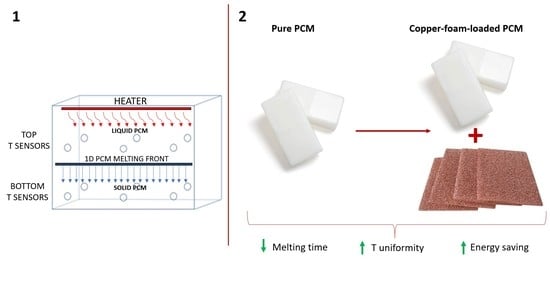
Experimental Investigation on Latent Thermal Energy Storages (LTESs) Based on Pure and Copper-Foam-Loaded PCMs
Abstract
:1. Introduction
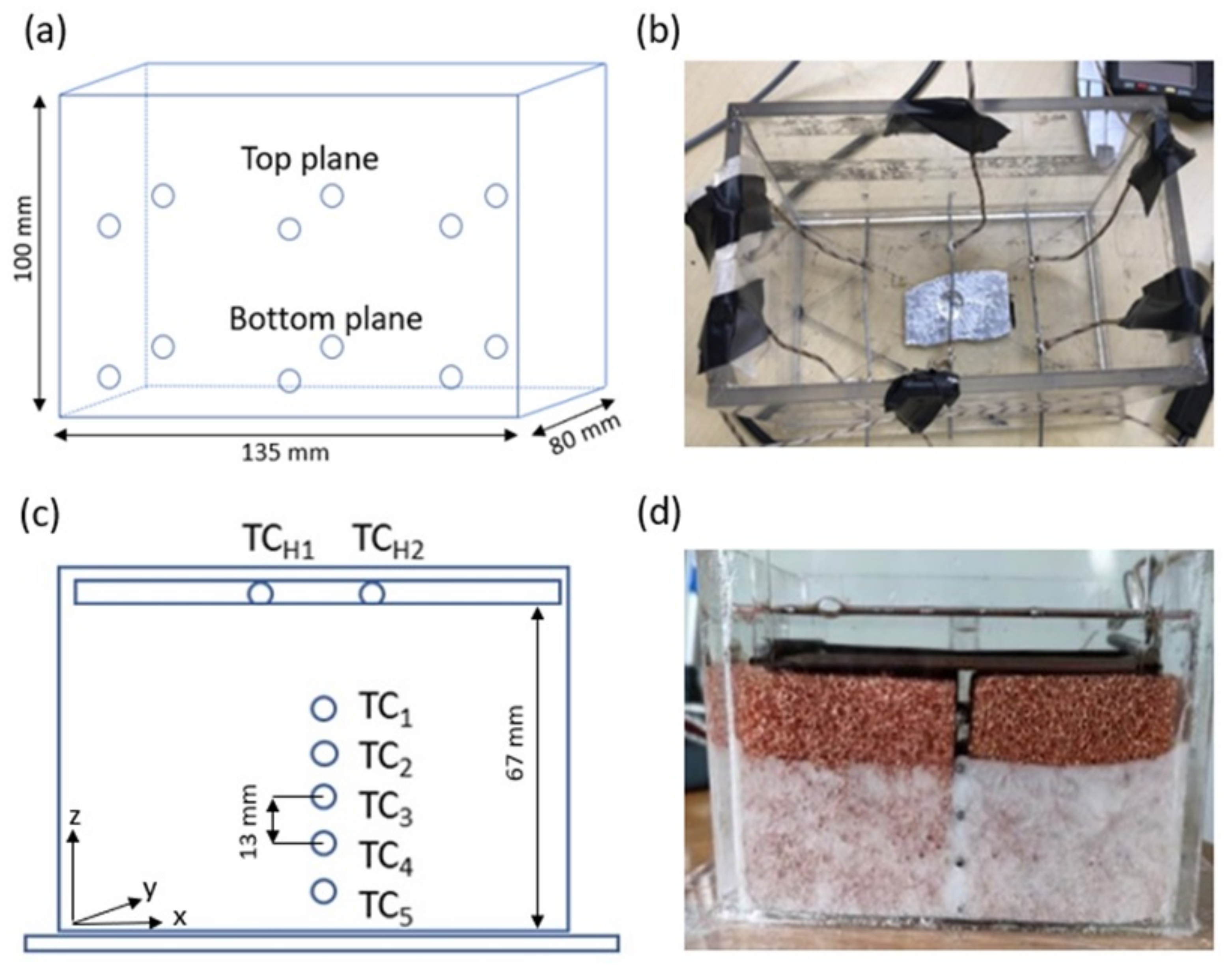
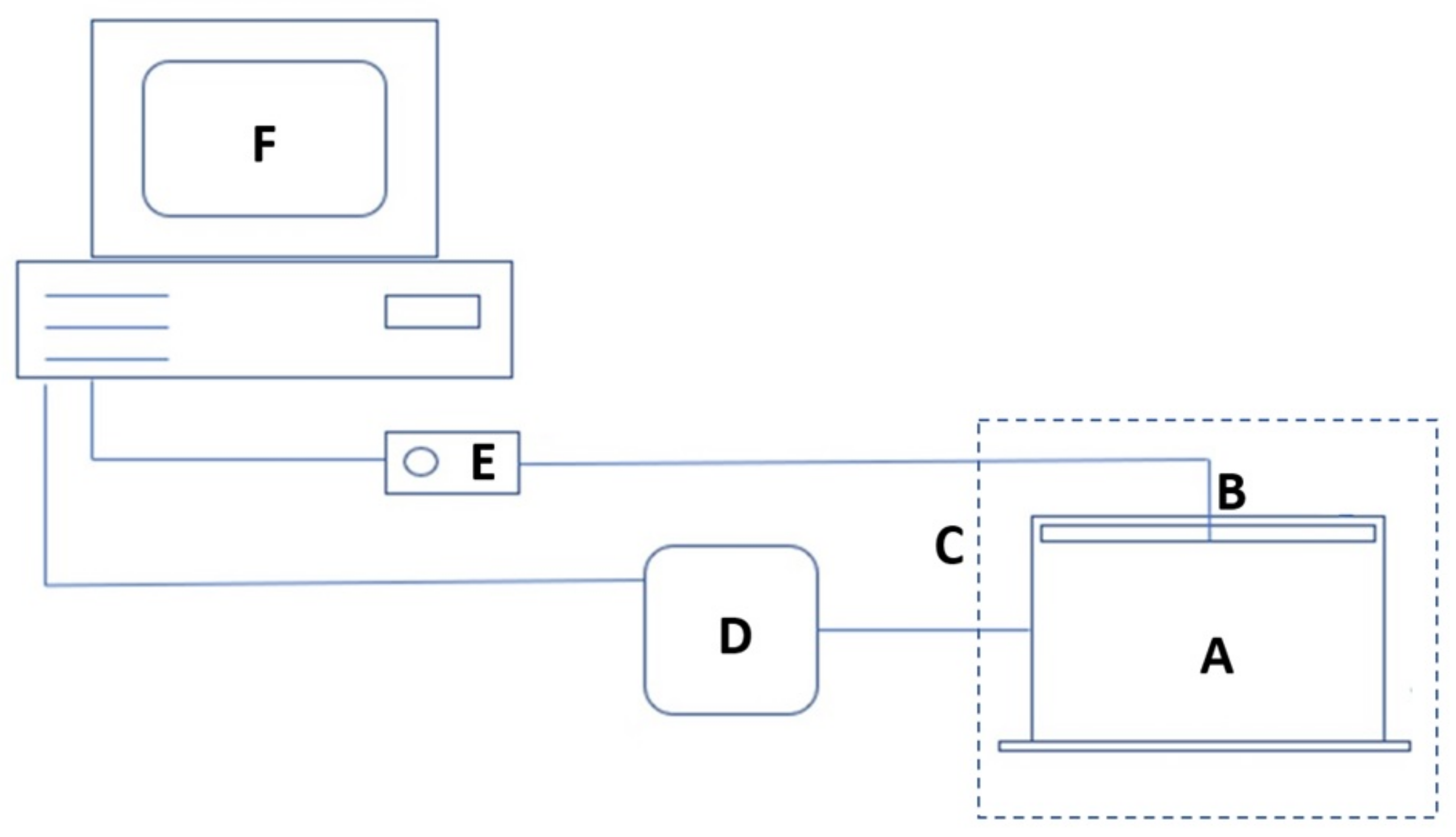
2. Materials and Methods
3. Results and Discussion
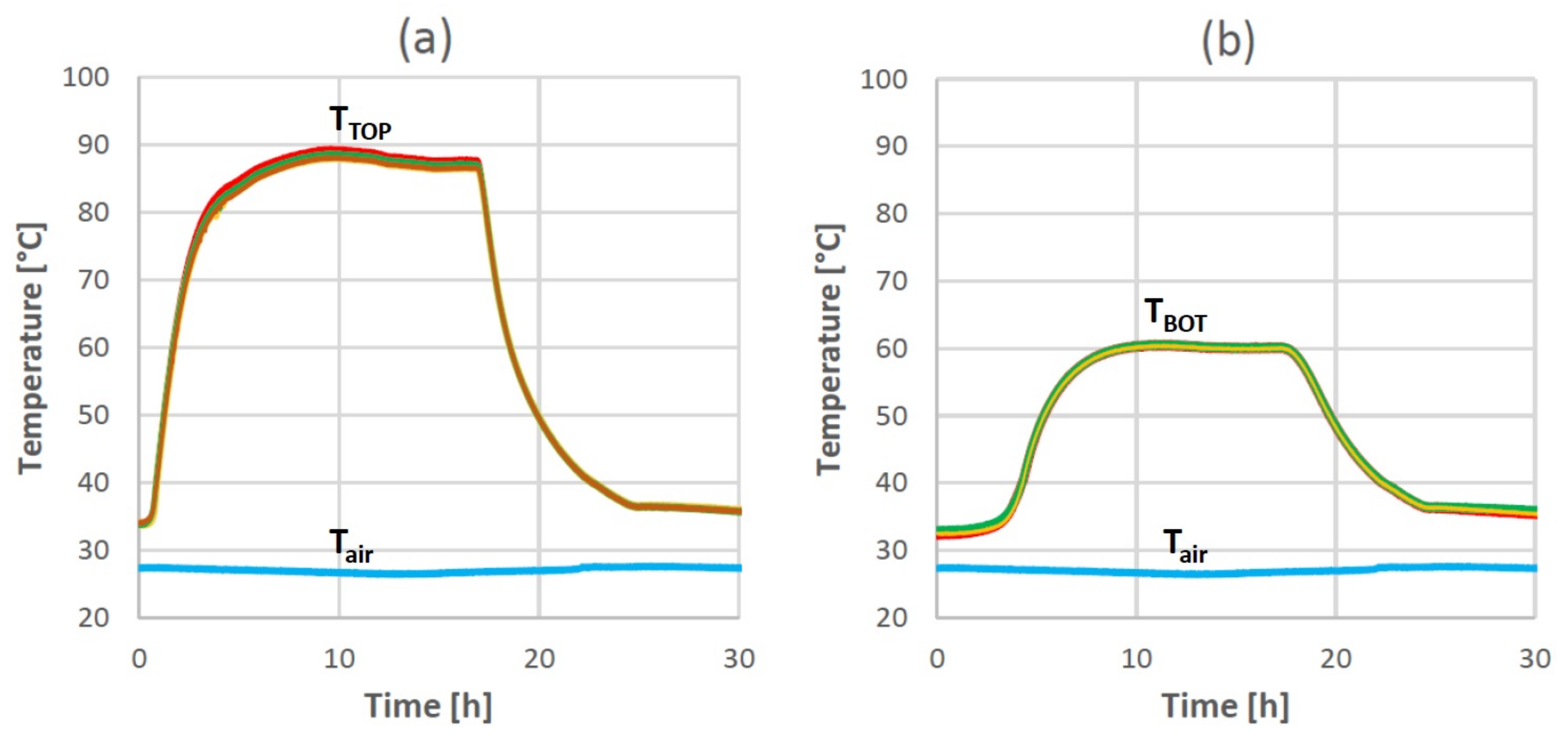
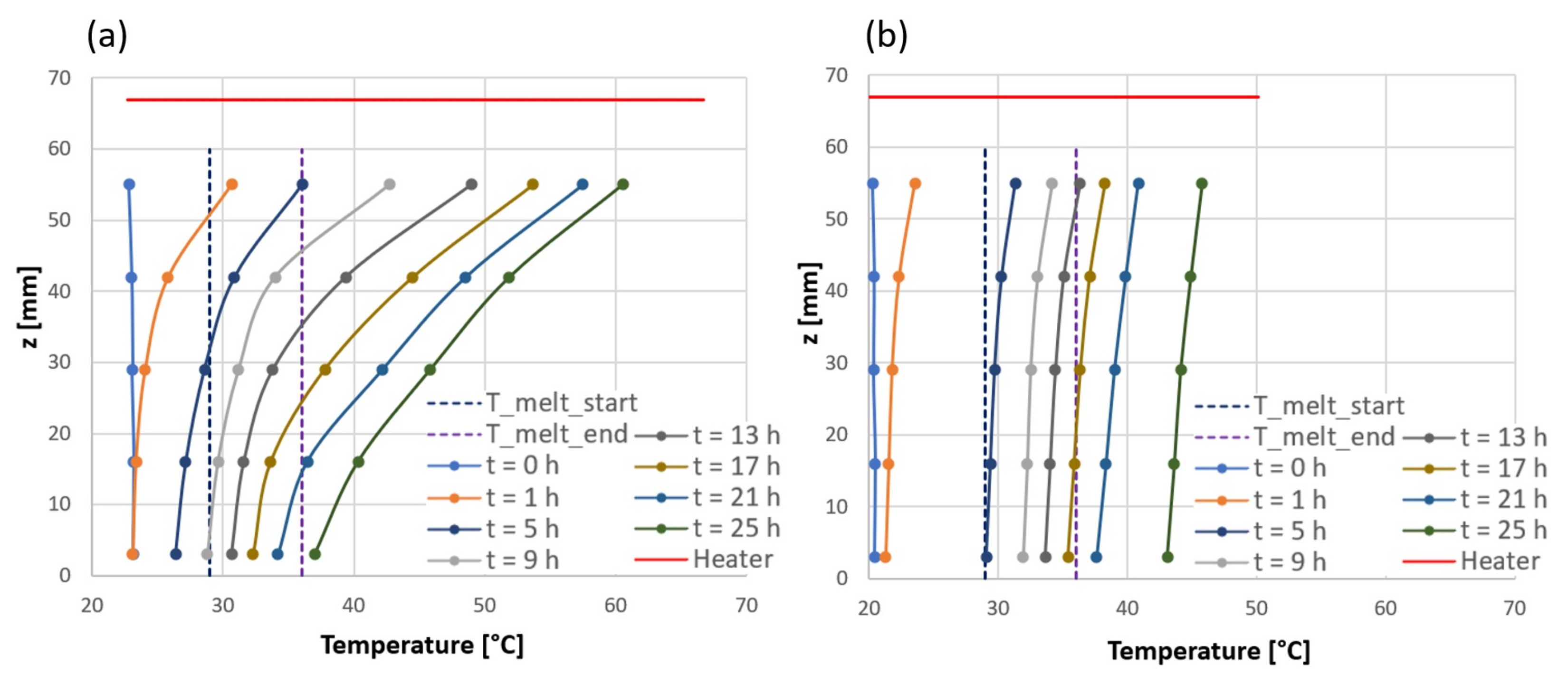
3.1. Temperature Homogeneity along the Horizontal Direction
3.2. Comparison between Pure PCM and Copper-Foam-Loaded PCM
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Additive manufacturing |
| BP | Battery pack |
| BTM | Battery thermal management |
| BTMS | Battery thermal management system |
| CSP | Concentrating solar power |
| EV | Electric vehicle |
| HP | Heat pipes |
| HX | Heat exchanger |
| LIB | Lithium-ion battery |
| LTES | Latent thermal energy storage |
| LTESS | Latent thermal energy storage system |
| MTHX | Multi-tube heat exchanger |
| PCM | Phase change material |
| PD | Pore density |
| PPI | Pores per inches |
| RTD | Resistance temperature detector |
| SOH | State of health |
| TC | Thermocouple |
| TESS | Thermal energy storage system |
References
- Zeinelabdein, R.; Omer, S.; Gan, G. Critical review of latent heat storage systems for free cooling in buildingserformance studies of refrigerated van having. Renew. Sustain. Energy Rev. 2018, 82, 2843–2868. [Google Scholar] [CrossRef]
- Fadl, M.; Eames, P.C. An experimental investigation of the heat transfer and energy storage characteristics of a compact latent heat thermal energy storage system for domestic hot water applications. Energy 2019, 188, 116083. [Google Scholar] [CrossRef]
- Alkhazaleh, A.; Kandola, B. Thermal and Flammability Properties of Paraffin/Nanoclay Composite Phase Change Materials Incorporated in Building Materials for Thermal Energy Storage. Int. J. Energy Power Eng. 2017, 11, 696–701. [Google Scholar]
