Novel Effective Yeast Strains and Their Performance in High Gravity and Very High Gravity Ethanol Fermentations from Sweet Sorghum Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Screening of Yeasts for Ethanol Tolerance and Ethanol Fermentation
2.1.1. Yeast Isolation
2.1.2. Inoculum Preparation
2.1.3. Ethanol Tolerance Test
2.1.4. Ethanol Fermentation Test
2.2. Batch Ethanol Fermentation under HG and VHG Conditions
2.2.1. Microorganisms and Inoculum Preparation
2.2.2. Raw Materials
2.2.3. Batch Ethanol Fermentation
2.3. Molecular Identification
2.3.1. Genomic DNA Extraction and PCR Amplification
2.3.2. Sequence Analyses
2.4. Analytical Methods
3. Results and Discussions
3.1. Isolation and Screening of Yeast for Ethanol Tolerance and Ethanol Fermentation
3.1.1. Yeast Isolation
3.1.2. Ethanol Tolerance Test
3.1.3. Ethanol Fermentation Test
3.2. Batch Ethanol Fermentation under HG and VHG Conditions
3.2.1. Ethanol Fermentation from SSJ Medium without Nutrient Addition
3.2.2. Ethanol Fermentation from SSJ Medium with Urea Addition
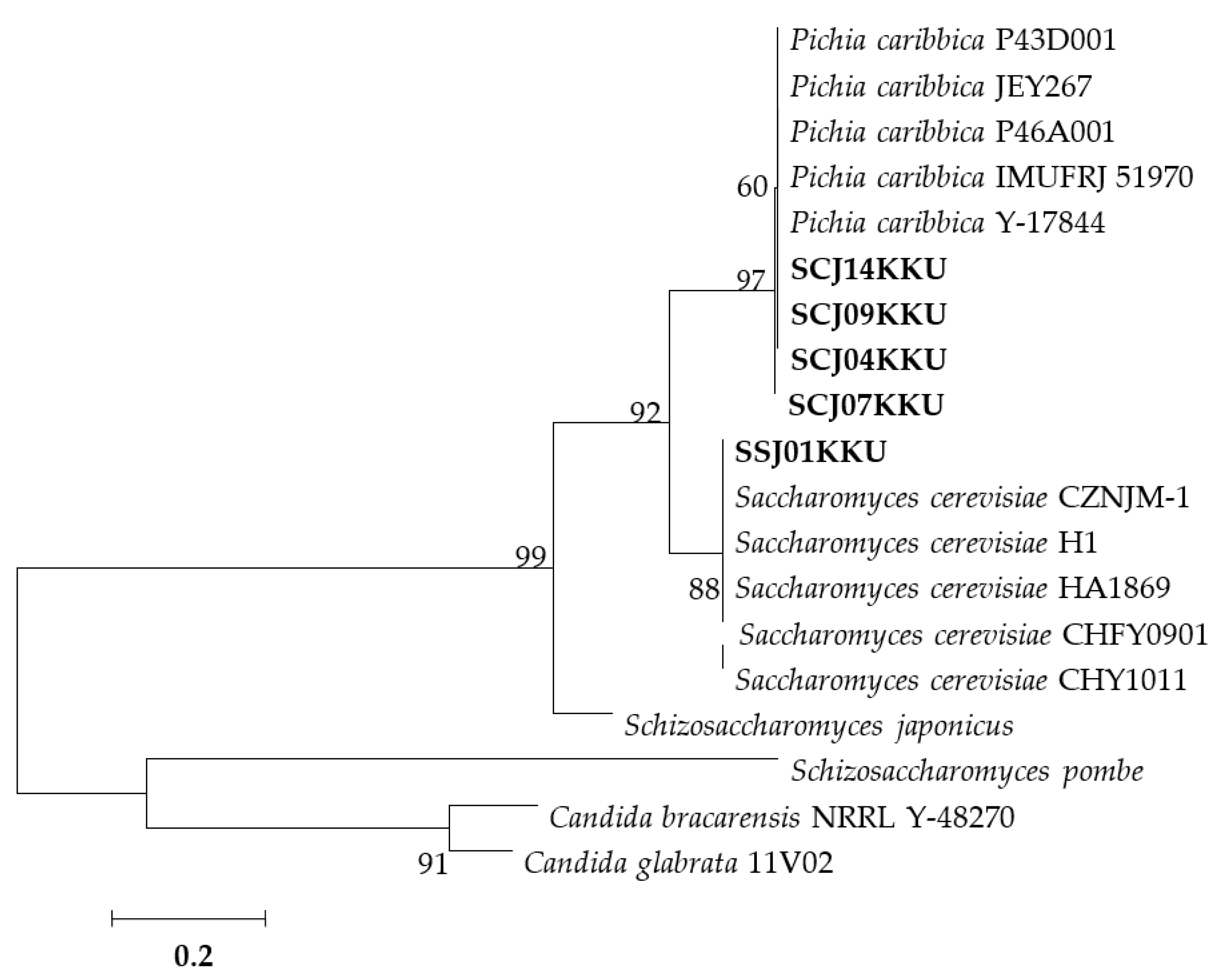
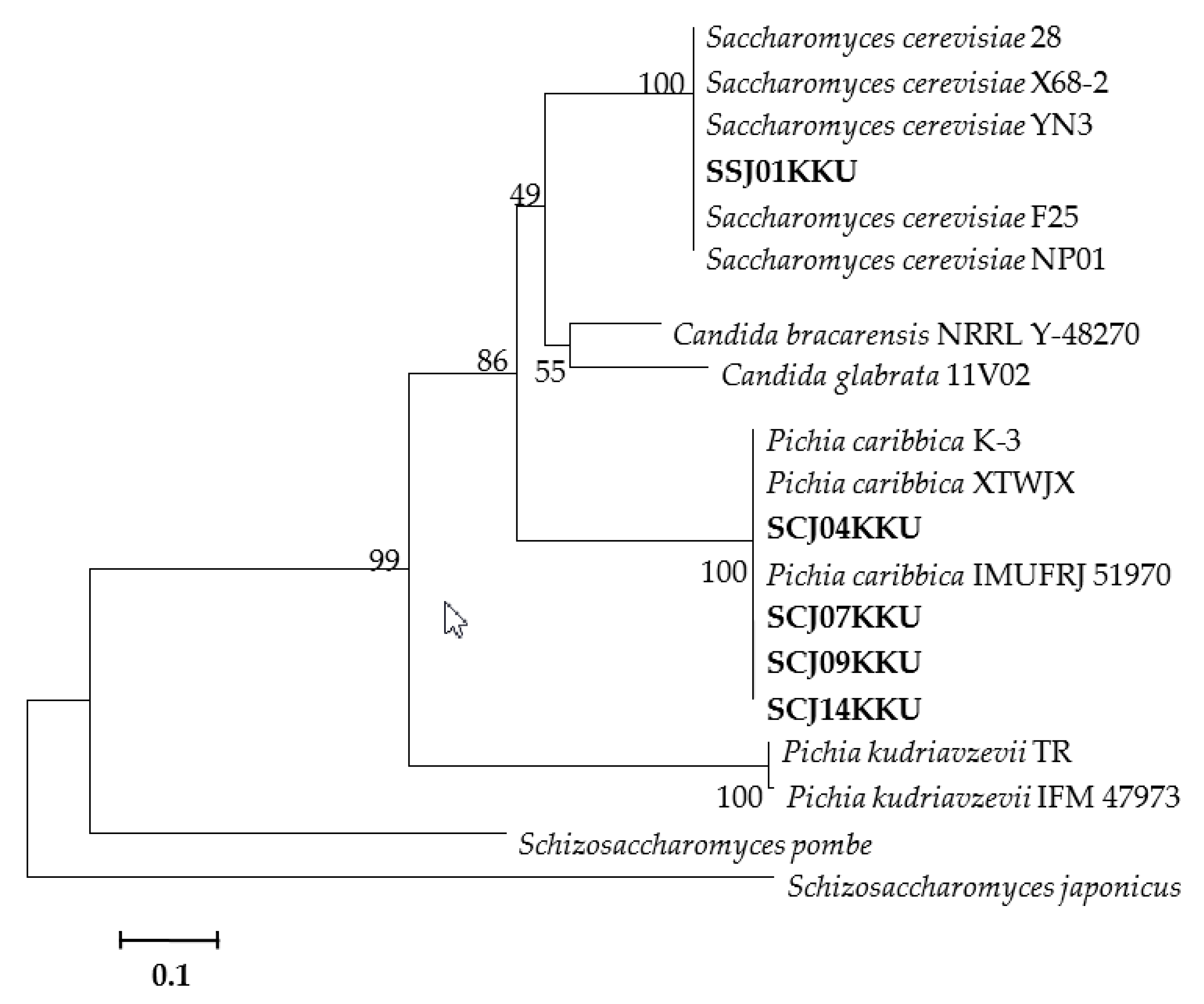
3.3. Molecular Identification of the Selected Yeasts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mathur, S.; Umakanth, A.V.; Tonapi, V.A.; Sharma, R.; Sharma, M.K. Sweet sorghum as biofuel feedstock: Recent advances and available resources. Biotechnol. Biofuels 2017, 10, 146. [Google Scholar] [CrossRef] [Green Version]
- Woods, J. Integrating Sweet Sorghum and Sugarcane for Bioenergy: Modelling the Potential for Electricity and Ethanol Production in SE Zimbabwe. Ph.D. Thesis, King’s College, London, UK, 2000. [Google Scholar]
- Gnansounou, E.; Dauriat, A.; Wyman, C.E. Refining sweet sorghum to ethanol and sugar: Economic trade-offs in the context of North China. Bioresour. Technol. 2005, 96, 885–1002. [Google Scholar] [CrossRef] [PubMed]
- Jaisil, P.; Pakdee, P.; Pothisoong, T.; Lertprasert-rat, K. Production cost of sweet sorghum (Sorghum bicolor (L.) Moench) and syrup production for ethanol plant. J. Nat. Res. Counc. Thail. 2009, 148–156. [Google Scholar]
