Analysis of Using Soot Application in the Processing of Zinc-Bearing Waste Materials
Abstract
:1. Introduction
1.1. Pyrometallurgical Processes
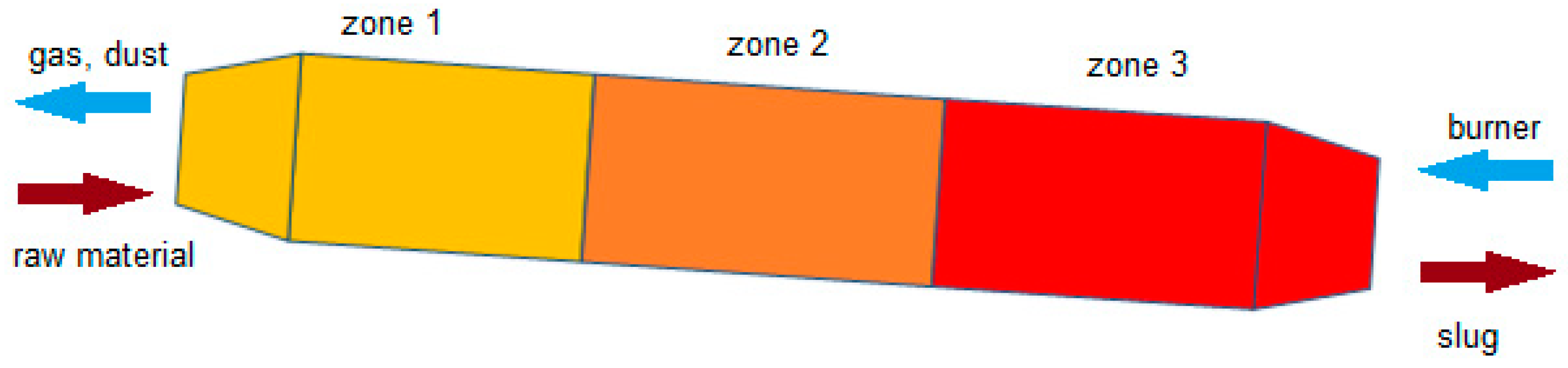
1.2. Waelz Process
1.3. Fire and Explosion Safety
2. Materials
3. Methods
- Determination of the characteristic properties of soot in terms of the applicability in pyrometallurgical processes for obtaining metals (soot grain analysis, thermogravimetric tests, and explosion tests);
- Testing the processing of waste zinc-bearing materials in a fusing furnace.
- The first stage consisted in heating the fuel sample to 1163 K in an inert gas atmosphere, at a heating rate of 10 K/min;
- The second, after exceeding 1163 K, synthetic air was introduced into the interior space of the furnace. At this stage, the heating rate was slowed down to obtain complete combustion of the fuel sample in the air atmosphere. The heating rate was set at 2 K/min. The experiment was completed at a temperature of 1298 K.
