Experimental and Photothermal Performance Evaluation of Multi-Wall Carbon-Nanotube-Enhanced Microencapsulation Phase Change Slurry for Efficient Photothermal Conversion and Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
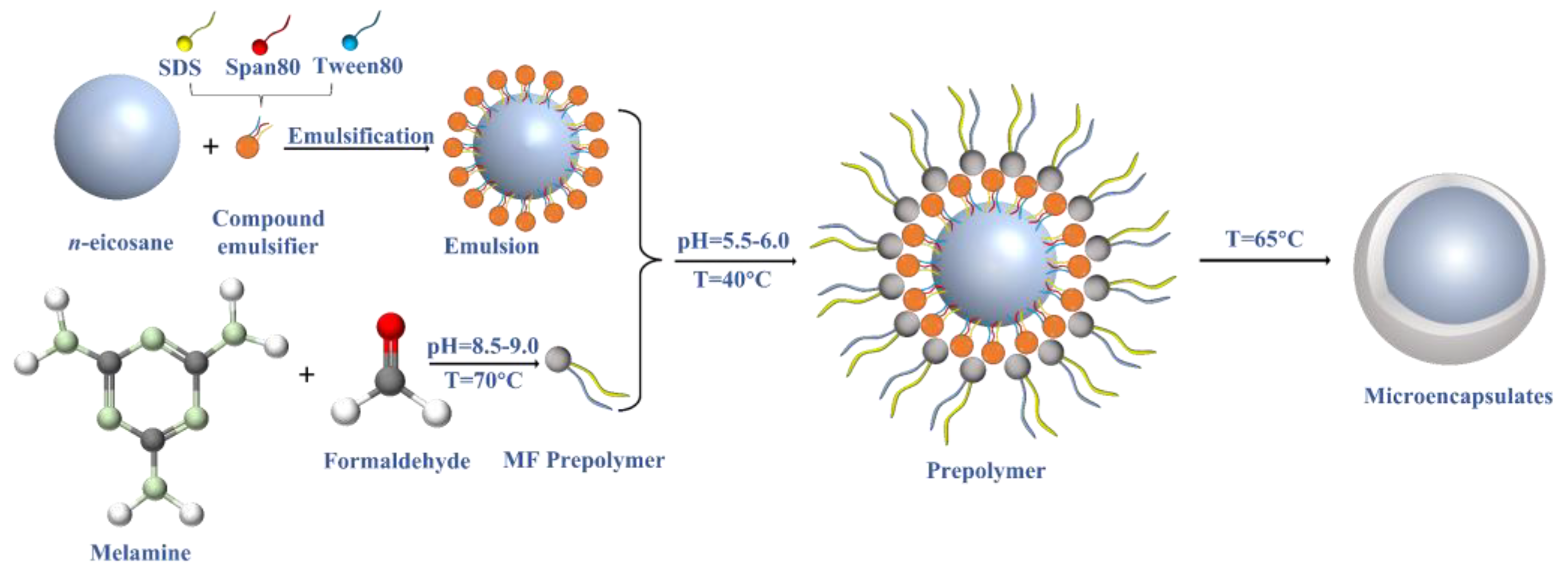
2.2. Synthesis of Microencapsulated Phase Change Materials
2.3. Preparation of the Nano-Enhanced MPCM Slurry
2.4. Characterization and Measurement
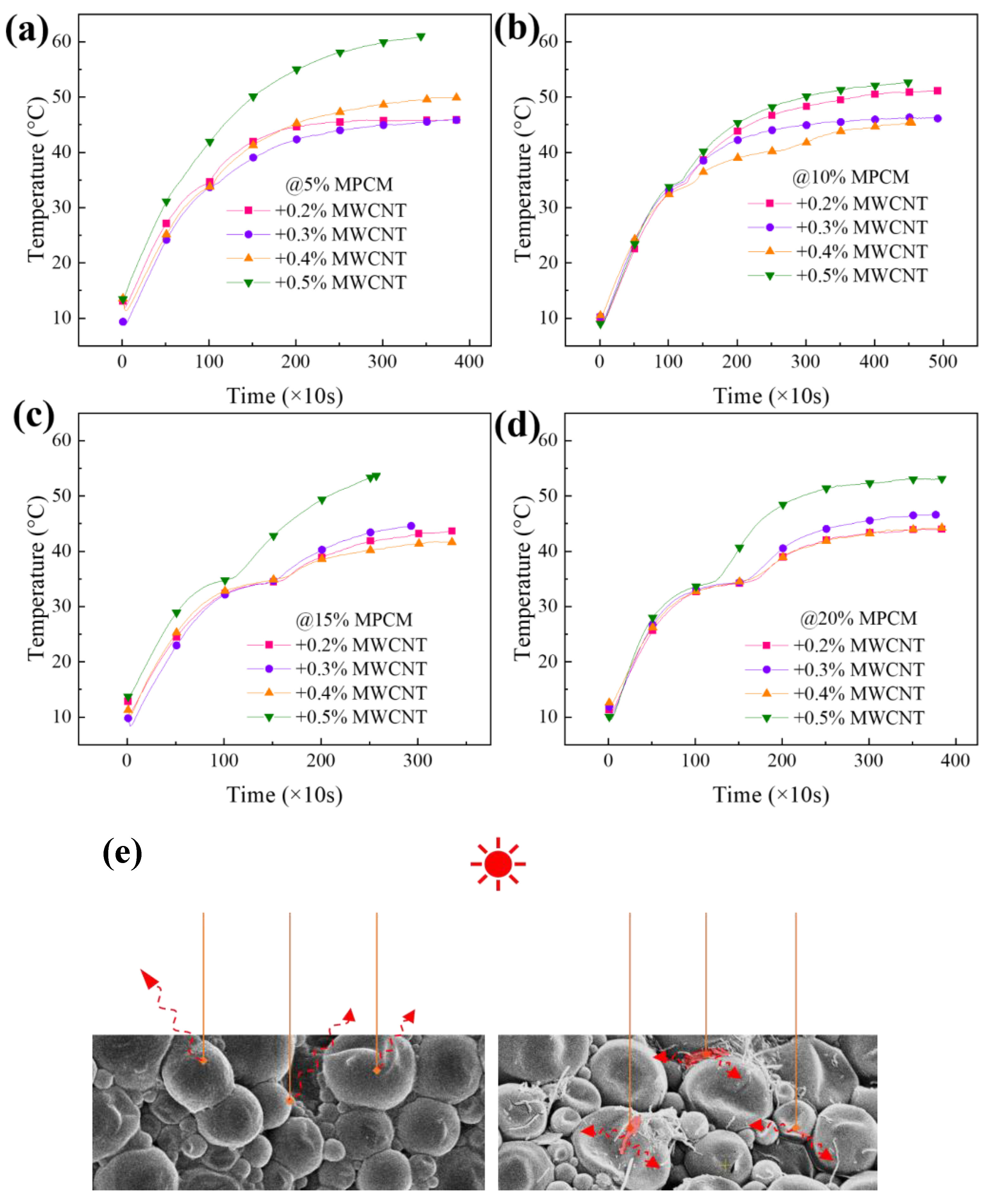
3. Results and Discussion
Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paul, J.; Kadirgama, K.; Samykano, M.; Pandey, A.K.; Tyagi, V.V. A comprehensive review on thermophysical properties and solar thermal applications of organic nano composite phase change materials. J. Energy Storage 2022, 45, 103415. [Google Scholar] [CrossRef]
- Robertson, N. Catching the rainbow: Light harvesting in dye-sensitized solar cells. Angew. Chem. Int. Ed. 2008, 47, 1012–1014. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhou, J.; Li, H.; Li, X. Cuprous oxide modified nanoencapsulated phase change materials fabricated by RAFT miniemulsion polymerization for thermal energy storage and photothermal conversion. Powder Technol. 2022, 399, 117189. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Yan, Y.; Chen, Z. Synthesis and characterization of hydroxylated carbon nanotubes modified microencapsulated phase change materials with high latent heat and thermal conductivity for solar energy storage. Sol. Energy Sol. Cells 2022, 236, 111546. [Google Scholar] [CrossRef]