- Deesuth, O.; Laopaiboon, P.; Jaisil, P.; Laopaiboon, L. Optimization of nitrogen and metal ions supplementation for very high gravity bioethanol fermentation from sweet sorghum juice using an orthogonal array design. Energies 2012, 5, 3178–3197. [Google Scholar] [CrossRef]
- Deesuth, O.; Laopaiboon, P.; Klanrit, P.; Laopaiboon, L. Improvement of ethanol production from sweet sorghum juice under high gravity and very high gravity conditions: Effects of nutrient supplementation and aeration. Ind. Crops Prod. 2015, 74, 95–102. [Google Scholar] [CrossRef]
- Phukoetphim, N.; Salakkam, A.; Laopaiboon, P.; Laopaiboon, L. Improvement of ethanol production from sweet sorghum juice under batch and fed-batch fermentations: Effects of sugar levels, nitrogen supplementation, and feeding regimes. Electron. J. Biotechnol. 2017, 26, 84–92. [Google Scholar] [CrossRef]
- Bai, F.W.; Anderson, W.A.; Moo-Young, M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, D.; Lin, Y.; Wang, X.; Kong, H.; Tanaka, S. Substrate and product inhibition on yeast performance in ethanol fermentation. Energy Fuels 2015, 29, 1019–1027. [Google Scholar] [CrossRef]
- Stanley, D.; Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010, 109, 13–24. [Google Scholar] [CrossRef]
- Bhima, B.; Marrivada, S.R.; Devi, T.A.; Reddy, Y.R.; Rao, L.V. Screening and characterization of stress tolerant Saccharomyces cerevisiae isolated from brewery effluents for animal probiotic applications. IIOAB J. 2010, 1, 32–39. [Google Scholar]
- Choi, G.W.; Um, H.J.; Kim, Y.; Kang, H.W.; Kim, M.; Chung, B.W.; Kim, Y.W. Isolation and characterization of two soil derived yeasts for bioethanol production on Cassava starch. Biomass Bioenergy 2010, 34, 1223–1231. [Google Scholar] [CrossRef]
- Kurtzman, C.P. Candida shehatae: Genetic diversity and phylogenetic relationships with other xylose-fermenting yeasts. Antonie Leeuwenhoek 1990, 57, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Chan-u-tit, P.; Laopaiboon, L.; Jaisil, P.; Laopaiboon, P. High level ethanol production by nitrogen and osmoprotectant supplementation under very high gravity fermentation conditions. Energies 2013, 6, 884–899. [Google Scholar] [CrossRef] [Green Version]
