Physically Motivated Water Modeling in Control-Oriented Polymer Electrolyte Membrane Fuel Cell Stack Models
Abstract
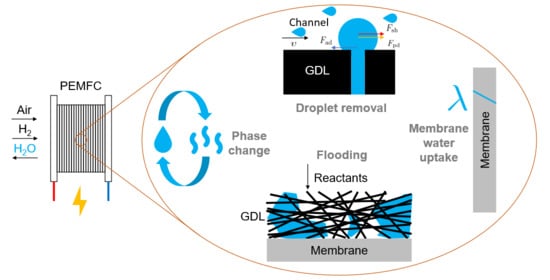
:1. Introduction
2. Fuel Cell Model
- The respective FC manifolds are lumped into one volume and do not have any spatial expansion;
- The gases are ideal;
- The gas inside a manifold has homogeneous properties;
- The gas in the exit manifold has the same composition as in the center manifold;
- Dry air only consists of nitrogen and oxygen;
- The FC has one uniform and externally controlled temperature;
- Steady-state conditions always hold in the supply and exit manifolds, as well as the GDL.
2.1. Model Description
2.1.1. Cathode
2.1.2. Anode
2.1.3. Membrane
2.1.4. Electrochemistry
2.1.5. Overview
2.2. Experimental Setup
2.3. Parametrization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CL | Catalyst layer |
| EMT | Effective medium theory |
| FC | Fuel cell |
| GDL | Gas diffusion layer |
| ODE | Ordinary differential equation |
| PEMFC | Polymer electrolyte membrane fuel cell |
Nomenclature
| Subscripts | ||
| Tunable parameters | ||
| Adhesion | ||
| Dry air | ||
| Anode | ||
| Atmosphere | ||
| Boltzmann | ||
| Cathode | ||
| Fuel cell | ||
| Channel | ||
| Center manifold | ||
| Contact | ||
| Dry | ||
| Droplet | ||
| Drag | ||
| Exit manifold | ||
| Gas diffusion layer | ||
| Gravitational | ||
| Hydrogen | ||
| Intrinsic exchange | ||
| Immobile | ||
| Injection | ||
| Inflow | ||
| Liquid water | ||
| Limit | ||
| Maximum | ||
| Minimum | ||
| Membrane | ||
| Nitrogen | ||
| Oxygen | ||
| Optimized | ||
| Outflow | ||
| Pressure drop | ||
| Phase change | ||
| Pushing | ||
| Recirculation | ||
| Reduced | ||
| Reference | ||
| Saturation | ||
| Shear | ||
| Sliding | ||
| Supply manifold | ||
| Sink and source | ||
| Vapor | ||
| Wetting | ||
| i | Gaseous species running index | |
| j | Membrane running index | |
| k | Sampling instant | |
| l | Tunable parameter running index | |
| n | Electrochemical running index | |
| Symbols | ||
| Valve position | 1 | |
| Molar volume | ||
| Adhesion parameter | 1 | |
| Vector containing the tunable parameters | ||
| Specific Gibbs free energy | J | |
| Specific entropy | J/K | |
| Thickness | m | |
| Surface tension | N/m | |
| Pressure drop parameter | 1 | |
| Membrane water content | 1 | |
| System function | ||
| Output function | ||
| Output weighting matrix | ||
| Input vector | ||
| State vector | ||
| Output vector | ||
| Measured output vector | ||
| Faraday constant | C/mol | |
| Universal or mass-specific gas constant | , or | |
| Gas viscosity | ||
| Switching function | 1 | |
| Density | ||
| Ionic conductivity | ||
| Time constant | s | |
| Θ | Tunable parameter | |
| Angle | rad | |
| Relative humidity | 1 | |
| A | Area | |
| a | Water activity | 1 |
| C | Unitless concentrations of the gaseous species | 1 |
| c | Membrane surface water concentration | |
| Combined diffusivity parameter | mol/s | |
| D | Water diffusion coefficient | |
| d | Droplet wetted diameter | m |
| E | Activation energy | J/mol |
| e | Elementary charge | C |
| F | Force | N |
| f | Volume fraction of water in the membrane | 1 |
| g | Gravitational acceleration | |
| H | Channel height | m |
| h | Droplet height | m |
| I | Current | A |
| J | Objective function | |
| K | Intrinsic exchange current parameter | |
| k | Nozzle, mass, volume flow coefficient, or constant | , , 1/s, 1/Pa, or J/K |
| M | Molar mass | kg/mol |
| m | Mass | kg |
| n | Number | 1, or |