- Yu, C.; Park, J.; Youn, J.R.; Song, Y.S. Sustainable solar energy harvesting using phase change material (PCM) embedded pyroelectric system. Energy Convers. Manag. 2022, 253, 115145. [Google Scholar] [CrossRef]
- Yan, D.; Li, M. TaON doped metal-organic frameworks-based composite phase change materials with excellent photo-thermal conversion performance. Sol. Energy Mater. Sol. Cells 2022, 243, 111790. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, T.; Wang, Y.; Li, J.; Liu, H.; Wang, X. Hierarchical microencapsulation of phase change material with carbon-nanotubes/polydopamine/silica shell for synergistic enhancement of solar photothermal conversion and storage. Sol. Energy Mater. Sol. Cells 2022, 236, 111539. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Han, N.; Zhang, X.; Li, W. Design and synthesis of microcapsules with cross-linking network supporting core for supercooling degree regulation. Energy Build. 2021, 253, 111437. [Google Scholar] [CrossRef]
- Qin, M.; Feaugas, O.; Zu, K. Novel metal-organic framework (MOF) based phase change material composite and its impact on building energy consumption. Energy Build. 2022, 273, 112382. [Google Scholar] [CrossRef]
- Yuan, S.; Yan, R.; Ren, B.; Du, Z.; Cheng, X.; Du, X.; Wang, H. Robust, double-layered phase-changing microcapsules with superior solar-thermal conversion capability and extremely high energy storage density for efficient solar energy storage. Renew. Energy 2021, 180, 725–733. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Farid, M.; Al-Muhtaseb, S.; Kurdi, J. Emulsion stability and cross-linking of PMMA microcapsules containing phase change materials. Sol. Energy Mater. Sol. Cells 2015, 132, 311–318. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, J.; Wang, B.; Li, Y.; Zhang, W.; Zhou, J.; Guo, H.; Liu, Y. Biodegradable wood plastic composites with phase change microcapsules of honeycomb-BN-layer for photothermal energy conversion and storage. Chem. Eng. J. 2022, 448, 137218. [Google Scholar] [CrossRef]
- Chen, X.; Kong, X.; Wang, S.; Fu, X.; Yu, B.; Yao, K.; Zhong, Y.; Li, J.; Xu, W.; Liu, Y. Microencapsulated phase change materials: Facile preparation and application in building energy conservation. J. Energy Storage 2022, 48, 104025. [Google Scholar] [CrossRef]
- Zhu, X.; Sheng, X.; Li, J.; Chen, Y. Thermal comfort and energy saving of novel heat-storage coatings with microencapsulated PCM and their application. Energy Build. 2021, 251, 111349. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Salazar, S.L.; Rao, Z.; Arıcı, M.; Wei, W. Photothermal properties and photothermal conversion performance of nano-enhanced paraffin as a phase change thermal energy storage material. Sol. Energy Mater. Sol. Cells 2021, 219, 110792. [Google Scholar] [CrossRef]
- Ma, X.; Liu, H.; Chen, C.; Liu, Y.; Zhang, L.; Xu, B.; Xiao, F. Synthesis of novel microencapsulated phase change material with SnO2/CNTs shell for solar energy storage and photo-thermal conversion. Mater. Res. Express 2020, 7, 015513. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, T.; Jiao, S.; Guo, C. Preparation of reduced graphene oxide modified magnetic phase change microcapsules and their application in direct absorption solar collector. Sol. Energy Mater. Sol. Cells 2020, 216, 110695. [Google Scholar] [CrossRef]