- Melzoch, K.; Rychtera, M.; Hábová, V. Effect of immobilization upon the properties and behaviour of Saccharomyces cerevisiae cells. J. Biotechnol. 1994, 32, 59–65. [Google Scholar] [CrossRef]
- Ariyajarearnwong, P.; Laopaiboon, L.; Jaisil, P.; Laopaiboon, P. Repeated-batch ethanol fermentation from sweet sorghum juice by free cells of Saccharomyces cerevisiae NP 01. Afr. J. Biotechnol. 2011, 10, 13909–13918. [Google Scholar] [CrossRef] [Green Version]
- Sridee, W.; Laopaiboon, L.; Jaisil, P.; Laopaiboon, P. The use of dried spent yeast as a low-cost nitrogen supplement in ethanol fermentation from sweet sorghum juice under very high gravity conditions. Electron. J. Biotechnol. 2011, 14, 3. [Google Scholar] [CrossRef]
- Laopaiboon, L.; Nuanpeng, S.; Srinophakun, P.; Klanrit, P.; Laopaiboon, P. Ethanol production from sweet sorghum juice using very high gravity technology: Effect carbon and nitrogen supplementations. Bioresour. Technol. 2009, 100, 4176–4182. [Google Scholar] [CrossRef]
- Harju, S.; Fedosyuk, H.; Peterson, K.R. Rapid isolation of yeast genomic DNA: Bust n’ Grab. BMC Biotechnol. 2004, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Zoecklien, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine Analysis and Production; Chapman & Hall: New York, NY, USA, 1995. [Google Scholar]
- Mecozzi, M. Estimation of total carbohydrate amount in environmental samples by the phenol-sulphuric acid method assisted by multivariate calibration. Chemometr. Intell. Lab. Syst. 2005, 9, 84–90. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, W.; Li, C.; Sakakibara, K.; Tanaka, S.; Kong, H. Factors affecting ethanol fermentation using Saccharomyces cerevisiae BY4742. Biomass Bioenergy 2012, 47, 395–401. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Xue, C.; Bai, F. Impact of osmotic stress and ethanol inhibition in yeast cells on process oscillation associated with continuous very-high-gravity ethanol fermentation. Biotechnol. Biofuels 2013, 6, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozmihci, S.; Kargi, F. Ethanol fermentation of cheese whey powder solution by repeated fed-batch operation. Enzym. Microb. Technol. 2007, 41, 169–174. [Google Scholar] [CrossRef]
