Reduction Reactivity of Low Grade Iron Ore-Biomass Pellets for a Sustainable Ironmaking Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Specification and Preparation
2.2. Experimental Methods
2.2.1. Thermal Analysis
2.2.2. Experiments on Reduction of Iron Ore-Biomass Pellets
2.3. Determination of Reduction and Metallization Degrees
3. Results and Discussion
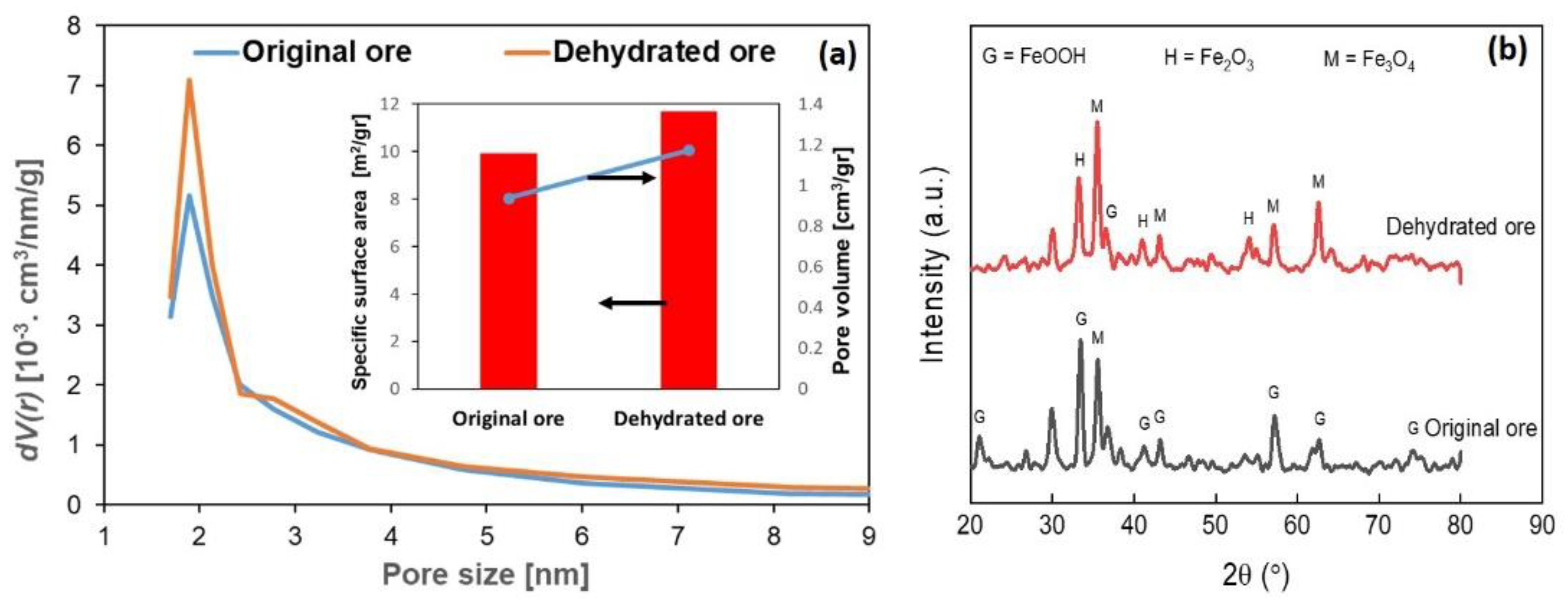
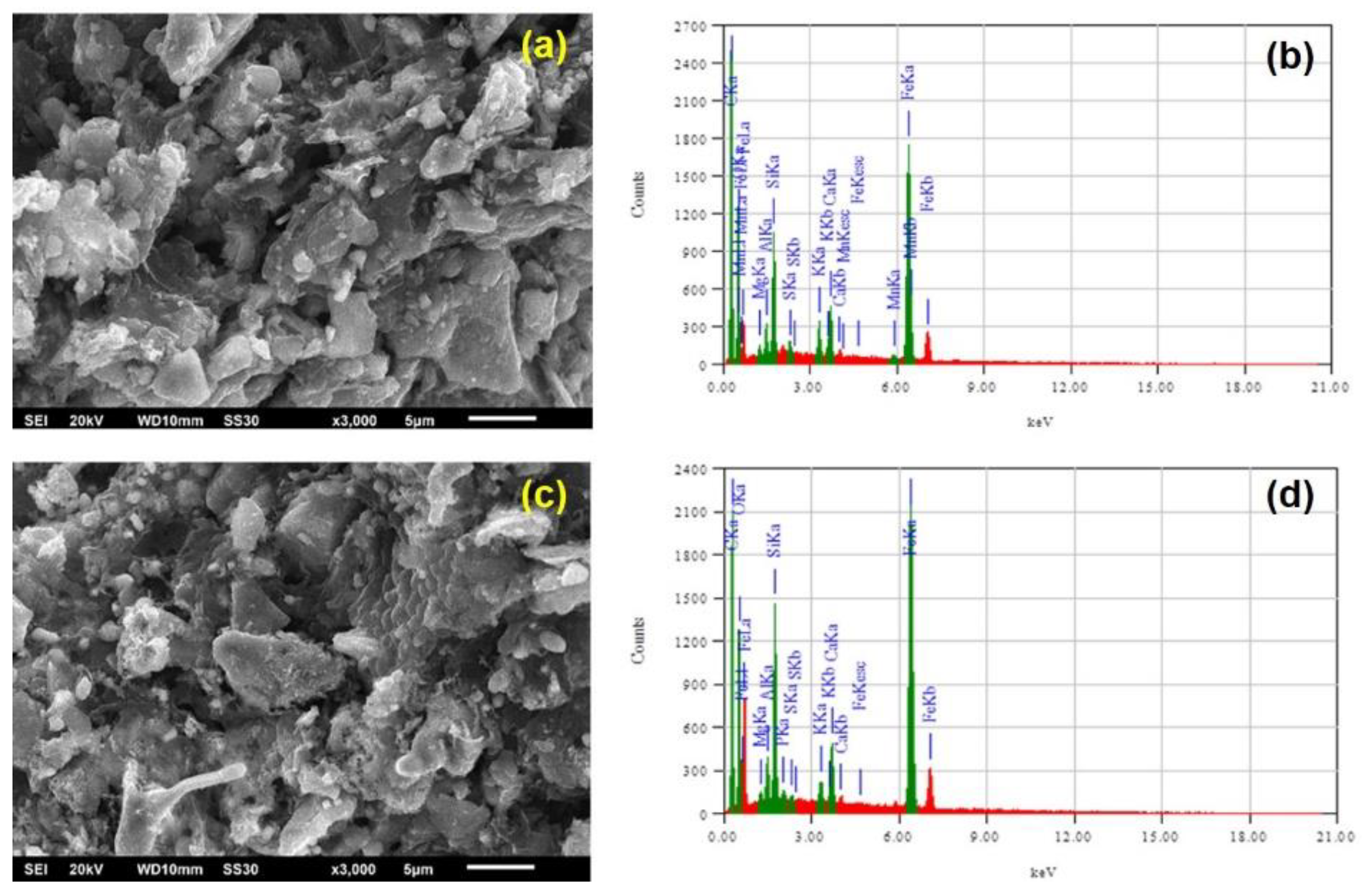