- Ahrari, M.; Khajavi, R.; Dolatabadi, M.K.; Toliyat, T.; Rashidi, A. A review on application of phase change materials in textiles finishing. Int. J. Mater. Metall. Eng. 2017, 11, 400–405. [Google Scholar]
- BM Information. BMW Motorrad Rider Equipment 2018; Technical Report; 2017. Available online: https://www.press.bmwgroup.com/global/article/detail/T0275552EN/bmw-motorrad-rider-equipment-2018?language=en (accessed on 17 May 2022).
- Sharma, M.K.; Buddhi, D. Performance studies of refrigerated van having PCM for generating off site refrigeration effect. J. Pure Appl. Sci. Technol. 2013, 3, 1–12. [Google Scholar]
- Oró, E.; Miró, L.; Farid, M.M.; Cabeza, L.F. Improving thermal performance of freezers using phase change materials. Int. J. Refrig. 2012, 35, 984–991. [Google Scholar] [CrossRef]
- Borhani, M.; Hosseini, M.J.; Pakrouh, R.; Ranjbar, A.A.; Nourian, A. Performance Enhancement of a Thermoelectric Harvester with a PCM/Metal Foam Composite. Renew. Energy 2021, 168, 1122–1140. [Google Scholar] [CrossRef]
- Li, S.F.; Liu, Z.h.; Wang, X.J. A comprehensive review on positive cold energy storage technologies and applications in air conditioning with phase change materials. Appl. Energy 2019, 255, 113667. [Google Scholar] [CrossRef]
- Kaur, I.; Singh, P. State-of-the-art in heat exchanger additive manufacturing. Int. J. Heat Mass Transf. 2021, 178, 121600. [Google Scholar] [CrossRef]
- Pakrouh, R.; Hosseini, M.J.; Ranjbar, A.A.; Bahrampoury, R. A numerical method for PCM-based pin fin heat sinks optimization. Energy Convers. Manag. 2015, 103, 542–552. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, P.; Zhu, J. Thermal management of electronic devices using pin-fin based cascade microencapsulated PCM/expanded graphite composite. Int. J. Heat Mass Transf. 2020, 149, 119199. [Google Scholar] [CrossRef]