- Meng, D.; Yang, S.; Guo, L.; Li, G.; Ge, J.; Huang, Y.; Bielawski, C.W.; Geng, J. The enhanced photothermal effect of graphene/conjugated polymer composites: Photoinduced energy transfer and applications in photocontrolled switches. Chem. Commun. 2014, 50, 14345–14348. [Google Scholar] [CrossRef]
- Xu, B.; Wei, Z.; Hong, X.; Zhou, Y.; Gan, S.; Shen, M. Preparation and characterization of novel microencapsulated phase change materials with SiO2/FeOOH as the shell for heat energy storage and photocatalysis. J. Energy Storage 2021, 43, 103251. [Google Scholar] [CrossRef]
- Lin, J.; Ouyang, Y.; Chen, L.; Wen, K.; Li, Y.; Mu, H.; Ren, Q.; Xie, X.; Long, J. Enhancing the solar absorption capacity of expanded graphite-paraffin wax composite phase change materials by introducing carbon nanotubes additives. Surf. Interfaces 2022, 30, 101871. [Google Scholar] [CrossRef]
- Zheng, X.; Gao, X.; Huang, Z.; Li, Z.; Fang, Y.; Zhang, Z. Form-stable paraffin/graphene aerogel/copper foam composite phase change material for solar energy conversion and storage. Sol. Energy Mater. Sol. Cells 2021, 226, 111083. [Google Scholar] [CrossRef]
- Maithya, O.M.; Li, X.; Feng, X.; Sui, X.; Wang, B. Microencapsulated phase change material via Pickering emulsion stabilized by graphene oxide for photothermal conversion. J. Mater. Sci. 2020, 55, 7731–7742. [Google Scholar] [CrossRef]
