The Effect of Chromium on Photosynthesis and Lipid Accumulation in Two Chlorophyte Microalgae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Culture and Treatment
2.2. Determination of Microalgae Growth Performance
2.3. Measurement of Toxicity, Modulated Fluorescence and Photosynthetic Inhibition
2.4. Lipid Extraction
2.5. Fatty Acid Analysis
2.6. Statistical Analysis
3. Results
3.1. Influence of Chromium on Microalgal Growth
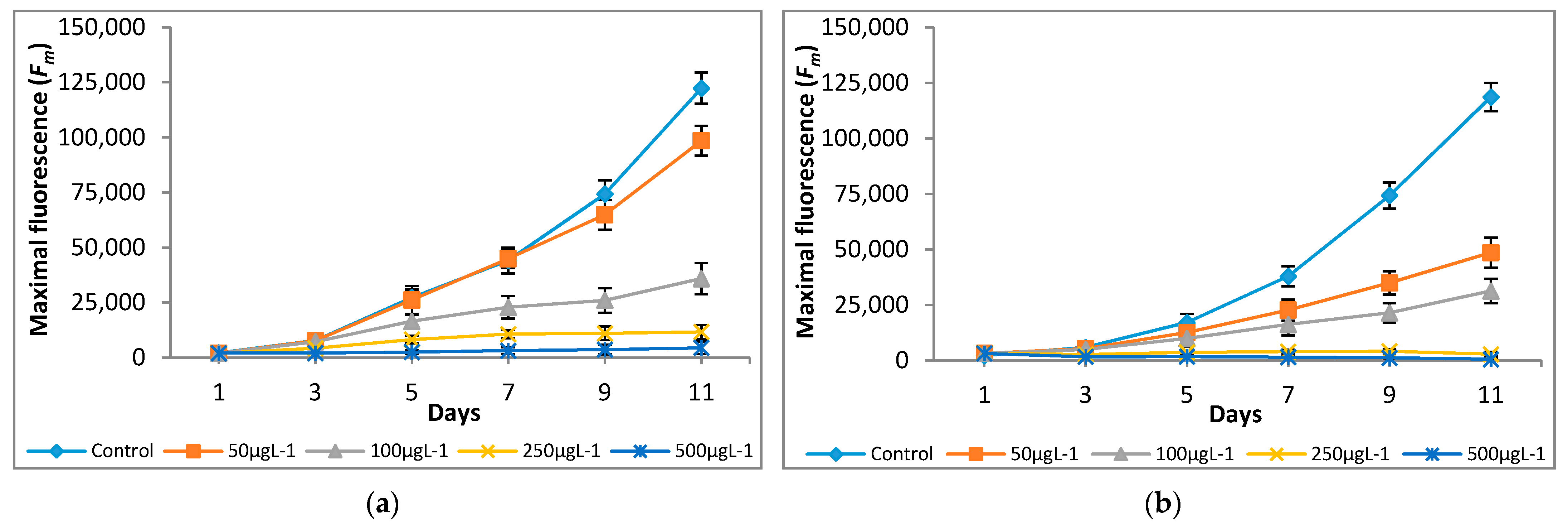
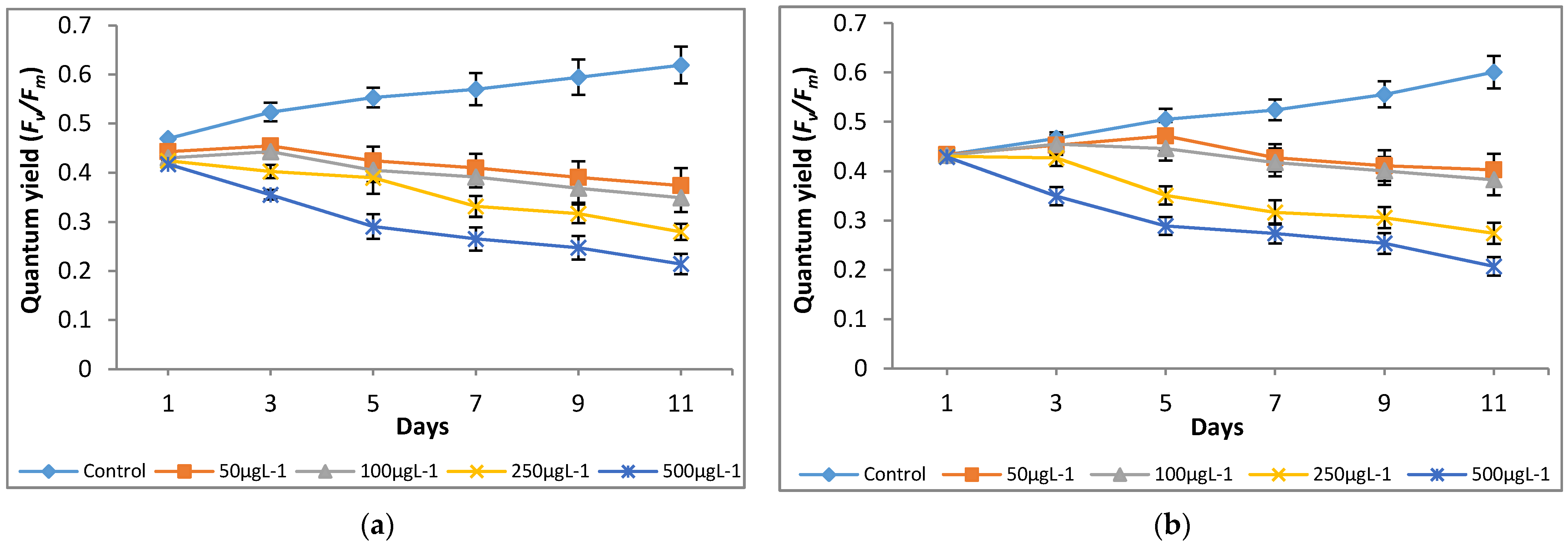
3.2. Effect of Chromium on Modulated Fluorescence and Photosynthetic Inhibition
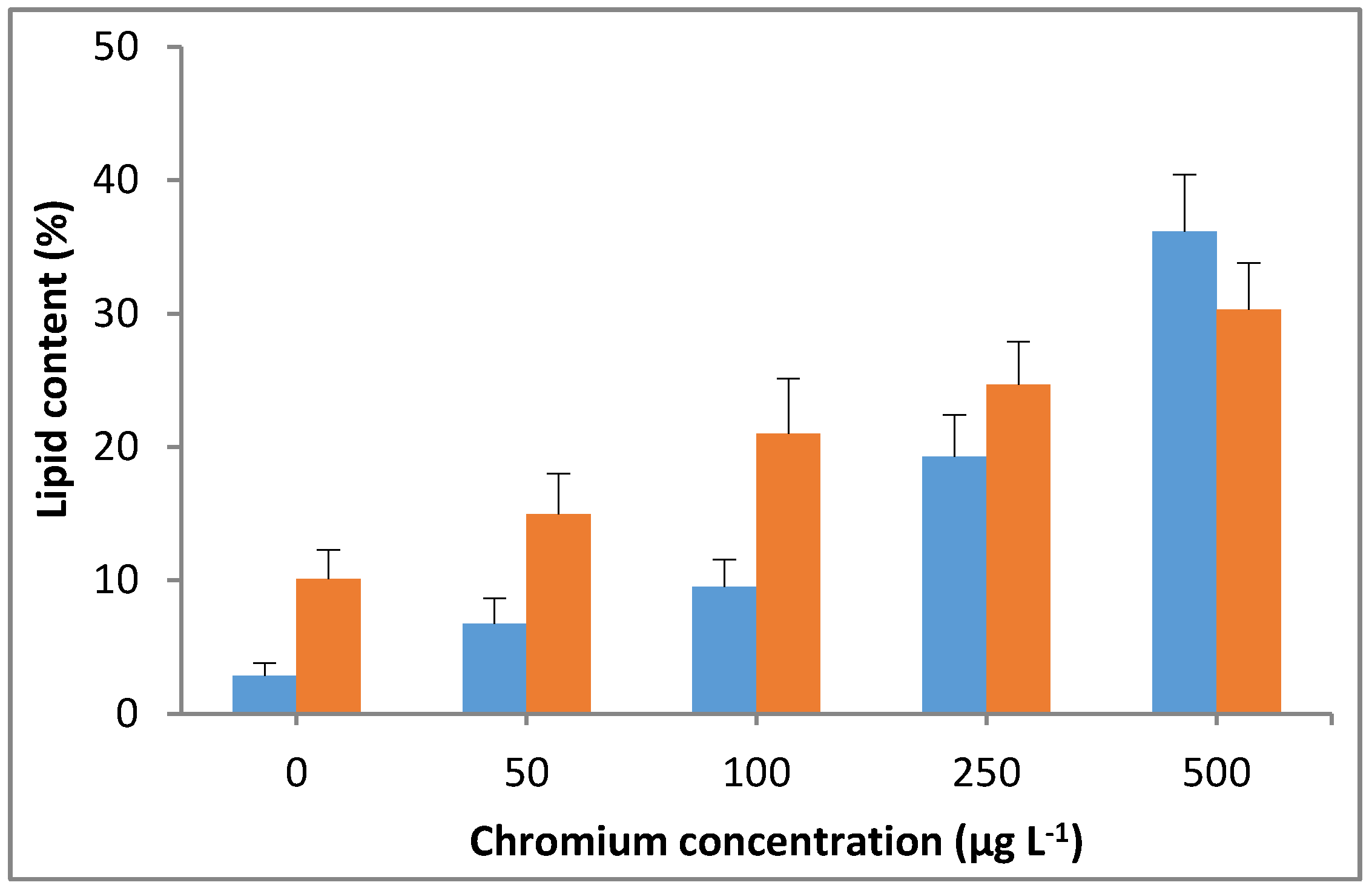
3.3. Effect of Chromium on Lipid Accumulation
3.4. Fatty Acid Composition
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bashir, K.M.I.; Mansoor, S.; Kim, N.-R.; Grohmann, F.R.; Shah, A.A.; Cho, M.-G. Effect of organic carbon sources and environmental factors on cell growth and lipid content of Pavlova lutheri. Ann. Microbiol. 2019, 69, 353–368. [Google Scholar] [CrossRef]
- Lu, C.M.; Chau, C.W.; Zhang, J.H. Acute toxicity of excess mercury on the photosynthetic performance of cyanobacterium, Spirulina platensis—Assessment by chlorophyll fluorescence analysis. Chemosphere 2000, 41, 191–196. [Google Scholar] [CrossRef]
- Juneau, P.; Dewez, D.; Matsui, S.; Kim, S.G.; Popovic, R. Evaluation of different algal species sensitivity to mercury and metolachlor by PAM-fluorometry. Chemosphere 2001, 45, 589–598. [Google Scholar] [CrossRef]
- Pena-Vazquez, E.; Perez-Conde, C. Development of a microalgal PAM test method for Cu (II) in water: Comparison of using spectrofluorometry. Ecotoxicology 2010, 19, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.M.I.; Cho, M.-G. The effect of kanamycin and tetracycline on growth and photosynthetic activity of two Chlorophyte algae. BioMed Res. Int. 2016, 2016, 5656304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miazek, K.; Iwanek, W.; Remacle, C.; Richel, A.; Goffin, D. Effect of metals, metalloids and metallic nanoparticles on microalgae growth and