4. Results
4.1. Particle Size Distribution
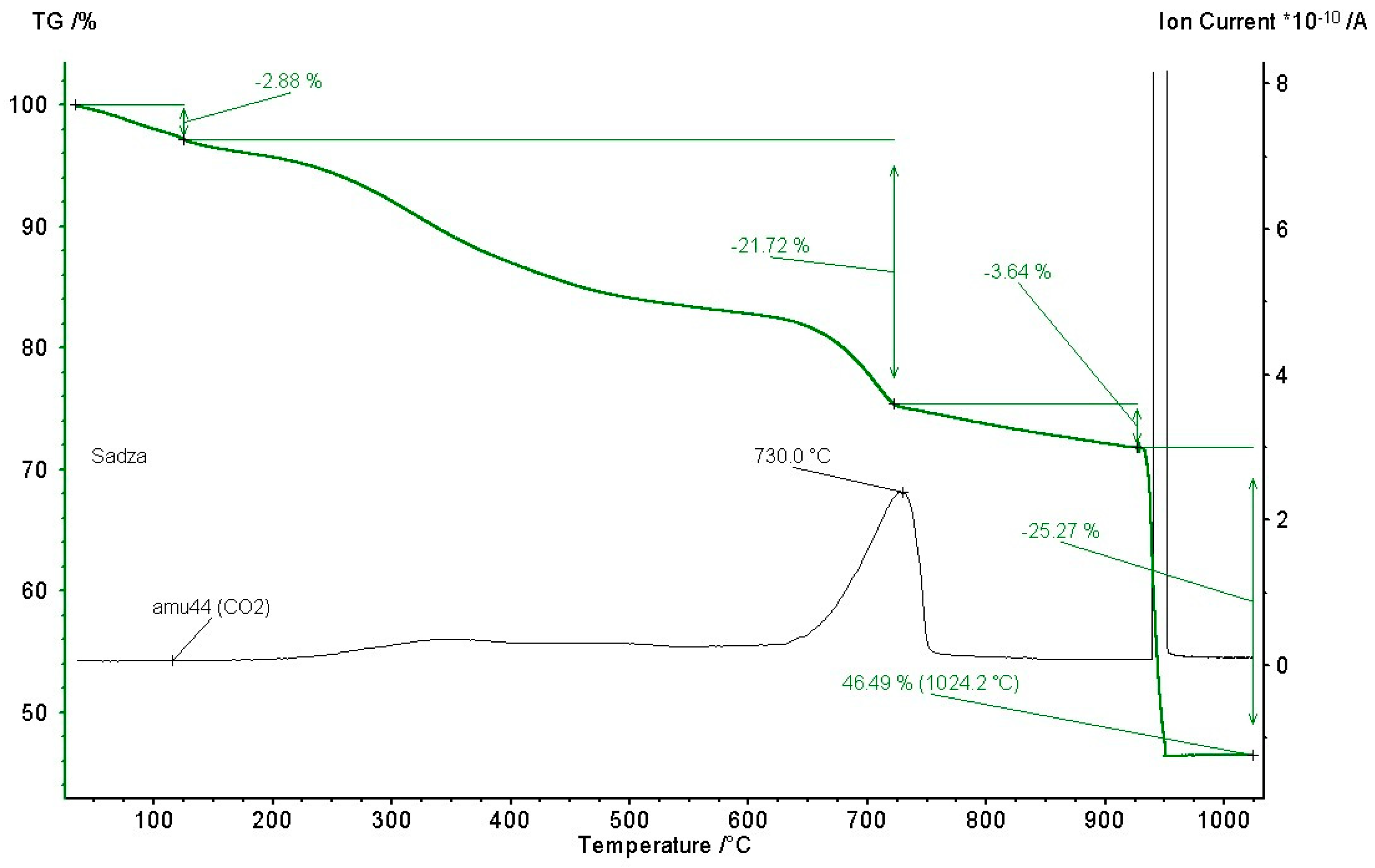
4.2. TG Analysis and Explosion Indices
- The first phase is connected with the evaporation of moisture present in the sample (around 2.88 wt.%), during which the CO2 concentration was equal to zero;
- After the moisture is evaporated, approx. constant ion current is observed (responsible for the molecular mass of 44, i.e., CO2), in the range of temperatures of approx. 524–914 K. During this phase, a constant decomposition of volatile matter is observed (<21.72 wt.%);
- At the temperature of 1004 K, the first peak value of the ion current was observed, which corresponds to Reaction (4), described previously, i.e., carbonates decomposition;
- At the temperature of 1214 K, the highest peak value was observed. During the following 2–3 min, the mass of the sample decreased by approx. 25 wt.%, which corresponds to the introduction of the air into the test chamber, causing sudden oxidation of the remaining carbon. Therefore, 46.49% of the total sample mass constitutes ash.