- Suwanapong, S.; Khongsay, N.; Laopaiboon, L.; Jaisil, P.; Laopaiboon, P. Dried spent yeast and its hydrolysate as nitrogen supplements for single batch and repeated-batch ethanol fermentation from sweet sorghum juice. Energies 2013, 6, 1618–1631. [Google Scholar] [CrossRef]
- Yue, G.; Yu, J.; Zhang, X.; Tan, T. The influence of nitrogen sources on ethanol production by yeast from concentrated sweet sorghum juice. Biomass Bioenerg. 2012, 39, 48–52. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Robnett, C.J. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 1997, 35, 1216–1233. [Google Scholar] [CrossRef] [Green Version]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef]
- Oda, Y.; Yabuki, M.; Tonomura, K.; Fukunaga, M. A phylogenetic analysis of Saccharomyces species by the sequence of 18S-28S rRNA spacer regions. Yeast 1997, 13, 1243–1250. [Google Scholar] [CrossRef]
- Belloch, C.; Fernández-Espinar, T.; Querol, A.; García, M.D.; Barrio, E. An analysis of inter- and intraspecific genetic variabilities in the Kluyveromyces marxianus group of yeast species for the reconsideration of the K. lactis taxon. Yeast 2002, 19, 257–268. [Google Scholar] [CrossRef]
- Chang, J.W.; Lin, Y.H.; Huang, L.Y.; Duan, K.J. The effect of fermentation configurations and FAN supplementation on ethanol production from sorghum grains under very-high-gravity conditions. J. Taiwan Inst. Chem. E 2011, 42, 1–4. [Google Scholar] [CrossRef]
- Khongsay, N.; Lin, Y.H.; Laopaiboon, P.; Laopaiboon, L. Improvement of very-high-gravity ethanol fermentation from sweet sorghum juice by controlling fermentation redox potential. J. Taiwan Inst. Chem. E 2014, 45, 302–307. [Google Scholar] [CrossRef]
- Thani, A.; Lin, Y.H.; Laopaiboon, P.; Laopaiboon, L. Variation of fermentation redox potential during cell-recycling continuous ethanol operation. J. Biotechnol. 2016, 239, 68–75. [Google Scholar] [CrossRef] [PubMed]
 ), SCJ02KKU (△), SCJ03KKU (✕), SCJ04KKU (
), SCJ02KKU (△), SCJ03KKU (✕), SCJ04KKU (  ), SCJ05KKU (▽), SCJ06KKU (