- Maithya, O.M.; Zhu, X.; Li, X.; Korir, S.J.; Feng, X.; Sui, X.; Wang, B. High-energy storage graphene oxide modified phase change microcapsules from regenerated chitin Pickering Emulsion for photothermal conversion. Sol. Energy Mater. Sol. Cells 2021, 222, 110924. [Google Scholar] [CrossRef]
- Zhao, Q.; He, F.; Zhang, Q.; Fan, J.; He, R.; Zhang, K.; Yan, H.; Yang, W. Microencapsulated phase change materials based on graphene Pickering emulsion for light-to-thermal energy conversion and management. Sol. Energy Mater. Sol. Cells 2019, 203, 110204. [Google Scholar] [CrossRef]
- Liu, H.; Tian, X.; Ouyang, M.; Wang, X.; Wu, D.; Wang, X. Microencapsulating n-docosane phase change material into CaCO3/Fe3O4 composites for high-efficient utilization of solar photothermal energy. Renew. Energy 2021, 179, 47–64. [Google Scholar] [CrossRef]
- Fan, X.; Qiu, X.; Lu, L.; Zhou, B. Full-spectrum light-driven phase change microcapsules modified by CuS-GO nanoconverter for enhancing solar energy conversion and storage capability. Sol. Energy Mater. Sol. Cells 2021, 223, 110937. [Google Scholar] [CrossRef]
- Eisapour, M.; Eisapour, A.H.; Hosseini, M.J.; Talebizadehsardari, P. Exergy and energy analysis of wavy tubes photovoltaic-thermal systems using microencapsulated PCM nano-slurry coolant fluid. Appl. Energy 2020, 266, 114849. [Google Scholar] [CrossRef]
- Ho, C.J.; Tanuwijava, A.O.; Lai, C.-M. Thermal and electrical performance of a BIPV integrated with a microencapsulated phase change material layer. Energy Build. 2012, 50, 331–338. [Google Scholar] [CrossRef]