- Mills, A.; Al-Hallaj, S. Simulation of passive thermal management system for lithium-ion battery packs. J. Power Sources 2005, 141, 307–315. [Google Scholar] [CrossRef]
- Ananno, A.A.; Masud, M.H.; Dabnichki, P.; Ahmed, A. Design and numerical analysis of a hybrid geothermal PCM flat plate solar collector dryer for developing countries. Sol. Energy 2020, 196, 270–286. [Google Scholar] [CrossRef]
- Eisapour, M.; Eisapour, A.H.; Hosseini, M.J.; Sardari, P.T. Exergy and energy analysis of wavy tubes photovoltaic-thermal systems using microencapsulated PCM nano-slurry coolant fluid. Appl. Energy 2020, 266, 114849. [Google Scholar] [CrossRef]
- Jaguemont, J.; Omar, N.; Van den Bossche, P.; Mierlo, J. Phase-change materials (PCM) for automotive applications: A review. Appl. Therm. Eng. 2018, 132, 308–320. [Google Scholar] [CrossRef]
- Ianniciello, L.; Biwolé, P.H.; Achard, P. Electric vehicles batteries thermal management systems employing phase change materials. J. Power Sources 2018, 378, 383–403. [Google Scholar] [CrossRef]
- Al-Hallaj, S.; Selman, J.R. A novel thermal management system for electric vehicle batteries using phase-change material. J. Electrochem. Soc. 2000, 147, 3231. [Google Scholar] [CrossRef]
- Hémery, C.V.; Pra, F.; Robin, J.F.; Marty, P. Experimental performances of a battery thermal management system using a phase change material. J. Power Sources 2014, 270, 349–358. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, B.; Li, B.; Yan, Y. A critical review of thermal management models and solutions of lithium-ion batteries for the development of pure electric vehicles. Renew. Sustain. Energy Rev. 2016, 64, 106–128. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, F. A review on passive and active strategies of enhancing the safety of lithium-ion batteries. Int. J. Heat Mass Transf. 2022, 184, 122288. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, Y.; Howey, D.A.; Perez, H.; Foley, A.; Pecht, M. Battery warm-up methodologies at subzero temperatures for automotive applications: Recent advances and perspectives. Prog. Energy Combust. Sci. 2020, 77, 100806. [Google Scholar] [CrossRef]