industrial product biosynthesis: A review. Int. J. Mol. Sci. 2015, 16, 23929–23969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Rodas, V.; Agrelo, M.; Carrillo, E.; Ferrero, L.; Larrauri, A.; Martín-Otero, L.; Costas, E. Resistance of microalgae to modern water contaminants as the result of rare spontaneous mutations. Eur. J. Phycol. 2001, 36, 179–190. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Gałczyńska, M. Sustainable production of Monoraphidium microalgae biomass as a source of bioenergy. Energies 2020, 13, 5975. [Google Scholar] [CrossRef]
- Bengtson Nash, S.M.; Quayle, P.A.; Schreiber, U.; Müller, J.F. The selection of a model microalgal species as biomaterial for a novel aquatic phytotoxicity assay. Aquat. Toxicol. 2005, 72, 315–326. [Google Scholar] [CrossRef]
- Herlory, O.; Bonzom, J.M.; Gilbin, R. Sensitivity evaluation of green alga Chlamydomonas reinhardtii to uranium by pulse amplitude modulated (PAM) fluorometry. Aquat. Toxicol. 2013, 140–141, 288–294. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.-U.; Lee, J.-S.; Kim, H.C.; Lee, W.C.; Shin, K.-H. Algal photosynthetic responses to toxic metals and herbicides assessed by chlorophyll a fluorescence. Ecotoxicol. Environ. Saf. 2014, 104, 51–71. [Google Scholar] [CrossRef]
- Ouyang, H.L.; Kong, X.Z.; He, W.; Qin, N.; He, Q.S.; Wang, Y.; Wang, R.; Xu, F.L. Effects of five heavy metals at sub-lethal concentrations on the growth and photosynthesis of Chlorella vulgaris. Chin. Sci. Bull. 2012, 57, 3363–3370. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, S.; Rovina, K.; Azad, S.A.; Naher, L.; Suryani, S.; Chaikaew, P. Heavy metal contaminants removal from wastewater using the potential filamentous fungi biomass: A review. J. Microb. Biochem. Technol. 2015, 7, 384–393. [Google Scholar] [CrossRef]
- Ng, C.C.; Motior Rahman, M.; Boyce, A.N.; Abas, M.R. Heavy metals phyto-assessment in commonly grown vegetables: Water spinach (I. aquatica) and okra (A. esculentus). SpringerPlus 2016, 5, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignati, D.A.L.; Dominik, J.; Beye, M.L.; Pettine, M.; Ferrari, B.J.D. Chromium (VI) is more toxic than chromium (III) to freshwater algae: A paradigm to revise? Ecotoxicol. Environ. Saf. 2010, 73, 743–749. [Google Scholar] [CrossRef]
