min4 | S, min−2 °C−3 | Hf, °C |
|---|---|---|---|---|
| WS | 0.0149 | 3.52 × 10−4 | 4.70 × 10−7 | 834 |
| WS_400 | 0.0105 | 2.15 × 10−4 | 2.66 × 10−7 | 1111 |
| WS_500 | 0.0246 | 5.80 × 10−4 | 5.82 × 10−7 | 1055 |
| SCG | 0.0078 | 1.47 × 10−4 | 3.60 × 10−7 | 1438 |
| SCG_400 | 0.0072 | 1.06 × 10−4 | 2.59 × 10−7 | 1687 |
| SCG_500 | 0.0077 | 1.61 × 10−4 | 2.12 × 10−7 | 1447 |
| BG | 0.0115 | 1.67 × 10−4 | 2.54 × 10−7 | 816 |
| BG_400 | 0.0027 | 0.482 × 10−4 | 0.72 × 10−7 | 1661 |
| BG_500 | 0.0033 | 0.554 × 10−4 | 0.77 × 10−7 | 1756 |
| Specific surface area (BET), m2/g | WS | WS_400 | WS_500 | SCG | BG | BG_400 | BG_500 |
| 2.25 | 2.68 | 1.82 | 0.06 | 0.36 | 0.67 | 0.27 |
| Specific surface area (BET), m2/g | WS_400 | SCG_400 | BG_400 |
| 1939.54 | 2510.06 | 2618.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sieradzka, M.; Kirczuk, C.; Kalemba-Rec, I.; Mlonka-Mędrala, A.; Magdziarz, A. Pyrolysis of Biomass Wastes into Carbon Materials. Energies 2022, 15, 1941. https://doi.org/10.3390/en15051941
Sieradzka M, Kirczuk C, Kalemba-Rec I, Mlonka-Mędrala A, Magdziarz A. Pyrolysis of Biomass Wastes into Carbon Materials. Energies. 2022; 15(5):1941. https://doi.org/10.3390/en15051941
Chicago/Turabian StyleSieradzka, Małgorzata, Cezary Kirczuk, Izabela Kalemba-Rec, Agata Mlonka-Mędrala, and Aneta Magdziarz. 2022. "Pyrolysis of Biomass Wastes into Carbon Materials" Energies 15, no. 5: 1941. https://doi.org/10.3390/en15051941