- Lin, J.; Liu, X.; Li, S.; Zhang, C.; Yang, S. A review on recent progress, challenges and perspective of battery thermal management system. Int. J. Heat Mass Transf. 2021, 167, 120834. [Google Scholar] [CrossRef]
- He, L.; Tang, X.; Luo, Q.; Liao, Y.; Luo, X.; Liu, J.; Ma, L.; Dong, D.; Gan, Y.; Li, Y. Structure optimization of a heat pipe-cooling battery thermal management system based on fuzzy grey relational analysis. Int. J. Heat Mass Transf. 2022, 182, 121924. [Google Scholar] [CrossRef]
- Yue, Q.; He, C.; Wu, M.; Zhao, T. Advances in thermal management systems for next-generation power batteries. Int. J. Heat Mass Transf. 2021, 181, 121853. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Liu, M.; Rao, Z.; Deng, H.; Wang, Q.; Qi, X.; Wang, S. An innovative battery thermal management with thermally induced flexible phase change material. Energy Convers. Manag. 2020, 221, 113145. [Google Scholar] [CrossRef]
- Esapour, M.; Hamzehnezhad, A.; Darzi, A.A.R.; Jourabian, M. Melting and solidification of PCM embedded in porous metal foam in horizontal multi-tube heat storage system. Energy Convers. Manag. 2018, 171, 398–410. [Google Scholar] [CrossRef]
- El Idi, M.M.; Karkri, M. Heating and cooling conditions effects on the kinetic of phase change of PCM embedded in metal foam. Case Stud. Therm. Eng. 2020, 21, 100716. [Google Scholar] [CrossRef]
- Biwole, P.H.; Groulx, D.; Souayfane, F.; Chiu, T. Influence of fin size and distribution on solid-liquid phase change in a rectangular enclosure. Int. J. Therm. Sci. 2018, 124, 433–446. [Google Scholar] [CrossRef]
- Al-Abidi, A.; Mat, S.; Sopian, K.B.; Sulaiman, M.Y.; Mohammad, A.T. Numerical study of PCM solidification in a triplex tube heat exchanger with internal and external fins. Int. J. Heat Mass Transf. 2013, 61, 684–695. [Google Scholar] [CrossRef]