3.1. Effect of Dehydration Process on Iron Ore Properties
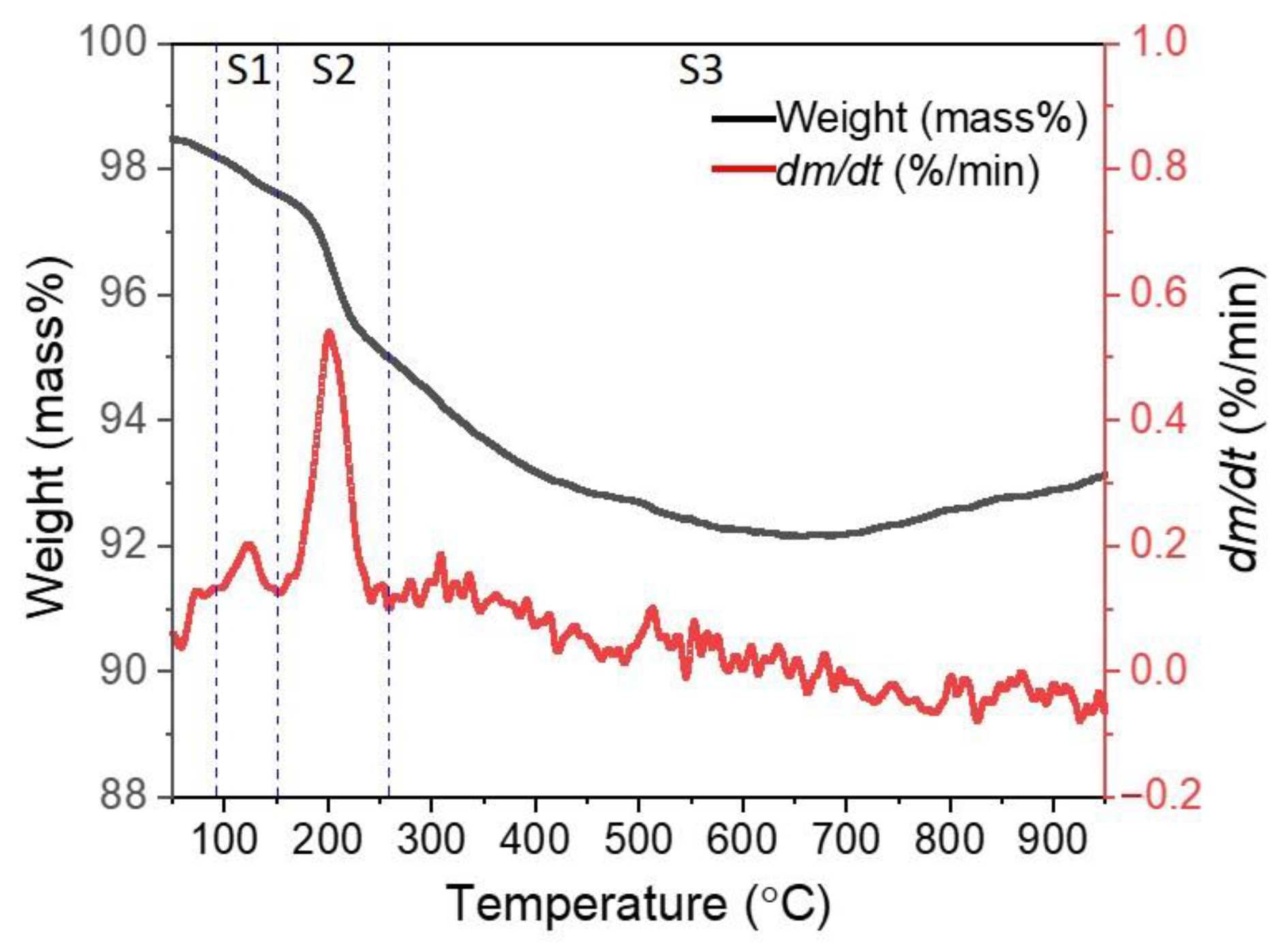
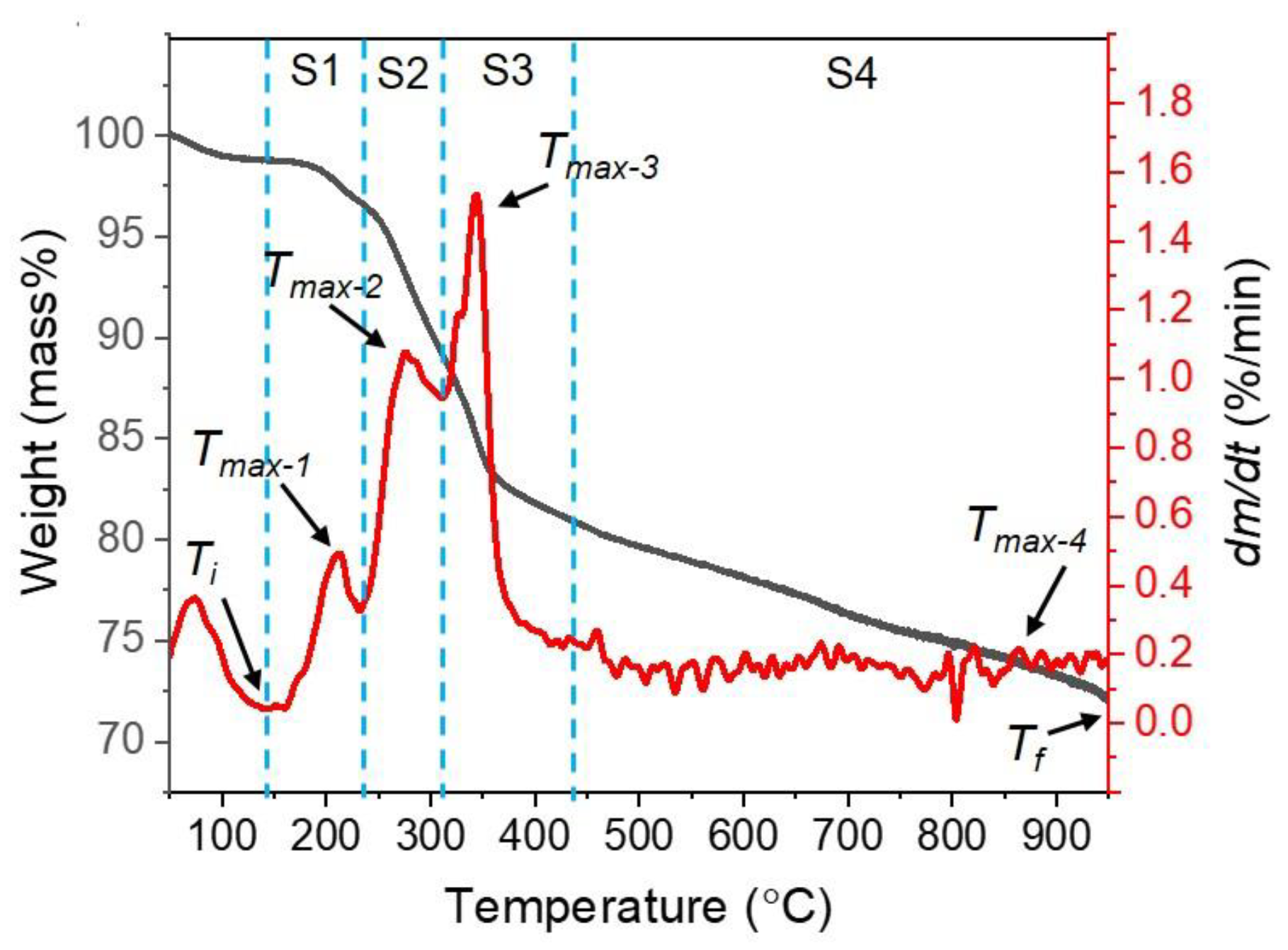
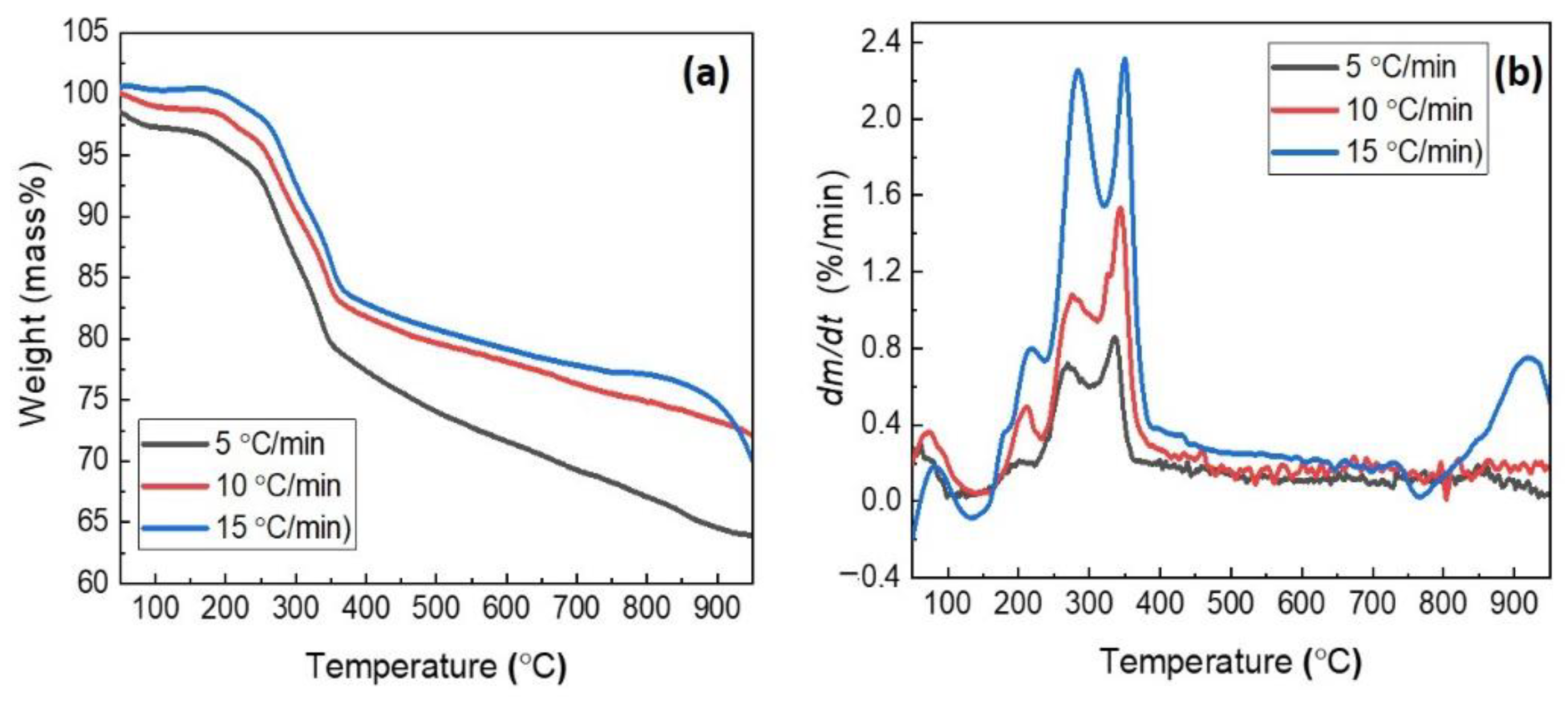
3.2. Thermogravimetric Characterization of the Composite Pellet