), SCJ05KKU (▽), SCJ06KKU (  ), SCJ07KKU (☐), SCJ08KKU (◆), SCJ09KKU (
), SCJ07KKU (☐), SCJ08KKU (◆), SCJ09KKU (  ), SCJ10KKU (
), SCJ10KKU (  ), SCJ11KKU (
), SCJ11KKU (  ), SCJ12KKU (
), SCJ12KKU (  ), SCJ13KKU (
), SCJ13KKU (  ) and SCJ14KKU (
) and SCJ14KKU (  ); (B): GR01KKU (
); (B): GR01KKU (  ), GR02KKU (△), GR03KKU (✕), GR04KKU (
), GR02KKU (△), GR03KKU (✕), GR04KKU (  ), GR05KKU (▽), GR06KKU (
), GR05KKU (▽), GR06KKU (  ), GR07KKU (☐), GR08KKU (◆), GR09KKU (
), GR07KKU (☐), GR08KKU (◆), GR09KKU (  ), GR10KKU (
), GR10KKU (  ) and SSJ01KKU (
) and SSJ01KKU (  ), NP01 (
), NP01 (  ) and ATCC4132 (▲).
) and ATCC4132 (▲).
 ), SCJ02KKU (△), SCJ03KKU (✕), SCJ04KKU (
), SCJ02KKU (△), SCJ03KKU (✕), SCJ04KKU (  ), SCJ05KKU (▽), SCJ06KKU (
), SCJ05KKU (▽), SCJ06KKU (  ), SCJ07KKU (☐), SCJ08KKU (◆), SCJ09KKU (
), SCJ07KKU (☐), SCJ08KKU (◆), SCJ09KKU (  ), SCJ10KKU (
), SCJ10KKU (  ), SCJ11KKU (
), SCJ11KKU (  ), SCJ12KKU (
), SCJ12KKU (  ), SCJ13KKU (
), SCJ13KKU (  ) and SCJ14KKU (
) and SCJ14KKU (  ); (B): GR01KKU (
); (B): GR01KKU (  ), GR02KKU (△), GR03KKU (✕), GR04KKU (
), GR02KKU (△), GR03KKU (✕), GR04KKU (  ), GR05KKU (▽), GR06KKU (
), GR05KKU (▽), GR06KKU (  ), GR07KKU (☐), GR08KKU (◆), GR09KKU (
), GR07KKU (☐), GR08KKU (◆), GR09KKU (  ), GR10KKU (
), GR10KKU (  ) and SSJ01KKU (
) and SSJ01KKU (  ), NP01 (
), NP01 (  ) and ATCC4132 (▲).
) and ATCC4132 (▲).
 ,☐), SCJ07KKU (
,☐), SCJ07KKU (  ,
,  ), SCJ09KKU (▼,∇), SCJ14KKU (◆,
), SCJ09KKU (▼,∇), SCJ14KKU (◆,  ) and SSJ01KKU (
) and SSJ01KKU (  ,
,  ); reference strains: S. cerevisiae NP01 (
); reference strains: S. cerevisiae NP01 (  ,
,  ) and ATCC4132 (▲,
) and ATCC4132 (▲,  ).
).
 ,☐), SCJ07KKU (
,☐), SCJ07KKU (  ,
,  ), SCJ09KKU (▼,∇), SCJ14KKU (◆,
), SCJ09KKU (▼,∇), SCJ14KKU (◆,  ) and SSJ01KKU (
) and SSJ01KKU (  ,
,  ); reference strains: S. cerevisiae NP01 (
); reference strains: S. cerevisiae NP01 (  ,
,  ) and ATCC4132 (▲,
) and ATCC4132 (▲,  ).
).
 ,☐), SCJ14KKU (◆,
,☐), SCJ14KKU (◆,  ) and SSJ01KKU (
) and SSJ01KKU (  ,
,  ); reference strains: S. cerevisiae NP01 (
); reference strains: S. cerevisiae NP01 (  ,
,  ) and ATCC4132 (▲,
) and ATCC4132 (▲,  ).
).