- WHO. Chromium in Drinking-Water Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2003; Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/chromium.pdf (accessed on 2 March 2021).
- Yang, L.; Chen, P. Biosorption of hexavalent chromium onto raw and chemically modified Sargassum sp. Bioresour. Technol. 2008, 99, 297–307. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Gao, L.; Zhao, W.; Chen, W.; Li, M. Effects of nitrogen forms and supply mode on lipid production of microalga Scenedesmus obliquus. Energies 2020, 13, 697. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.; Phang, S.-M.; Tong, S.-L.; Brown, M.T. A modified toxicity testing method using tropical marine microalgae. Environ. Monit. Assess 2002, 75, 145–154. [Google Scholar] [CrossRef]
- Baumann, H.A.; Morrison, L.; Stenge, D.B. Metal accumulation and toxicity measured by PAM-chlorophyll fluorescence in seven species of marine macroalgae. Ecotoxicol. Environ. Saf. 2009, 72, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.M.I.; Lee, J.-H.; Petermann, M.J.; Shah, A.A.; Jeong, S.-J.; Kim, M.-S.; Park, N.G.; Cho, M.-G. Estimation of antibacterial properties of Chlorophyta, Rhodophyta and Haptophyta microalgae species. Microbiol. Biotechnol. Lett. 2018, 46, 225–233. [Google Scholar] [CrossRef]
- Watanabe, M.M.; Kawachi, M.; Hiroki, M.; Kasai, F. NIES Collection List of Strains. In Microalgae and Protozoa. Microbial Culture Collections, 6th ed.; National Institute for Environmental Studies: Tsukuba, Japan, 2000; p. 159. [Google Scholar]