- Ji, C.; Qin, Z.; Low, Z.; Dubey, S.; Choo, F.H.; Duan, F. Non-uniform heat transfer suppression to enhance PCM melting by angled fins. Appl. Therm. Eng. 2018, 129, 269–279. [Google Scholar] [CrossRef]
- Arıcı, M.; Tütüncü, E.; Kan, M.; Karabay, H. Melting of nanoparticle-enhanced paraffin wax in a rectangular enclosure with partially active walls. Int. J. Heat Mass Transf. 2017, 104, 7–17. [Google Scholar] [CrossRef]
- Colla, L.; Fedele, L.; Mancin, S.; Danza, L.; Manca, O. Nano-PCMs for enhanced energy storage and passive cooling applications. Appl. Therm. Eng. 2017, 110, 584–589. [Google Scholar] [CrossRef]
- Krishna, J.; Kishore, P.S.; Solomon, A.B. Heat pipe with nano enhanced-PCM for electronic cooling application. Exp. Therm. Fluid Sci. 2017, 81, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Arasu, V.; Mujumdar, A.S. Numerical study on melting of paraffin wax with Al2O3 in a square enclosure. Int. Commun. Heat Mass Transf. 2012, 39, 8–16. [Google Scholar] [CrossRef]
- Darzi, A.A.R.; Jourabian, M.; Farhadi, M. Melting and solidification of PCM enhanced by radial conductive fins and nanoparticles in cylindrical annulus. Energy Convers. Manag. 2016, 118, 253–263. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Mehling, H.; Hiebler, S.; Ziegler, F. Heat transfer enhancement in water when used as PCM in thermal energy storage. Appl. Therm. Eng. 2002, 22, 1141–1151. [Google Scholar] [CrossRef]
- Samimi, F.; Babapoor, A.; Azizi, M.; Karimi, G. Thermal management analysis of a Li-ion battery cell using phase change material loaded with carbon fibers. Energy 2016, 96, 355–371. [Google Scholar] [CrossRef]
- Fan, L.; Khodadadi, J.M. Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2011, 15, 24–46. [Google Scholar] [CrossRef]