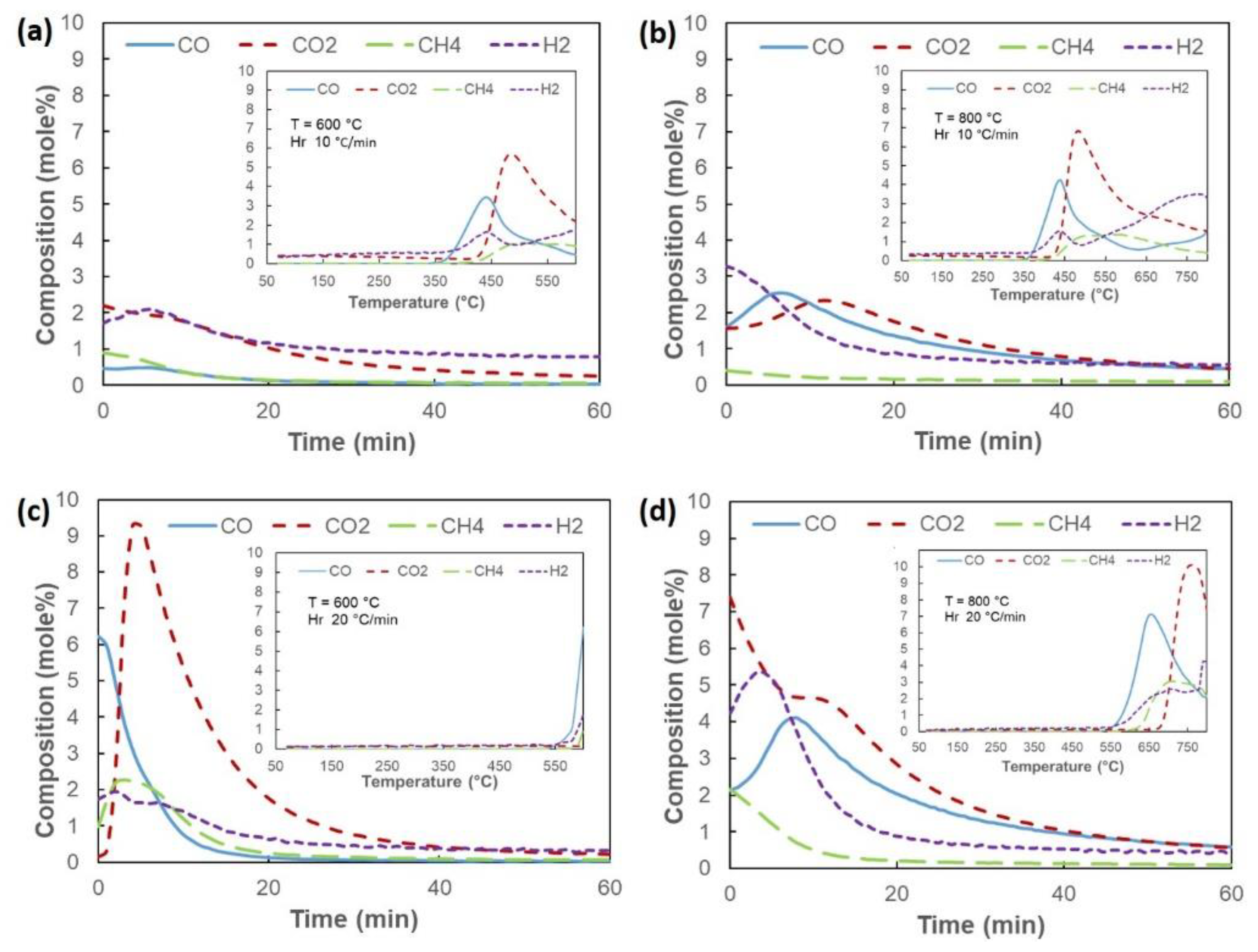
3.3. Reduction Behavior of Composite Pellet
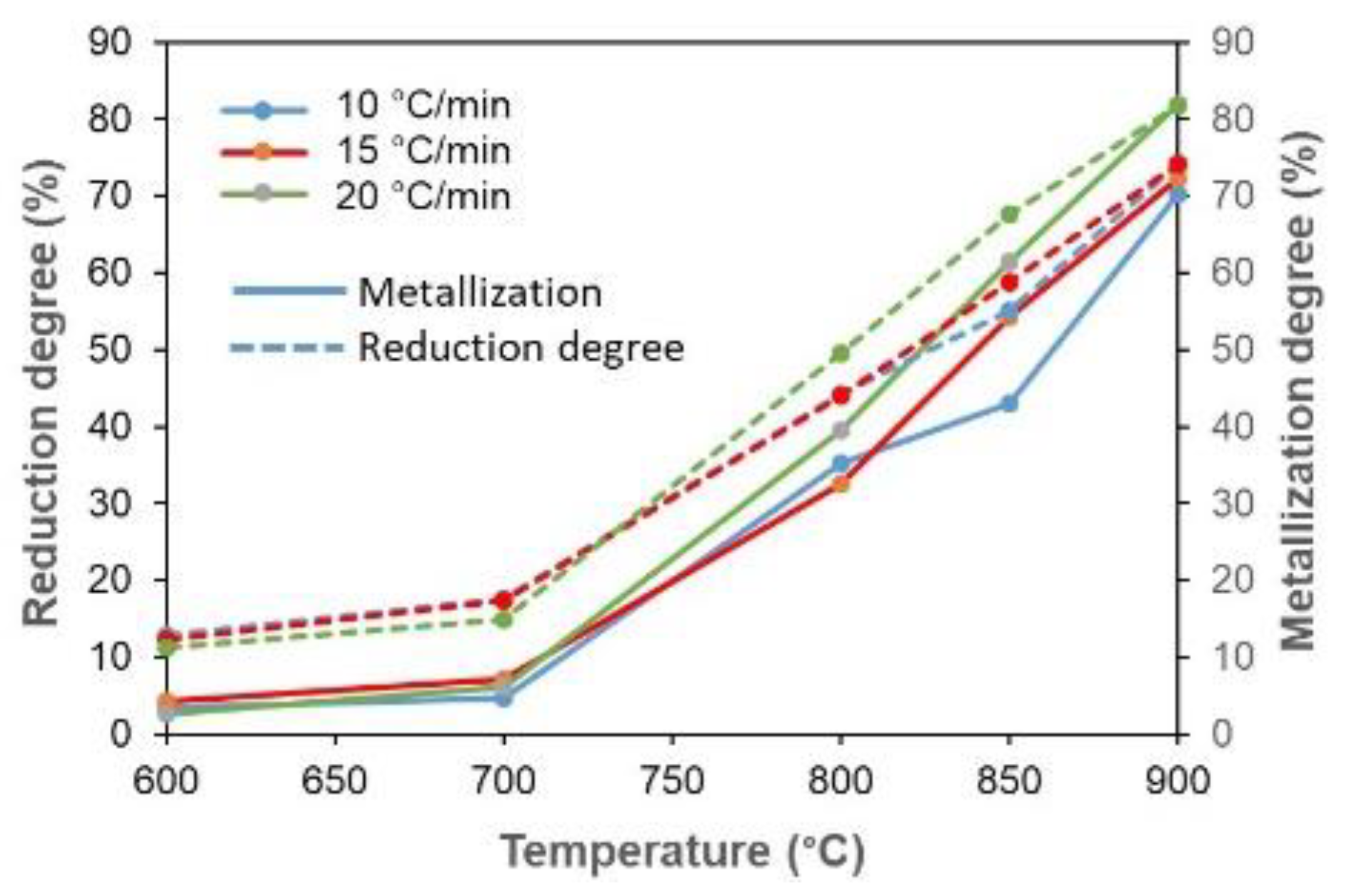
3.4. Reduction and Metallization Degree of Composite Pellet
4. Conclusions
- The dehydration process of low-grade iron ore could remove water content from the iron ore powder, which had an impact through increasing the specific surface area and the occurrence of a reduction reaction that transforms Fe2O3 to Fe3O4.