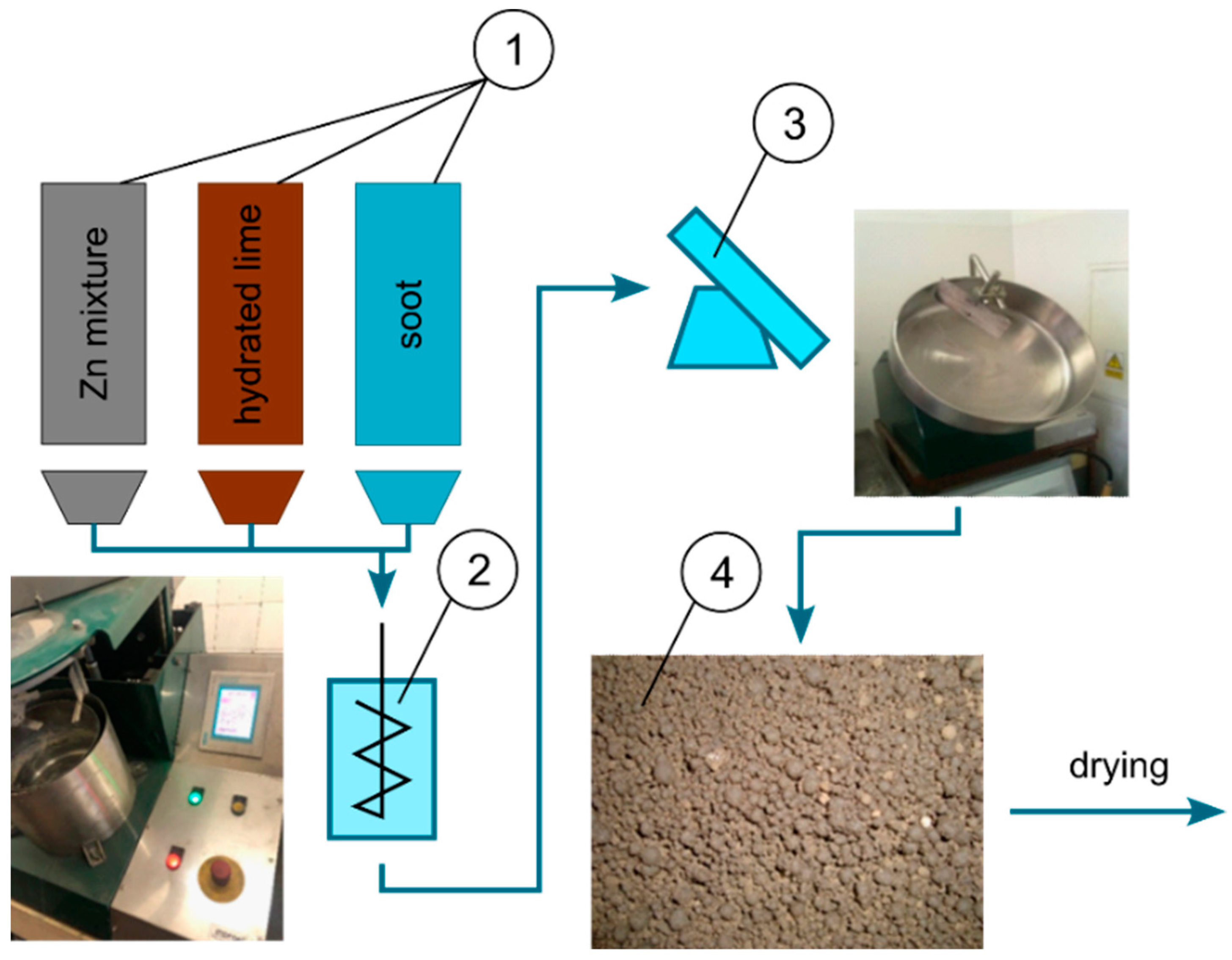
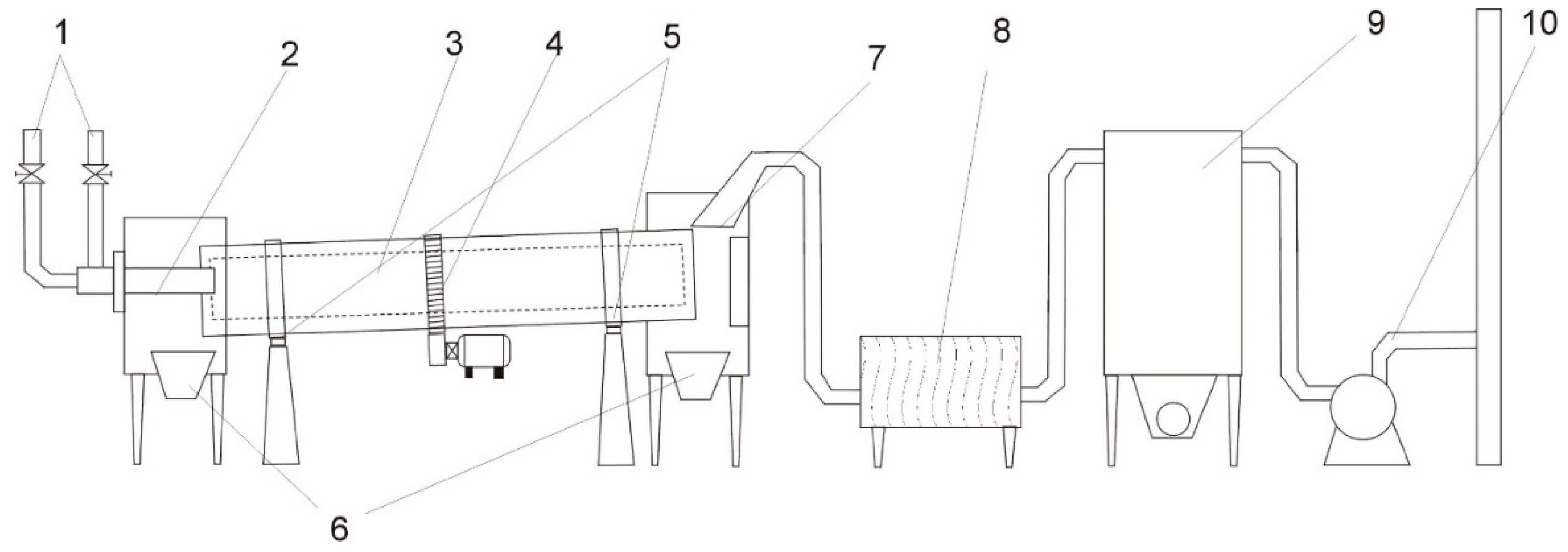
4.3. Processing of Zinc-Bearing Waste with the Use of Soot in a Rotary Kiln
5. Conclusions
- The use of fine-grained (dusty) soot in the processing of waste zinc-bearing materials in a rotary kiln is not possible due to the risk of ignition and explosion.
- A method that allows the use of soot includes introducing them into the furnace in the form of an agglomerate with zinc-bearing charge materials.
- The content of zinc and lead in the crude oxide obtained in laboratory conditions in the process with the use of soot is at a similar level to the case of zinc oxides obtained in industrial conditions in the process with the use of coke breeze.
- Based on the test results and the analysis of the physicochemical properties of the materials used, it was found that soot, with the appropriate procedures at the stage of agglomerate preparation, meets the requirements for reducers and may be an alternative to coke breeze in the metallurgical process.
- From the point of view of fire and explosion protection, the use of the materials described in the article, as the redactors, is permissible, in particular for anthracite, whose flammability and explosive parameters are more favorable than soot and typical charges are made of carbon-bearing materials, e.g., coke. Typical ignition sources, such as surface temperature [24], will not have sufficient characteristics to ignite the dust.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coal Mining and Consumption in the EU. How is Poland Doing? (Language: PL). Available online: https://demagog.org.pl/analizy_i_raporty/wydobycie-i-zuzycie-wegla-w-ue-jak-wypada-polska/ (accessed on 9 May 2022).