 ,☐), SCJ14KKU (◆,
,☐), SCJ14KKU (◆,  ) and SSJ01KKU (
) and SSJ01KKU (  ,
,  ); reference strains: S. cerevisiae NP01 (
); reference strains: S. cerevisiae NP01 (  ,
,  ) and ATCC4132 (▲,
) and ATCC4132 (▲,  ).
).
| Isolate | Sugar Consumption (%) | Parameter | ||
|---|---|---|---|---|
| PE (g/L) | QP (g/L·h) | YP/S (g/g) | ||
| NP01 (control) | 55.97 ± 1.86 h,j | 56.46 ± 0.43 l,n | 1.18 ± 0.02 n | 0.51 ± 0.00 a |
| ATCC4132 (control) | 61.03 ± 0.21 k,l | 61.31 ± 0.56 m | 1.28 ± 0.02 j,m | 0.49 ± 0.01 a |
| SCJ01KKU | 33.70 ± 1.46 c | 25.71 ± 0.21 b | 0.54 ± 0.01 b | 0.37 ± 0.00 a |
| SCJ02KKU | 38.29 ± 1.43 d,e | 31.54 ± 0.27 c | 0.66 ± 0.01 b | 0.40 ± 0.01 a |
| SCJ03KKU | 44.13 ± 0.17 f | 42.01 ± 041 d,e,f | 0.88 ± 0.02 c,d,e | 0.48 ± 0.00 a |
| SCJ04KKU | 68.64 ± 1.87 n | 58.17 ± 0.27 l,m | 1.21 ± 0.02 j,m | 0.46 ± 0.00 a |
| SCJ05KKU | 33.53 ± 1.42 c | 31.27 ± 0.52 c | 0.65 ± 0.01 b | 0.46 ± 0.01 a |
| SCJ06KKU | 46.62 ± 1.03 f | 41.71 ± 0.42 d,e,f | 0.87 ± 0.02 c,d | 0.49 ± 0.01 a |
| SCJ07KKU | 67.42 ± 1.35 n | 55.88 ± 0.46 k,l | 1.16 ± 0.01 g,h,j | 0.46 ± 0.00 a |
| SCJ08KKU | 53.59 ± 1.56 g,h | 41.56 ± 0.30 d,e,f | 0.87 ± 0.02 c,d | 0.38 ± 0.01 a |
| SCJ09KKU | 66.34 ± 1.50 m,n | 56.37 ± 0.34 k,l | 1.17 ± 0.02 h,j | 0.42 ± 0.01 a |
| SCJ10KKU | 53.23 ± 1.07 g,h | 43.61 ± 0.40 d,e,f | 0.91 ± 0.02 c,d,e,f | 0.40 ± 0.01 a |
| SCJ11KKU | 53.23 ± 1.26 g,h | 44.97 ± 0.35 e,f,g | 0.94 ± 0.02 c,d,e,f | 0.41 ± 0.00 a |
| SCJ12KKU | 53.23 ± 1.40 g,h | 50.00 ± 0.28 h,j | 1.04 ± 0.02 g,h | 0.46 ± 0.01 a |
| SCJ13KKU | 63.82 ± 1.68 l,m | 48.88 ± 0.30 g,h,j | 1.02 ± 0.02 d,e,f | 0.38 ± 0.00 a |
| SCJ14KKU | 58.32 ± 0.06 j,k | 60.12 ± 0.48 l,m,n | 1.25 ± 0.03 j,m | 0.50 ± 0.00 a |
| GR01KKU | 43.90 ± 1.73 h | 42.45 ± 0.35 h | 0.88 ± 0.01 h | 0.40 ± 0.01 a |
| GR02KKU | 50.48 ± 1.53 h | 43.43 ± 0.27 h | 0.90 ± 0.02 h | 0.42 ± 0.01 a |
| GR03KKU | 51.30 ± 1.24 g | 46.23 ± 0.32 f,g,h | 0.96 ± 0.072 c,d,e,f | 0.44 ± 0.00 a |
| GR04KKU | 53.20 ± 1.84 g,h | 49.36 ± 0.41 j,k | 1.03 ± 0.02 f,g | 0.45± 0.00 a |
| GR05KKU | 9.82 ± 0.68 a | 13.06 ± 0.19 a | 0.27 ± 0.00 a | 0.49 ± 0.00 a |
| GR06KKU | 52.72 ± 1.22 g,h | 49.01 ± 0.53 g,h,j | 1.02 ± 0.01 e,f | 0.45 ± 0.01 a |
| GR07KKU | 21.41 ± 0.81 b | 14.13 ± 0.15 a | 0.29 ± 0.00 a | 0.32 ± 0.00 a |
| GR08KKU | 39.64 ± 0.85 e | 40.51 ± 0.25 d,e | 0.84 ± 0.02 c | 0.49 ± 0.00 a |