- Liu, L.; Jia, Y.; Lin, Y.; Alva, G.; Fang, G. Performance evaluation of a novel solar photovoltaic–thermal collector with dual channel using microencapsulated phase change slurry as cooling fluid. Energy Convers. Manag. 2017, 145, 30–40. [Google Scholar] [CrossRef]
- Liu, L.; Jia, Y.; Lin, Y.; Alva, G.; Fang, G. Numerical study of a novel miniature compound parabolic concentrating photovoltaic/thermal collector with microencapsulated phase change slurry. Energy Convers. Manag. 2017, 153, 106–114. [Google Scholar] [CrossRef]
- Moradi, H.; Mirjalily, S.A.A.; Oloomi, S.A.A.; Karimi, H. Performance evaluation of a solar air heating system integrated with a phase change materials energy storage tank for efficient thermal energy storage and management. Renew. Energy 2022, 191, 974–986. [Google Scholar] [CrossRef]
- Hu, D.; Han, L.; Zhou, W.; Li, P.; Huang, Y.; Yang, Z.; Jia, X. Flexible phase change composite based on loading paraffin into cross-linked CNT/SBS network for thermal management and thermal storage. Chem. Eng. J. 2022, 437, 135056. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Wi, S.; Yun, B.Y.; Kim, S. Engineering biochar with multiwalled carbon nanotube for efficient phase change material encapsulation and thermal energy storage. Energy 2021, 216, 119294. [Google Scholar] [CrossRef]
- Ong, P.J.; Png, Z.M.; Soo, X.Y.D.; Wang, X.; Suwardi, A.; Chua, M.H.; Xu, J.W.; Zhu, Q. Surface modification of microencapsulated phase change materials with nanostructures for enhancement of their thermal conductivity. Mater. Chem. Phys. 2022, 277, 125438. [Google Scholar] [CrossRef]