- Righetti, G.; Doretti, L.; Zilio, C.; Longo, G.A.; Mancin, S. Experimental investigation of phase change of medium/high temperature paraffin wax embedded in 3D periodic structure. Int. J. Thermofluids 2020, 5, 100035. [Google Scholar] [CrossRef]
- Righetti, G.; Savio, G.; Meneghello, R.; Doretti, L.; Mancin, S. Experimental study of phase change material (PCM) embedded in 3D periodic structures realized via additive manufacturing. Int. J. Therm. Sci. 2020, 153, 106376. [Google Scholar] [CrossRef]
- Rehman, T.-U.; Ali, H.; Janjua, M.; Sajjad, U.; Yan, W.M. A critical review on heat transfer augmentation of phase change materials embedded with porous materials/foams. Int. J. Heat Mass Transf. 2019, 135, 649–673. [Google Scholar] [CrossRef]
- Alhusseny, A.; Al-Zurfi, N.; Nasser, A.; Al-Fatlawi, A.; Aljanabi, M. Impact of using a PCM-metal foam composite on charging/discharging process of bundled-tube LHTES units. Int. J. Heat Mass Transf. 2020, 150, 119320. [Google Scholar] [CrossRef]
- Ghahremannezhad, A.; Xu, H.; Salimpour, M.R.; Wang, P.; Vafai, K. Thermal performance analysis of phase change materials (PCMs) embedded in gradient porous metal foams. Appl. Therm. Eng. 2020, 179, 115731. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Nsofor, E.C. Multiple-segment metal foam application in the shell-and-tube PCM thermal energy storage system. J. Energy Storage 2018, 20, 529–541. [Google Scholar] [CrossRef]
- Rehman, T.-U.; Ali, H. Experimental study on the thermal behavior of RT-35HC paraffin within copper and Iron-Nickel open cell foams: Energy storage for thermal management of electronics. Int. J. Heat Mass Transf. 2020, 146, 118852. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, Z.; Zhu, H.; Wang, Y.; Peng, S. Melting heat transfer characteristics of a composite phase change material fabricated by paraffin and metal foam. Appl. Energy 2017, 185, 1971–1983. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Calmidi, V.V.; Mahajan, R.L. Thermophysical properties of high porosity metal foams. Int. J. Heat Mass Transf. 2002, 45, 1017–1031. [Google Scholar] [CrossRef]