- Schreiber, U. Detection of rapid induction kinetics with a new type of high-frequency modulated chlorophyll fluorometer. Photosynth. Res. 1986, 9, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balan, R.; Suraishkumar, G.K. Simultaneous increases in specific growth rate and specific lipid content of Chlorella vulgaris through UV-induced reactive species. Biotechnol. Prog. 2014, 30, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Eary, L.E.; Zachara, J.M. Environmental chemistry of chromium. Sci. Total Environ. 1989, 86, 15–23. [Google Scholar] [CrossRef]
- Gomez, V.; Callao, M.P. Chromium determination and speciation since 2000. TRAC-Trend Anal. Chem. 2006, 25, 1006–1015. [Google Scholar] [CrossRef]
- Nogales, B.; Lanfranconi, M.P.; Pina-Villalonga, J.M.; Bosch, R. Anthropogenic perturbations in marine microbial communities. FEMS Microbiol. Rev. 2011, 35, 275–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagchi, D.; Stohs, S.J.; Downs, B.W.; Bagchi, M.; Preuss, H.G. Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 2002, 180, 5–22. [Google Scholar] [CrossRef]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in agricultural soils and crops: A review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Cieslak-Golonka, M. Toxic and mutagenic effects of chromium (VI). In Polyhedron Rep; Elsevier Science Ltd: Amsterdam, The Netherlands, 1996; Volume 61, p. 3667. [Google Scholar]
- Haglund, K. The use of algae in aquatic toxicity assessment. In Progress in Phycological Research; Biopress: Bristol, UK, 1997; Volume 12, pp. 181–212. [Google Scholar]
- Vajpayee, P.; Rai, U.N.; Ali, M.B.; Tripathi, V.; Yadav, V.; Sinha, S.; Singh, S.N. Chromium-induced physiologic changes in Vallisneria spiralis L. and its role in phytoremediation of tannery effluent. Bull. Environ. Contam. Toxicol. 2001, 67, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Irfanullah, H.M.D.; Moss, B. Ecology of Dictyosphaerium pulchellum Wood (Chlorophyta, Chlorococcales) in a shallow, acid, forest lake. Aquat. Ecol. 2006, 40, 1–12. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Kim, M.-S.; Stahl, U.; Cho, M.-G. Agrobacterium-mediated genetic transformation of Dictyosphaerium pulchellum for the expression of erythropoietin. J. Appl. Phycol. 2018, 30, 3503–3518. [Google Scholar] [CrossRef]