| P | Power | W |
| p | Pressure | Pa |
| R | Ohmic resistance | Ω |
| r | Radius | m |
| s | Liquid water saturation | 1 |
| T | Fuel cell temperature | K |
| t | Time | s |
| U | Voltage | V |
| V | Volume | |
| v | Gas velocity | m/s |
| W | Channel width | m |
| w | Mass fraction | 1 |
| x | Humidity ratio | 1 |
| Z | Number of electrons transferred in the electrochemical reaction | 1 |
References
- Nehrir, M.H.; Wang, C. Principles of Operation of Fuel Cells. In Modeling and Control of Fuel Cells: Distributed Generation Applications; IEEE: Piscataway, NJ, USA, 2009; pp. 29–56. [Google Scholar] [CrossRef]
- Mench, M.M. Fuel Cell Engines; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Pukrushpan, J.T.; Peng, H.; Stefanopoulou, A.G. Control-Oriented Modeling and Analysis for Automotive Fuel Cell Systems. J. Dyn. Syst. Meas. Control 2004, 126, 14–25. [Google Scholar] [CrossRef]
- Xu, L.; Fang, C.; Hu, J.; Cheng, S.; Li, J.; Ouyang, M.; Lehnert, W. Parameter extraction and uncertainty analysis of a proton exchange membrane fuel cell system based on Monte Carlo simulation. Int. J. Hydrogen Energy 2017, 42, 2309–2326. [Google Scholar] [CrossRef]
- Müller, E.A.; Stefanopoulou, A.G. Analysis, Modeling, and Validation for the Thermal Dynamics of a Polymer Electrolyte Membrane Fuel Cell System. J. Fuel Cell Sci. Technol. 2006, 3, 99–110. [Google Scholar] [CrossRef]
- Pukrushpan, J.T.; Stefanopoulou, A.G.; Peng, H. Fuel Cell System Model: Fuel Cell Stack. In Control of Fuel Cell Power Systems; Advances in Industrial Control; Springer: London, UK, 2004; pp. 31–56. [Google Scholar] [CrossRef]
- Schultze, M.; Horn, J. A Control Oriented Simulation Model of an Evaporation Cooled Polymer Electrolyte Membrane Fuel Cell System. IFAC Proc. Vol. 2011, 44, 14790–14795. [Google Scholar] [CrossRef]
- Bao, C.; Ouyang, M.; Yi, B. Modeling and control of air stream and hydrogen flow with recirculation in a PEM fuel cell system-I. Control-oriented modeling. Int. J. Hydrogen Energy 2006, 31, 1879–1896. [Google Scholar] [CrossRef]
- Xu, L.; Fang, C.; Hu, J.; Cheng, S.; Li, J.; Ouyang, M.; Lehnert, W. Parameter extraction of polymer electrolyte membrane fuel cell based on quasi-dynamic model and periphery signals. Energy 2017, 122, 675–690. [Google Scholar] [CrossRef]
- Ritzberger, D.; Höflinger, J.; Du, Z.P.; Hametner, C.; Jakubek, S. Data-driven parameterization of polymer electrolyte membrane fuel cell models via simultaneous local linear structured state space identification. Int. J. Hydrogen Energy 2021, 46, 11878–11893. [Google Scholar] [CrossRef]
- Ritzberger, D.; Hametner, C.; Jakubek, S. A Real-Time Dynamic Fuel Cell System Simulation for Model-Based Diagnostics and Control: Validation on Real Driving Data. Energies 2020, 13, 3148. [Google Scholar] [CrossRef]
- McKay, D.A.; Siegel, J.B.; Ott, W.; Stefanopoulou, A.G. Parameterization and prediction of temporal fuel cell voltage behavior during flooding and drying conditions. J. Power Sources 2008, 178, 207–222. [Google Scholar] [CrossRef]
- Kravos, A.; Kregar, A.; Penga, Ž.; Barbir, F.; Katrašnik, T. Real Time Capable Transient Model of Liquid Water Dynamics in Proton Exchange Membrane Fuel Cells. J. Power Sources 2021. submitted. [Google Scholar]
- Xu, L.; Fang, C.; Li, J.; Ouyang, M.; Lehnert, W. Nonlinear dynamic mechanism modeling of a polymer electrolyte membrane fuel cell with dead-ended anode considering mass transport and actuator properties. Appl. Energy 2018, 230, 106–121. [Google Scholar] [CrossRef]