- Righetti, G.; Lazzarin, R.; Noro, M.; Mancin, S. Phase change materials embedded in porous matrices for hybrid thermal energy storages: Experimental results and modeling. Int. J. Refrig. 2019, 106, 266–277. [Google Scholar] [CrossRef]
- Dinesh, B.V.S.; Bhattacharya, A. Comparison of energy absorption characteristics of PCM-metal foam systems with different pore size distributions. J. Energy Storage 2020, 28, 101190. [Google Scholar] [CrossRef]
- Marri, G.K.; Balaji, C. Experimental and numerical investigations on the effect of porosity and PPI gradients of metal foams on the thermal performance of a composite phase change material heat sink. Int. J. Heat Mass Transf. 2021, 164, 120454. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Z.; Zhang, H.; Sun, X.; Dou, B.; Wu, W.; Zhang, G.; Jiang, L. Experimental investigation of the comprehensive heat transfer performance of PCMs filled with CMF in a heat storage device. Int. J. Heat Mass Transf. 2022, 188, 122582. [Google Scholar] [CrossRef]
- Naldi, C.; Dongellini, M.; Salvi, F.; Silvestrini, M.; Falcone, M.; Morini, G.L. Numerical model calibration for composite-PCM LTES. In Proceedings of the PCM2021 13th IIR - Phase Change Materials and Slurries for Refrigeration and Air Conditioning Conference, Vicenza, Italy, 1–3 September 2021. [Google Scholar] [CrossRef]
- Naldi, C.; Dongellini, M.; Morini, G.L. The evaluation of the effective thermal conductivity of metal-foam loaded phase change materials. J. Energy Storage 2022, 51, 104450. [Google Scholar] [CrossRef]
- Eurobatex®. Eurobatex® Data Sheet. Available online: https://www.unionfoam.it/doc/up/338698001630987597.pdf (accessed on 17 May 2022).