- Łędzki, A.; Klimczyk, A.; Stachura, R.; Bernasowski, M. Rola Własności Koksu w Wybranych Piecach Szybowych. Wiad Hut 2014, 18, 765–768. [Google Scholar]
- Tochowicz, S. Wytapianie Stali w Piecach Elektrycznych, 1st ed.; Wydawnictwo Śląsk: Katowice, Poland, 1988. [Google Scholar]
- Cholewa, M.; Gawroński, J.; Przybył, M. Podstawy Procesów Metalurgicznych, 1st ed.; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2004. [Google Scholar]
- Lis, T. Współczesne Metody Otrzymywania Stali, 1st ed.; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2000. [Google Scholar]
- Łędzki, A.; Zieliński, K.; Klimczyk, A. Podstawy Technologii Wytwarzania i Przetwarzania—Część V stalownictwo; Akademia Górniczo—Hutnicza: Kraków, Poland, 2020. [Google Scholar]
- Łabaj, J. Możliwości wykorzystania odpadowych surowców węglonośnych do otrzymywania cynku i ołowiu. Rudy Met. Nieżel. 2008, 6, 350–354. [Google Scholar]
- An Introduction to Iron and Steel Processing. Available online: http://www.jfe-21st-cf.or.jp/index2.html (accessed on 8 October 2021).
- Niesler, M. (Ed.) Najlepsze Dostępne Techniki (BAT) Wytyczne dla Produkcji Stali, Stalownie Elektryczne z Odlewaniem Stali. Available online: http://www.ekoportal.gov.pl/fileadmin/Ekoportal/Pozwolenia_zintegrowane/poradniki_branzowe/5._Najlepsze_Dostepne_Techniki__BAT__wytyczne_dla_branzy_metali_zelaznych_-_stalownie_elektryczne_z_odlewaniem_stali.pdf (accessed on 23 November 2021).
- World Steel in Figures 2008, 2nd Edition. Available online: http://www.worldsteel.org/?action=publicationlist (accessed on 19 September 2021).
- Suetens, T.; Klaasen, K.; Van Acker, K.; Blanpain, B. Comparison of electric arc furnace dust treatment technologies using. J Clean Prod. 2014, 65, 152–167. [Google Scholar] [CrossRef]
- NTN Develops. “EAF Dust Briquetter” New Product and Technology Info. Available online: http://www.ntn.co.jp/english/news/news_files/new_products/2006.html (accessed on 20 October 2021).
- Czernecki, J.; Stós, E.; Botor, J.; Prajsnar, R.; Czekaj, J.; Galicki, J.; Jasiński, J. Technologia przerobu pyłów stalowniczych w piecach obrotowych. Pr. Inst. Metal Żel. 2002, 1, 17–23. [Google Scholar]
- EN IEC 60079; Explosive Atmospheres—Part 0: Equipment—General Requirements. International Electrotechnical Commission: Geneva, Switzerland, 2017.
- Eckhoff, R.K. Understanding Dust Explosions. The Role of Power Science and Technology. J. Loss Prevent. Proc. 2009, 22, 105–116. [Google Scholar] [CrossRef]
- Półka, M.; Ptak, S. Impact of Biomass Implementation on Coal Burning Installations. Proc. Eng. 2017, 192, 743–747. [Google Scholar]
- Frank, W.L. Dust explosion prevention and the critical importance of housekeeping. Proc. Saf. Prog. 2004, 23, 175–184. [Google Scholar] [CrossRef]
- Półka, M. An Analysis of Flammability and Explosion Parameters of Coke Dust and Use of Preliminary Hazard Analysis for Qualitative Risk Assessment. Sustainability 2020, 12, 4130. [Google Scholar] [CrossRef]
- ISO 13320:2020; Particle Size Analysis—Laser Diffraction Methods. International Electrotechnical Commission: Geneva, Switzerland, 2020.
- EN 14034; Part 1. Determination of Explosion Characteristics of Dust Clouds—Part 1: Determination of the Maximum Explosion Pressure pmax of Dust Clouds. International Electrotechnical Commission: Geneva, Switzerland, 2011.
- EN 14034; Part 2. Determination of Explosion Characteristics of Dust Clouds—Part 2: Determination of the Maximum Rate of Explosion Pressure Rise (dp/dt) max of Dust Clouds. International Electrotechnical Commission: Geneva, Switzerland, 2011.