- From thermogravimetric analysis, as the heating rate rose, the initial temperature of the weight loss shifted to a higher temperature. In addition, increasing the heating rate induces elevating the comprehensive reduction reactivity index (S) and shortening the reduction time.
- Analysis of phase transformation based on temperature changes showed that, at a temperature of 600–700 °C, most of the formed phase was magnetite (Fe3O4). By increasing the temperature to 800 °C, wustite (FeO) formation was very significant. The reduction reaction at 900 °C indicated that Fe2O3 → Fe3O4 → FeO → Fe reaction was fast. According to the reduced gas analysis, carbon gasification significantly enhanced the reduction rate starting at 800 °C. Furthermore, a heating rate of 20 °C/min resulted in higher peak Fe intensity when compared to 10 °C/min. This could be due to the carbon gasification rate at a heating rate of 20 °C/min higher than at 10 °C/min.
- The changing trend of the reduction and metallization degrees from 600 to 700 °C was slight. However, increasing the temperature to 800 °C showed a consistently high escalation of both degrees up to 900 °C. The degrees of reduction and metallization at a heating rate of 20 °C/min showed a more considerable increase than 10 and 15 °C/min. In addition, the current study can achieve the same degree of reduction at lower temperatures compared to the iron-coke mixture in the blast furnace method. This indicates that there is energy saving in this direct reduction process.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass applications in iron and steel industry: An overview of challenges and opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Kemppainen, A.; Haapakangas, J.; Fabritius, T. Extensive review of the opportunities to use biomass-based fuels in iron and steelmaking processes. J. Clean. Prod. 2017, 148, 709–734. [Google Scholar] [CrossRef]
- Purwanto, H.; Zakiyuddin, A.M.; Rozhan, A.N.; Mohamad, A.S.; Salleh, H.M. Effect of charcoal derived from oil palm empty fruit bunch on the sinter characteristics of low grade iron ore. J. Clean. Prod. 2018, 200, 954–959. [Google Scholar] [CrossRef]
- Ubando, A.T.; Chen, W.-H.; Ong, H.C. Iron oxide reduction by graphite and torrefied biomass analyzed by TG-FTIR for mitigating CO2 emissions. Energy 2019, 180, 968–977. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, J.; Zhou, L.; Liu, Y.; Guo, Z.; Wang, Q. Experimental study of commercial charcoal as alternative fuel for coke breeze in iron ore sintering process. Energy Convers. Manag. 2016, 125, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.-B.; Zong, Y.-B.; Wei, R.; Gao, W.; Liu, X.-M. Direct Reduction of High-phosphorus Oolitic Hematite Ore Based on Biomass Pyrolysis. J. Iron Steel Res. Int. 2016, 23, 874–883. [Google Scholar] [CrossRef]
- Wei, R.; Cang, D.; Bai, Y.; Huang, D.; Liu, X. Reduction characteristics and kinetics of iron oxide by carbon in biomass. Ironmak. Steelmak. 2016, 43, 144–152. [Google Scholar] [CrossRef]
- Nayak, D.; Dash, N.; Ray, N.; Rath, S.S. Utilization of waste coconut shells in the reduction roasting of overburden from iron ore mines. Powder Technol. 2019, 353, 450–458. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Song, Q.; Liu, S.; Ma, Q.; Ma, L.; Shu, X. Catalytic reforming of volatiles from co-pyrolysis of lignite blended with corn straw over three different structures of iron ores. J. Anal. Appl. Pyrolysis 2019, 144, 104714. [Google Scholar] [CrossRef]
- Cahyono, R.B.; Rozhan, A.N.; Yasuda, N.; Nomura, T.; Hosokai, S.; Kashiwaya, Y.; Akiyama, T. Catalytic coal-tar decomposition to enhance reactivity of low-grade iron ore. Fuel Process. Technol. 2013, 113, 84–89. [Google Scholar] [CrossRef]