- Izgi, B.; Arslan, M. Numerical analysis of solidification of PCM in a closed vertical cylinder for thermal energy storage applications. Heat Mass Transf. 2020, 56, 2909–2922. [Google Scholar] [CrossRef]


| PCM | Symbol | Unit | Value |
|---|---|---|---|
| Phase change temperature range from solid to liquid | [°C] | 29–36 | |
| Phase change temperature range from liquid to solid | [°C] | 36–31 | |
| Heat storage capacity | H | [kJ/kg] | 160 |
| Specific heat capacity | [kJ/kg K] | 2 | |
| Density of the solid phase | [kg/L] | 0.86 | |
| Density of the liquid phase | [kg/L] | 0.77 | |
| Heat conductivity for both solid and liquid phases | [W/m K] | 0.2 | |
| Volume expansion | [%] | 12 | |
| Flash temperature | [°C] | 167 | |
| Maximum working temperature | [°C] | 65 | |
| Copper foam | |||
| Heat conductivity | [W/m K] | 385 | |
| Porosity | [%] | 95 | |
| Pore density | 20 | ||
| Polycarbonate | |||
| Density | [g/cm3] | 1.2 | |
| Softening temperature | [°C] | 115 |
| Instrument | Quantity | Unit | Measured Value | FS | U(%FS) |
|---|---|---|---|---|---|
| Ammeter | i | [A] | 0.2 | 1 | 2 |
| Voltmeter | V | [V] | 17.5 | 30 | 3.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcone, M.; Rehman, D.; Dongellini, M.; Naldi, C.; Pulvirenti, B.; Morini, G.L. Experimental Investigation on Latent Thermal Energy Storages (LTESs) Based on Pure and Copper-Foam-Loaded PCMs. Energies 2022, 15, 4894. https://doi.org/10.3390/en15134894
Falcone M, Rehman D, Dongellini M, Naldi C, Pulvirenti B, Morini GL. Experimental Investigation on Latent Thermal Energy Storages (LTESs) Based on Pure and Copper-Foam-Loaded PCMs. Energies. 2022; 15(13):4894. https://doi.org/10.3390/en15134894
Chicago/Turabian StyleFalcone, Morena, Danish Rehman, Matteo Dongellini, Claudia Naldi, Beatrice Pulvirenti, and Gian Luca Morini. 2022. "Experimental Investigation on Latent Thermal Energy Storages (LTESs) Based on Pure and Copper-Foam-Loaded PCMs" Energies 15, no. 13: 4894. https://doi.org/10.3390/en15134894