| GR09KKU | 36.80 ± 1.54 c,d,e | 39.59 ± 0.38 d | 0.82 ± 0.02 c | 0.49 ± 0.01 a |
| GR10KKU | 35.56 ± 0.84 c,d | 32.19 ± 0.50 c | 0.67 ± 0.01 b | 0.44 ± 0.00 a |
| SSJ01KKU | 60.82 ± 0.21 k,l | 63.96 ± 0.63 n | 1.33 ± 0.03 m | 0.50 ± 0.00 a |
| Initial Sugar (g/L) | Parameter | Strains | ||||||
|---|---|---|---|---|---|---|---|---|
| NP01 (Control) | ATCC4132 (Control) | SCJ04KKU | SCJ07KKU | SCJ09KKU | SCJ14KKU | SSJ01KKU | ||
| 200 (HG) | SC (%) | 93.06 ± 1.09 b,c | 94.43 ± 1.08 b | 90.83 ± 0.92 c | 74.31 ± 1.37 d | 61.89 ± 0.78 e | 96.11 ± 0.68 a,b | 98.96 ± 0.17 a |
| PE (g/L) | 93.05 ± 0.57 b | 98.18 ± 0.44 a | 88.89 ± 0.27 c | 78.28 ± 0.45 d | 55.79 ± 0.46 e | 98.49 ± 0.19 a | 98.89 ± 0.20 a | |
| QP (g/L·h) | 1.11 ± 0.01 c | 1.36 ± 0.02 a | 1.06 ± 0.02 d | 0.93 ± 0.02 e | 0.66 ± 0.02 f | 1.17 ± 0.01 b | 1.18 ± 0.01 b | |
| YP/S (g/g) | 0.51 ± 0.01 a | 0.50 ± 0.01 a | 0.50 ± 0.01 a | 0.51 ± 0.00 a | 0.45 ± 0.00 b | 0.50 ± 0.00 b | 0.50 ± 0.01 a | |
| t (h) | 84 | 72 | 84 | 84 | 84 | 84 | 84 | |
| 240 (HG) | SC (%) | 94.68 ± 0.22 b | 95.82 ± 0.41 a | 85.47 ± 0.69 c | - | - | 74.17 ± 0.69 d | 95.79 ± 0.13 a |
| PE (g/L) | 114.94 ± 0.40 b | 115.32 ± 0.30 b | 103.78 ± 0.45 c | - | - | 87.06 ± 0.39 d | 118.12 ± 0.57 a | |
| QP (g/L·h) | 1.37 ± 0.02 b | 1.37 ± 0.00 b | 1.24 ± 0.01 c | - | - | 1.04 ± 0.00 d | 1.41 ± 0.01 a | |
| YP/S (g/g) | 0.51 ± 0.00 a | 0.50 ± 0.01 a,b | 0.51 ± 0.00 a | - | - | 0.49 ± 0.00 a | 0.51 ± 0.00 a | |
| t (h) | 84 | 84 | 84 | - | - | 84 | 84 | |
| 280 (VHG) | SC (%) | 84.55 ± 0.04 d | 81.20 ± 0.17 c | 80.65 ± 0.04 b | - | - | - | 85.59 ± 0.17 a |
| PE (g/L) | 80.40 ± 0.31 b | 79.32 ± 0.58 c | 80.03± 0.76 c,b | - | - | - | 82.29± 0.10 a | |
| QP (g/L·h) | 0.96 ± 0.00 b | 0.94 ± 0.01 c | 0.96 ± 0.01 b | - | - | - | 0.98 ± 0.00 a | |
| YP/S (g/g) | 0.34 ± 0.00 a | 0.35 ± 0.00 a | 0.35 ± 0.01 a | - | - | - | 0.34 ± 0.00 a | |
| t (h) | 84 | 84 | 84 | - | - | - | 84 | |
| Initial Sugar (g/L) | Parameter | Strains | ||||
|---|---|---|---|---|---|---|
| NP01 (Control) | ATCC4132 (Control) | SCJ04KKU | SCJ14KKU | SSJ01KKU | ||
| 200 (HG) | SC (%) | 96.34 ± 0.76 b | 95.62 ± 0.09 b | 96.18 ± 0.46 b | 93.59 ± 0.41 a | 96.91 ± 0.12 b |
| PE (g/L) | 97.97 ± 0.33 b | 113.26 ± 0.13 c | 97.94 ± 0.11 b | 94.44 ± 0.13 a | 97.10 ± 0.05 b | |