- Khudhair, A.M.; Farid, M.M. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers. Manag. 2004, 45, 263–275. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Kaushik, S.C.; Tyagi, S.K.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Chen, X.; Yang, H. Research progress on utilization of phase change materials in photovoltaic/thermal systems: A critical review. Renew. Sustain. Energy Rev. 2021, 149, 111313. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, H.; Chen, X.; Zhang, Y.; Li, A.; Wang, G. Advanced multifunctional composite phase change materials based on photo-responsive materials. Nano Energy 2021, 80, 105454. [Google Scholar] [CrossRef]
- Li, M.; Mu, B. Effect of different dimensional carbon materials on the properties and application of phase change materials: A review. Appl. Energy 2019, 242, 695–715. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Innovative design of microencapsulated phase change materials for thermal energy storage and versatile applications: A review. Sustain. Energy Fuels 2019, 3, 1091–1149. [Google Scholar] [CrossRef]
- Su, W.; Hu, M.; Wang, L.; Kokogiannakis, G.; Chen, J.; Gao, L.; Li, A.; Xu, C. Microencapsulated phase change materials with graphene-based materials: Fabrication, characterisation and prospects. Renew. Sustain. Energy Rev. 2022, 168, 112806. [Google Scholar] [CrossRef]
- Rodríguez-Cumplido, F.; Pabón-Gelves, E.; Chejne-Jana, F. Recent developments in the synthesis of microencapsulated and nanoencapsulated phase change materials. J. Energy Storage 2019, 24, 100821. [Google Scholar] [CrossRef]