- Vrlić, M.; Ritzberger, D.; Jakubek, S. Safe and Efficient Polymer Electrolyte Membrane Fuel Cell Control Using Successive Linearization Based Model Predictive Control Validated on Real Vehicle Data. Energies 2020, 13, 5353. [Google Scholar] [CrossRef]
- Böhler, L.; Ritzberger, D.; Hametner, C.; Jakubek, S. Constrained extended Kalman filter design and application for on-line state estimation of high-order polymer electrolyte membrane fuel cell systems. Int. J. Hydrogen Energy 2021, 46, 18604–18614. [Google Scholar] [CrossRef]
- Vrlić, M.; Ritzberger, D.; Jakubek, S. Model-Predictive-Control-Based Reference Governor for Fuel Cells in Automotive Application Compared with Performance from a Real Vehicle. Energies 2021, 14, 2206. [Google Scholar] [CrossRef]
- Du, Z.P.; Steindl, C.; Jakubek, S. Efficient Two-Step Parametrization of a Control-Oriented Zero-Dimensional Polymer Electrolyte Membrane Fuel Cell Model Based on Measured Stack Data. Processes 2021, 9, 713. [Google Scholar] [CrossRef]
- Sonntag, R.E.; Borgnakke, C.; Van Wylen, G.J. Properties of a Pure Substance. In Fundamentals of Thermodynamics, 5th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1998; pp. 40–69. [Google Scholar]
- O’Hayre, R.; Cha, S.W.; Colella, W.; Prinz, F.B. Overview of Fuel Cell Systems. In Fuel Cell Fundamentals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; Chapter 10; pp. 347–391. [Google Scholar] [CrossRef]
- Kravos, A.; Ritzberger, D.; Tavcar, G.; Hametner, C.; Jakubek, S.; Katrasnik, T. Thermodynamically consistent reduced dimensionality electrochemical model for proton exchange membrane fuel cell performance modelling and control. J. Power Sources 2020, 454, 227930. [Google Scholar] [CrossRef]
- Ramírez-Cruzado, A.; Ramírez-Peña, B.; Vélez-García, R.; Iranzo, A.; Guerra, J. Experimental Analysis of the Performance and Load Cycling of a Polymer Electrolyte Membrane Fuel Cell. Processes 2020, 8, 608. [Google Scholar] [CrossRef]
- Nam, J.H.; Kaviany, M. Effective diffusivity and water-saturation distribution in single- and two-layer PEMFC diffusion medium. Int. J. Heat Mass Transf. 2003, 46, 4595–4611. [Google Scholar] [CrossRef]
- Niblett, D.; Niasar, V.J.; Holmes, S. Water Distribution in Fuel Cell Gas Channels Using a Mechanistic Discrete Particle Model. ECS Meet. Abstr. 2020, MA2020-02, 2090. [Google Scholar] [CrossRef]
- Extrand, C.W.; Kumagai, Y. Liquid Drops on an Inclined Plane: The Relation between Contact Angles, Drop Shape, and Retentive Force. J. Colloid Interface Sci. 1995, 170, 515–521. [Google Scholar] [CrossRef]
- Santamaria, A.D.; Das, P.K.; MacDonald, J.C.; Weber, A.Z. Liquid-Water Interactions with Gas-Diffusion-Layer Surfaces. J. Electrochem. Soc. 2014, 161, F1184–F1193. [Google Scholar] [CrossRef] [Green Version]
- Weber, A.Z.; Borup, R.L.; Darling, R.M.; Das, P.K.; Dursch, T.J.; Gu, W.; Harvey, D.; Kusoglu, A.; Litster, S.; Mench, M.M.; et al. A Critical Review of Modeling Transport Phenomena in Polymer-Electrolyte Fuel Cells. J. Electrochem. Soc. 2014, 161, F1254–F1299. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Hu, J.; Cheng, S.; Fang, C.; Li, J.; Ouyang, M.; Lehnert, W. Robust control of internal states in a polymer electrolyte membrane fuel cell air-feed system by considering actuator properties. Int. J. Hydrogen Energy 2017, 42, 13171–13191. [Google Scholar] [CrossRef]
- Hinatsu, J.T.; Mizuhata, M.; Takenaka, H. Water Uptake of Perfluorosulfonic Acid Membranes from Liquid Water and Water Vapor. J. Electrochem. Soc. 1994, 141, 1493–1498. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer Electrolyte Fuel Cell Model. J. Electrochem. Soc. 1991, 138, 2334–2342. [Google Scholar] [CrossRef]