- Abe, K.; Kurniawan, A.; Ohashi, K.; Nomura, T.; Akiyama, T. Ultrafast Iron-Making Method: Carbon Combustion Synthesis from Carbon-Infiltrated Goethite Ore. ACS Omega 2018, 3, 6151–6157. [Google Scholar] [CrossRef]
- Hosokai, S.; Matsui, K.; Okinaka, N.; Ohno, K.; Shimizu, M.; Akiyama, T. Kinetic Study on the Reduction Reaction of Biomass-Tar-Infiltrated Iron. Energy Fuels 2012, 26, 7274–7279. [Google Scholar] [CrossRef]
- Cahyono, R.B.; Yasuda, N.; Nomura, T.; Akiyama, T. Utilization of Low Grade Iron Ore (FeOOH) and Biomass Through Integrated Pyrolysis-tar Decomposition (CVI process) in Ironmaking Industry: Exergy Analysis and its Application. ISIJ Int. 2015, 55, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, Y.; Nishio, M.; Tsubouchi, N.; Akiyama, T. Preparation of a Carbon-Containing Pellet with High Strength and High Reactivity by Vapor Deposition of Tar to a Cold-Bonded Pellet. Energy Fuels 2017, 31, 8877–8885. [Google Scholar] [CrossRef]
- Zulkania, A.; Rochmadi, R.; Cahyono, R.B.; Hidayat, M. Investigation into Biomass Tar-Based Carbon Deposits as Reduction Agents on Iron Ore Using the Tar Impregnation Method. Metals 2021, 11, 1623. [Google Scholar] [CrossRef]
- Luo, S.; Yi, C.; Zhou, Y. Direct reduction of mixed biomass-Fe2O3 briquettes using biomass-generated syngas. Renew. Energy 2011, 36, 3332–3336. [Google Scholar] [CrossRef]
- Guo, D.; Hu, M.; Pu, C.; Xiao, B.; Hu, Z.; Liu, S.; Zhu, X. Kinetics and mechanisms of direct reduction of iron ore-biomass composite pellets with hydrogen gas. Int. J. Hydrogen Energy. 2015, 40, 4733–4740. [Google Scholar] [CrossRef]
- Yuan, P.; Shen, B.; Duan, D.; Adweek, G.; Mei, X.; Lu, F. Study on the formation of direct reduced iron by using biomass as reductants of carbon-containing pellets in RHF process. Energy 2017, 141, 472–482. [Google Scholar] [CrossRef]
- Yuan, X.; Luo, F.; Liu, S.; Zhang, M.; Zhou, D. Comparative Study on the Kinetics of the Isothermal Reduction of Iron Ore Composite Pellets Using Coke, Charcoal, and Biomass as Reducing Agents. Metals 2021, 11, 340. [Google Scholar] [CrossRef]
- Rashid, R.Z.A.; Mohd-Salleh, H.; Ani, M.H.; Yunus, N.A.; Akiyama, T.; Purwanto, H. Reduction of low grade iron ore pellet using palm kernel shell. Renew. Energy 2014, 63, 617–623. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Zhang, G.; Wang, H.; Zhao, D. Experiments and Kinetic Modeling for Reduction of Ferric Oxide-biochar Composite Pellets. ISIJ Int. 2017, 57, 1374–1383. [Google Scholar] [CrossRef] [Green Version]
- Saito, G.; Nomura, T.; Sakaguchi, N.; Akiyama, T. Optimization of the Dehydration Temperature of Goethite to Control Pore Morphology. ISIJ Int. 2016, 56, 1598–1605. [Google Scholar] [CrossRef] [Green Version]
- Kurniawan, A.; Abe, K.; Ohashi, K.; Nomura, T.; Akiyama, T. Reduction of mild-dehydrated, low-grade iron ore by ethanol. Fuel Process. Technol. 2018, 178, 156–165. [Google Scholar] [CrossRef]
- Hubbard, C.R.; Snyder, R.L. RIR-Measurement and Use in Quantitative XRD. Powder Diffr. 1988, 3, 74–77. [Google Scholar] [CrossRef] [Green Version]
- Walter, D.; Buxbaum, G.; Laqua, W. The Mechanism of the Thermal Transformation from Goethite to Hematite. J. Therm. Anal. Calorim. 2001, 63, 733–748. [Google Scholar] [CrossRef]
- Pradhan, N.; Nayak, R.R.; Mishra, D.K.; Priyadarshini, E.; Sukla, L.B.; Mishra, B.K. Microbial Treatment of Lateritic Ni-ore for Iron Beneficiation and Their Characterization. World Environ. 2012, 2, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Zhang, J.; Qin, B.; Dong, Y.; Lu, Y.; Li, Y.; Hao, W.; Zhang, Y. Reduction kinetics of hematite ore fines with H2 in a rotary drum reactor. Powder Technol. 2018, 332, 18–26. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Song, Q.; Liu, S.; Ma, L.; Shu, X. Catalytic reforming of volatiles from co-pyrolysis of lignite blended with corn straw over three iron ores: Effect of iron ore types on the product distribution, carbon-deposited iron ore reactivity and its mechanism. Fuel 2021, 286, 119398. [Google Scholar] [CrossRef]