- EN 14034; Part 3. Determination Of Explosion Characteristics of Dust Clouds Determination of The Lower Explosion Limit LEL Of Dust Clouds. International Electrotechnical Commission: Geneva, Switzerland, 2011.
- EN 14034; Part 4. Determination of Explosion Characteristics of Dust Clouds. Determination of the Limiting Oxygen Concentration LOC of Dust Clouds. International Electrotechnical Commission: Geneva, Switzerland, 2011.
- Perka, B.; Piwowarski, K. A Method for Determining the Impact of Ambient Temperature on an Electrical Cable during a Fire. Energies 2021, 14, 7260. [Google Scholar] [CrossRef]






| Technology | Carbon-Bearing Material Used | Consumption of Carbon-Bearing Material |
|---|---|---|
| Preparation of pig iron in a blast furnace [2] | coke | 300–500 kg/t of pig iron |
| Production of zinc in ISP technology | coke | 0.8-1.2 kg/kg Zn |
| Processing of steel dust in rotary kilns | coke breeze | 1.5–2 kg/kg Zn |
| Processing of lead secondary raw materials in rotary and shuttle furnaces | coke breeze | 50 kg/Mg Pb |
| Processing of copper slags in an electric furnace | coke | 0.25–0.4 kg/kg Cu |
| Zinc-Bearing Material | Component Content, wt.% | ||||
|---|---|---|---|---|---|
| Zn | Pb | Cd | Fe | H2O | |
| Material I | 19.9 | 11.5 | 0.4 | - | 17.8 |
| Material II | 22.7 | 0.5 | 0.2 | - | 52.7 |
| Material III | 11.5 | 0.3 | 0.02 | - | 41.3 |
| Steel dust | 22.1 | 2.2 | - | 31.5 | - |
| Parameter | Unit | Value |
|---|---|---|
| Total carbon content | wt.% | 66–68.4 |
| Calorific value | kJ kg−1 | 22.8–33.0 |
| Sulfur content | wt.% | 0.32 |
| Volatile matter content | wt.% | 9.91 |
| Ash content | wt.% | 22.1 |
| Moisture content | wt.% | 1.7–3.5 |
| Parameter | Soot | Anthracite |
|---|---|---|
| 5.9 bar @500 g/m3 | 4.4 bar @500 g/m3 | |
| 420 bar/s @750 g/m3 | 249 bar/s @250 g/m3 | |
114 m∙bar/s @750 g/m3 | 68 m∙bar/s @250 g/m3 | |
| LEC | 125 g/m3 | n/a |
| LOC | 16% | n/a |
| Parameter | Soot | Anthracite Dust |
|---|---|---|
| TCL | 674 K | >1274 K 1 |
| T5mm | 564 K | >674 K 2 |
| Zinc-Bearing Material Used (Zn Content, wt.%) | Content, wt.% | |||
|---|---|---|---|---|
| Zn | Pb | Cd | Fe | |
| Material I (19.9) | 49.60 | 16.50 | 4.10 | - |
| Material II (22.7) | 51.43 | 17.27 | 1.66 | - |
| Material III (11.5) | 48.61 | 14.49 | 1.67 | - |
| Steel dust (22.1) | 58.87 | 8.21 | - | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smalcerz, A.; Ptak, S.; Łabaj, J.; Półka, M.; Kula, A.; Blacha, L. Analysis of Using Soot Application in the Processing of Zinc-Bearing Waste Materials. Energies 2022, 15, 7969. https://doi.org/10.3390/en15217969
Smalcerz A, Ptak S, Łabaj J, Półka M, Kula A, Blacha L. Analysis of Using Soot Application in the Processing of Zinc-Bearing Waste Materials. Energies. 2022; 15(21):7969. https://doi.org/10.3390/en15217969
Chicago/Turabian StyleSmalcerz, Albert, Szymon Ptak, Jerzy Łabaj, Marzena Półka, Adam Kula, and Leszek Blacha. 2022. "Analysis of Using Soot Application in the Processing of Zinc-Bearing Waste Materials" Energies 15, no. 21: 7969. https://doi.org/10.3390/en15217969