| QP(g/L·h) | 2.04 ± 0.02 c | 2.04 ± 0.00 c | 1.63 ± 0.00 b | 1.57 ± 0.01 a | 2.02 ± 0.00 c | |
| YP/S (g/g) | 0.51 ± 0.00 a | 0.51 ± 0.00 a | 0.51 ± 0.00 a | 0.50 ± 0.01 a | 0.50 ± 0.01 a | |
| t (h) | 48 | 48 | 60 | 60 | 48 | |
| 240 (HG) | SC (%) | 90.97 ± 0.01 b | 92.41 ± 0.13 b | 91.31 ± 0.08 b | 72.30 ± 2.86 a - | 91.89 ± 0.11 b |
| PE (g/L) | 110.32 ± 0.65 b | 115.32 ± 0.30 b | 109.03 ± 1.27 b | 78.95 ± 1.21 a | 112.47 ± 0.31 c | |
| QP(g/L·h) | 2.30 ± 0.01 c | 2.36 ± 0.00 d | 1.82 ± 0.02 b | 1.10 ± 0.02 a | 2.34 ± 0.01 d | |
| YP/S (g/g) | 0.51 c | 0.51 c | 0.50 b | 0.46 a | 0.51 c | |
| t (h) | 48 | 48 | 60 | 72 | 48 | |
| 280 (VHG) | SC (%) | 88.23 ± 0.09 c | 86.98 ± 0.39 b | 80.66 ± 0.52 a | - | 88.47 ± 0.04 c |
| PE (g/L) | 118.23 ± 0.70 b,c | 117.06 ± 0.71 b | 103.20 ± 0.13 a | - | 119.06 ± 0.64 c | |
| QP(g/L·h) | 1.64 ± 0.01 b,c | 1.63 ± 0.00 b | 1.43 ± 0.00 a | - | 1.65 ± 0.01 c | |
| YP/S (g/g) | 0.48 ± 0.00 b | 0.48 ± 0.01 b | 0.46 ± 0.00 a | - | 0.48 ± 0.01 b | |
| t (h) | 72 | 72 | 72 | - | 72 | |
| 280 (VHG **) | SC (%) | 88.85 ± 0.48 b | 87.68 ± 0.25 a | - | - | 89.07 ± 0.10 b |
| PE (g/L) | 119.91 ± 0.32 b | 119.05 ± 0.09 a | - | - | 121.83 ± 0.49 c | |
| QP (g/L·h) | 1.67 ± 0.00 b | 1.65 ± 0.01 a | - | - | 1.69 ± 0.00 c | |
| YP/S (g/g) | 0.48 ± 0.00 b | 0.48 ± 0.00 b | - | - | 0.49 ± 0.00 a | |
| t (h) | 72 | 72 | - | - | 72 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laopaiboon, L.; Suporn, S.; Klanrit, P.; Phukoetphim, N.; Daengbussadee, C.; Laopaiboon, P. Novel Effective Yeast Strains and Their Performance in High Gravity and Very High Gravity Ethanol Fermentations from Sweet Sorghum Juice. Energies 2021, 14, 557. https://doi.org/10.3390/en14030557
Laopaiboon L, Suporn S, Klanrit P, Phukoetphim N, Daengbussadee C, Laopaiboon P. Novel Effective Yeast Strains and Their Performance in High Gravity and Very High Gravity Ethanol Fermentations from Sweet Sorghum Juice. Energies. 2021; 14(3):557. https://doi.org/10.3390/en14030557
Chicago/Turabian StyleLaopaiboon, Lakkana, Suntaree Suporn, Preekamol Klanrit, Niphaphat Phukoetphim, Chalida Daengbussadee, and Pattana Laopaiboon. 2021. "Novel Effective Yeast Strains and Their Performance in High Gravity and Very High Gravity Ethanol Fermentations from Sweet Sorghum Juice" Energies 14, no. 3: 557. https://doi.org/10.3390/en14030557