- Zuo, H.-B.; Hu, Z.-W.; Zhang, J.-L.; Li, J.; Liu, Z.-J. Direct reduction of iron ore by biomass char. Int. J. Miner. Met. Mater. 2013, 20, 514–521. [Google Scholar] [CrossRef]
- Chen, J.; Fan, X.; Jiang, B.; Mub, L.; Yao, P.; Yin, H.; Song, X. Pyrolysis of oil-plant wastes in a TGA and a fixed-bed reactor: Thermochemical behaviors, kinetics, and products characterization. Bioresour. Technol. 2015, 192, 592–602. [Google Scholar] [CrossRef]
- Somerville, M.; Deev, A. The effect of heating rate, particle size and gas flow on the yield of charcoal during the pyrolysis of radiata pine wood. Renew. Energy 2020, 151, 419–425. [Google Scholar] [CrossRef]
- Wu, C.; Budarin, V.L.; Gronnow, M.J.; De Bruyn, M.; Onwudili, J.A.; Clark, J.H.; Williams, P.T. Conventional and microwave-assisted pyrolysis of biomass under different heating rates. J. Anal. Appl. Pyrolysis 2014, 107, 276–283. [Google Scholar] [CrossRef]
- Guo, D.; Li, Y.; Cui, B.; Chen, Z.; Luo, S.; Xiao, B.; Zhu, H.; Hu, M. Direct reduction of iron ore/biomass composite pellets using simulated biomass-derived syngas: Experimental analysis and kinetic modelling. Chem. Eng. J. 2017, 327, 822–830. [Google Scholar] [CrossRef]
- Mishra, S. Review on Reduction Kinetics of Iron Ore–Coal Composite Pellet in Alternative and Sustainable Ironmaking. J. Sustain. Metall. 2020, 6, 541–556. [Google Scholar] [CrossRef]
- Oh, J.; Noh, D. The reduction kinetics of hematite particles in H2 and CO atmospheres. Fuel 2017, 196, 144–153. [Google Scholar] [CrossRef]
- Castro, J.A.; Nogami, H.; Yagi, J. Transient Mathematical Model of Blast Furnace Based on Multi-fluid Concept, with Application to High PCI Operation. ISIJ Int. 2000, 40, 637–646. [Google Scholar] [CrossRef] [Green Version]




| Composition | TFe | Fe2O3 | Si | Ca | Al | O | CW | C |
|---|---|---|---|---|---|---|---|---|
| Weight (mass%) | 46.835 | 18.941 | 9.225 | 5.366 | 0.534 | 37.492 | 4.93 | 5.62 |
| Sample | Proximate Analysis (Mass%, Air-Dried Basis) | Ultimate Analysis (Mass%, Air-Dried Basis) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fixed Carbon | Volatile Matter | Water | Ash | C | H | O | N | S | |
| PKS | 30.771 | 65.579 | 2.700 | 0.950 | 50.720 | 6.170 | 0.220 | 41.760 | 0.030 |
| Molasses | 4.968 | 56.441 | 35.191 | 3.430 | 29.690 | 7.730 | 0.560 | 57.400 | 0.260 |
| Heating Rate (°C/min) | Ti (°C) | Tmax-1 (°C) | Tmax-2 (°C) | Tmax-3 (°C) | Tmax-4 (°C) | Tf (°C) | dm/dtmax-1 (%/min) | dm/dtmax-2 (%/min) | dm/dtmax-3 (%/min) | dm/dtmax-4 (%/min) | dm/dtmean (%/min) | S | tr (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 109.02 | 200.57 | 269.60 | 336.22 | 847.98 | 950 | 0.21 | 0.72 | 0.86 | 0.19 | 0.17 | 1.5 × 10-8 | 168.21 |
| 10 | 138.00 | 212.49 | 276.10 | 343.69 | 862.93 | 950 | 0.49 | 1.08 | 1.53 | 0.21 | 0.29 | 1.6 × 10-8 | 82.07 |
| 15 | 147.32 | 218.44 | 284.05 | 349.66 | 920.00 | 950 | 0.79 | 2.25 | 2.31 | 0.75 | 0.48 | 2.3 × 10-8 | 55.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkania, A.; Rochmadi, R.; Hidayat, M.; Cahyono, R.B. Reduction Reactivity of Low Grade Iron Ore-Biomass Pellets for a Sustainable Ironmaking Process. Energies 2022, 15, 137. https://doi.org/10.3390/en15010137
Zulkania A, Rochmadi R, Hidayat M, Cahyono RB. Reduction Reactivity of Low Grade Iron Ore-Biomass Pellets for a Sustainable Ironmaking Process. Energies. 2022; 15(1):137. https://doi.org/10.3390/en15010137
Chicago/Turabian StyleZulkania, Ariany, Rochmadi Rochmadi, Muslikhin Hidayat, and Rochim Bakti Cahyono. 2022. "Reduction Reactivity of Low Grade Iron Ore-Biomass Pellets for a Sustainable Ironmaking Process" Energies 15, no. 1: 137. https://doi.org/10.3390/en15010137