- Dutta, S.; Shimpalee, S.; Van Zee, J. Numerical prediction of mass-exchange between cathode and anode channels in a PEM fuel cell. Int. J. Heat Mass Transf. 2001, 44, 2029–2042. [Google Scholar] [CrossRef]
- Ahluwalia, R.; Wang, X. Buildup of nitrogen in direct hydrogen polymer-electrolyte fuel cell stacks. J. Power Sources 2007, 171, 63–71. [Google Scholar] [CrossRef]
- Weber, A.Z.; Newman, J. Transport in Polymer-Electrolyte Membranes. J. Electrochem. Soc. 2004, 151, A311. [Google Scholar] [CrossRef]
- Kravos, A.; Ritzberger, D.; Hametner, C.; Jakubek, S.; Katrašnik, T. Methodology for efficient parametrisation of electrochemical PEMFC model for virtual observers: Model based optimal design of experiments supported by parameter sensitivity analysis. Int. J. Hydrogen Energy 2021, 46, 13832–13844. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. III. Die elastischen Konstanten der quasiisotropen Mischkörper aus isotropen Substanzen. Ann. Phys. 1937, 421, 160–178. [Google Scholar] [CrossRef]
- Hwang, G.S.; Weber, A.Z. Effective-Diffusivity Measurement of Partially-Saturated Fuel-Cell Gas-Diffusion Layers. J. Electrochem. Soc. 2012, 159, F683–F692. [Google Scholar] [CrossRef]
- Owejan, J.P.; Trabold, T.A.; Mench, M.M. Oxygen transport resistance correlated to liquid water saturation in the gas diffusion layer of PEM fuel cells. Int. J. Heat Mass Transf. 2014, 71, 585–592. [Google Scholar] [CrossRef]
- Nijmeijer, H.; van der Schaft, A. Introduction. In Nonlinear Dynamical Control Systems; Springer: New York, NY, USA, 1990; pp. 1–22. [Google Scholar] [CrossRef]
- Nelles, O. Introduction to Optimization. In Nonlinear System Identification; Springer: Berlin/Heidelberg, Germany, 2001; pp. 23–34. [Google Scholar] [CrossRef]
- Atkinson, K. Numerical Methods for Ordinary Differential Equations. In An Introduction to Numerical Analysis, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1989; pp. 333–460. [Google Scholar]
- MathWorks Find Minimum of Function Using Genetic Algorithm—MATLAB Ga. Available online: https://www.mathworks.com/help/gads/ga.html (accessed on 15 February 2021).
- Kang, S.; Zhou, B.; Cheng, C.H.; Shiu, H.R.; Lee, C.I. Liquid water flooding in a proton exchange membrane fuel cell cathode with an interdigitated design. Int. J. Energy Res. 2011, 35, 1292–1311. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, H.; Hu, J.; Cai, Y.; Yi, B. Diagnostic tool to detect liquid water removal in the cathode channels of proton exchange membrane fuel cells. J. Power Sources 2006, 162, 469–473. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.P.; Kravos, A.; Steindl, C.; Katrašnik, T.; Jakubek, S.; Hametner, C. Physically Motivated Water Modeling in Control-Oriented Polymer Electrolyte Membrane Fuel Cell Stack Models. Energies 2021, 14, 7693. https://doi.org/10.3390/en14227693
Du ZP, Kravos A, Steindl C, Katrašnik T, Jakubek S, Hametner C. Physically Motivated Water Modeling in Control-Oriented Polymer Electrolyte Membrane Fuel Cell Stack Models. Energies. 2021; 14(22):7693. https://doi.org/10.3390/en14227693
Chicago/Turabian StyleDu, Zhang Peng, Andraž Kravos, Christoph Steindl, Tomaž Katrašnik, Stefan Jakubek, and Christoph Hametner. 2021. "Physically Motivated Water Modeling in Control-Oriented Polymer Electrolyte Membrane Fuel Cell Stack Models" Energies 14, no. 22: 7693. https://doi.org/10.3390/en14227693