- Pan, X.; Chen, X.; Zhang, D.; Wang, J.; Deng, C.; Mu, G.; Zhu, H. Effect of chromium(VI) on photosystem II activity and heterogeneity of Synechocystis sp. (Cyanophyta): Studied with in vivo chlorophyll fluorescence tests. J. Phycol. 2009, 45, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Hörcsik, Z.; Oláh, V.; Balogh, A.; Mészáros, I.; Simon, L.; Lakatos, G. Effect of Chromium(VI) on growth, element, and photosynthetic pigment composition of Chlorella pyrenoidosa. Acta. Biol. Szeged. 2006, 50, 19–23. [Google Scholar]
- Barsanti, L.; Bastianini, A.; Passarelli, V.; Tredici, M.R.; Gualtieri, P. Fatty acid content in wild type and WZSL mutant of Euglena gracilis. J. Appl. Phycol. 2000, 12, 515–520. [Google Scholar] [CrossRef]
- Rocchetta, I.; Mazzuca, M.; Conforti, V.; Ruiz, L.; Balzaretti, V.; Ríos de Molina, M.d.C. Effect of chromium on the fatty acid composition of two strains of Euglena gracilis. Environ. Pollut. 2006, 141, 353–358. [Google Scholar] [CrossRef] [PubMed]





| Species | Cr(VI) Concentration | ||||
|---|---|---|---|---|---|
| 0 μg L−1 | 50 μg L−1 | 100 μg L−1 | 250 μg L−1 | 500 μg L−1 | |
| M. pulchellum | μ = 0.1912 | μ = 0.1605 | 0.3688 × 106 * | 0.2139 × 106 * | 0.1311 × 106 * |
| M. pusillum | 0.6007 × 106 * | 0.4706 × 106 * | 0.3937 × 106 * | 0.1678 × 106 * | 0.1110 × 106 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, K.M.I.; Lee, H.-J.; Mansoor, S.; Jahn, A.; Cho, M.-G. The Effect of Chromium on Photosynthesis and Lipid Accumulation in Two Chlorophyte Microalgae. Energies 2021, 14, 2260. https://doi.org/10.3390/en14082260
Bashir KMI, Lee H-J, Mansoor S, Jahn A, Cho M-G. The Effect of Chromium on Photosynthesis and Lipid Accumulation in Two Chlorophyte Microalgae. Energies. 2021; 14(8):2260. https://doi.org/10.3390/en14082260
Chicago/Turabian StyleBashir, Khawaja Muhammad Imran, Hyeon-Jun Lee, Sana Mansoor, Alexander Jahn, and Man-Gi Cho. 2021. "The Effect of Chromium on Photosynthesis and Lipid Accumulation in Two Chlorophyte Microalgae" Energies 14, no. 8: 2260. https://doi.org/10.3390/en14082260