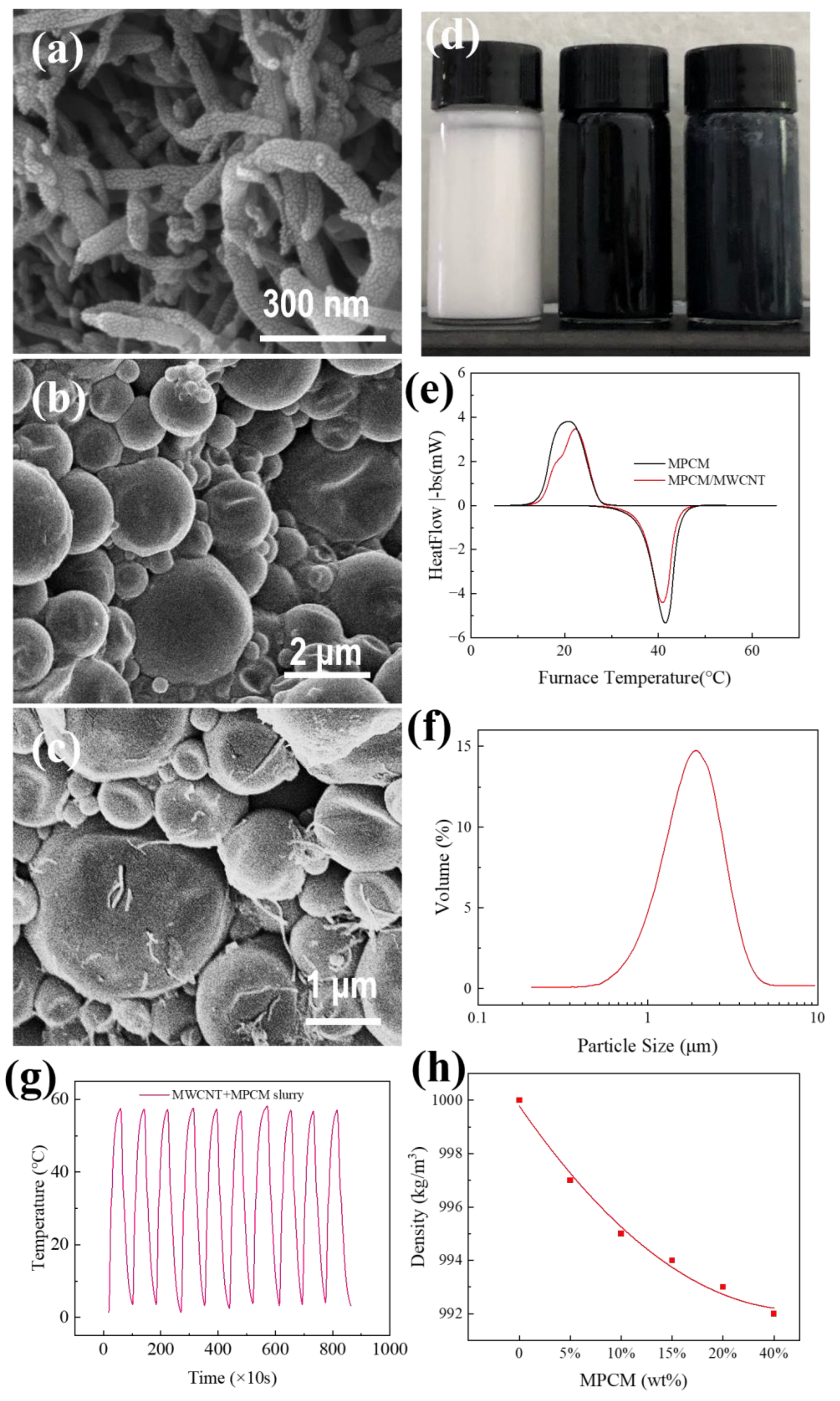
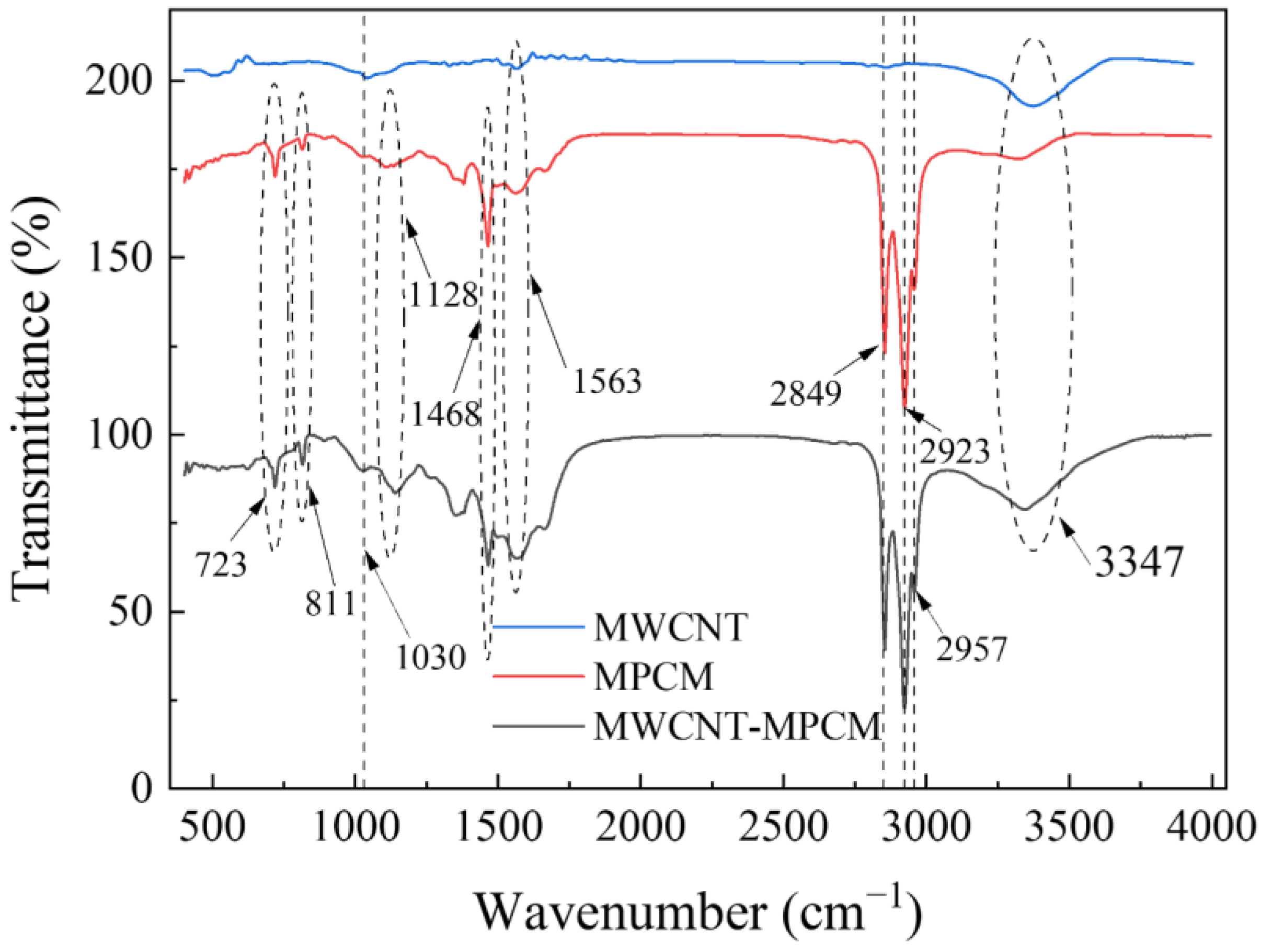
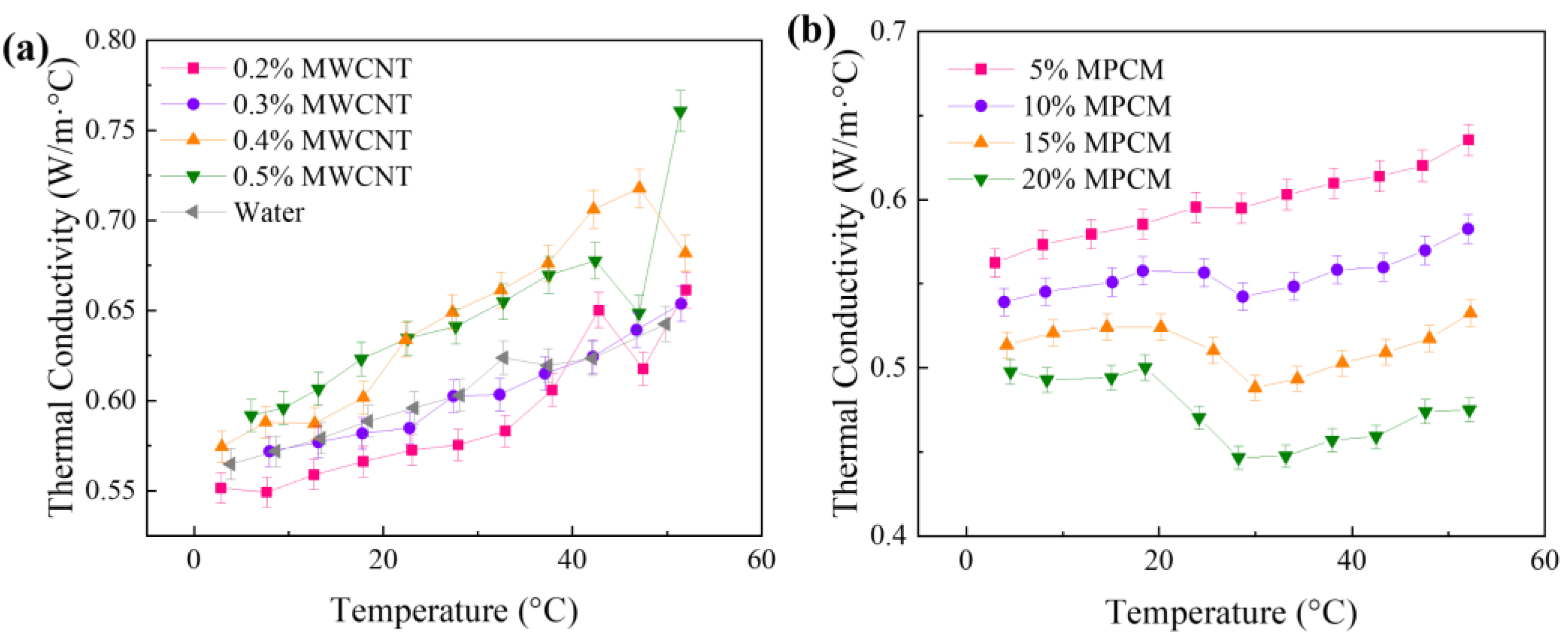



| Properties | MPCM | MWCNT-MPCM |
|---|---|---|
| Melting onset temperature (°C) | 36.19 | 36.24 |
| Melting offset temperature (°C) | 44.36 | 43.77 |
| Melting peak temperature (°C) | 41.41 | 40.87 |
| Solidifying onset temperature (°C) | 26.88 | 27.26 |
| Solidifying offset temperature (°C) | 14.99 | 14.57 |
| Solidifying peak temperature (°C) | 20.25 | 22.30 |
| Latent heat-melting (kJ/kg) | 135.92 | 107.72 |
| Latent heat-solidifying (kJ/kg) | 139.79 | 111.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhang, G.; Zhang, X. Experimental and Photothermal Performance Evaluation of Multi-Wall Carbon-Nanotube-Enhanced Microencapsulation Phase Change Slurry for Efficient Photothermal Conversion and Storage. Energies 2022, 15, 7627. https://doi.org/10.3390/en15207627
Wang C, Zhang G, Zhang X. Experimental and Photothermal Performance Evaluation of Multi-Wall Carbon-Nanotube-Enhanced Microencapsulation Phase Change Slurry for Efficient Photothermal Conversion and Storage. Energies. 2022; 15(20):7627. https://doi.org/10.3390/en15207627
Chicago/Turabian StyleWang, Changling, Guiling Zhang, and Xiaosong Zhang. 2022. "Experimental and Photothermal Performance Evaluation of Multi-Wall Carbon-Nanotube-Enhanced Microencapsulation Phase Change Slurry for Efficient Photothermal Conversion and Storage" Energies 15, no. 20: 7627. https://doi.org/10.3390/en15207627





