Future Material Developments for Electric Vehicle Battery Cells Answering Growing Demands from an End-User Perspective
Abstract
:1. Introduction
2. Projected Demand from End-User Side
3. Projected Improvements on Component and Cell Level
3.1. Anodes
3.1.1. Insertion/Intercalation Materials
Graphite
Lithium Titanate Oxide
Other Alternatives
3.1.2. Conversion/Alloying Materials
Silicon (Si)
Silicon Oxide (SiOx)
Other Alternatives
3.2. Cathodes
3.2.1. Nickel-Rich Cathodes
3.2.2. Lithium-Rich Oxides
3.3. Electrolytes
3.3.1. Lithium Salts
3.3.2. Solvents
3.3.3. Additives
3.3.4. Solid-State Electrolytes
3.4. Cell Considerations
3.5. Alternatives to LIB: Lithium-Sulfur and Potassium-Ion as an Alternative for Li-Ion
4. Safety Considerations
5. Conclusions and Outlook
- Anodes. Although graphite is the dominating active anode material in Li-ion technologies, its energy and power density limitations in combination with natural graphite being in the critical raw material list have raised the need for researching new compositions such as Si-based and other metal sulphides, which can provide capacities above 650 mAh·g−1 and could be recycled from other products, contributing to a circular economy.
- Cathodes. Ni-rich and Li-rich oxides are gaining attention, showing that Co-free Li-rich materials bring improved kinetics and cycling performances. However, these still need further research to improve cycling performance and rate capability.
- Electrolytes. The impact of electrolyte composition on the battery’s energy density, cycle-life, power, cost and safety has shifted the industry’s attention to improving all its components—salts, solvents and additives—looking at a considerable number of diverse compositions. Solid-state electrolytes are currently thought to be a promising alternative as they increase safety, chemical stability and energy density with the possibility to use Li metal, while reducing costs.
- Further considerations for safety enhancement. From the authors’ knowledge, there is no literature available that compares the exact characteristics of solid-state battery to liquid-state battery under abuse conditions, henceforth this area can be researched further. Nonetheless, based on this research it can be said that the solid-state solutions can be combined with a good packing design, positioning of cells and the battery within the vehicle, inclusion of contingency measures and signalling strategies to ensure optimal safety standards.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsiropoulos, I.; Tarvydas, D.; Lebedeva, N. Li-ion batteries for mobility and stationary storage applications. Publ. Off. Eur. Union Luxemb. 2018, 1–72. [Google Scholar] [CrossRef]
- Helmbrecht, M.; Olaverri-Monreal, C.; Bengler, K.; Vilimek, R.; Keinath, A. How electric vehicles affect driving behavioral patterns. IEEE Intell. Transp. Syst. Mag. 2014, 6, 22–32. [Google Scholar] [CrossRef]
- Sun, X.H.; Yamamoto, T.; Morikawa, T. Fast-charging station choice behavior among battery electric vehicle users. Transp. Res. Part D Transp. Environ. 2016, 46, 26–39. [Google Scholar] [CrossRef]
- Christensen, L.; Nørrelund, A.V.; Olsen, A. Travel behaviour of potential Electric Vehicle drivers. The need for changing A contribution to the Edison project. In Proceedings of the European Transport Conference 2010, Glasgow, UK, 11–13 October 2010. [Google Scholar]
- Battery Pack Prices Cited below $100/kWh for the First Time in 2020, While Market Average Sits at $137/kWh. Available online: https://about.bnef.com/blog/battery-pack-prices-cited-below-100-kwh-for-the-first-time-in-2020-while-market-average-sits-at-137-kwh/ (accessed on 3 March 2021).
- Interactive Map: Affordability of Electric Cars, Correlation between Market Uptake and GDP in the EU. Available online: https://www.acea.be/news/article/interactive-map-affordability-of-electric-cars-correlation-between-market-u (accessed on 3 March 2021).
- Electrification of the Transport System—Expert Group Report; European Commision: Brussels, Belgium, 2017.
- Zhang, Z.; Wang, D.; Zhang, C.; Chen, J. Electric vehicle range extension strategies based on improved AC system in cold climate—A review. Int. J. Refrig. 2018, 88, 141–150. [Google Scholar] [CrossRef]
- Yuksel, T.; Michalek, J.J. Effects of Regional Temperature on Electric Vehicle Efficiency, Range, and Emissions in the United States. Environ. Sci. Technol. 2015, 49, 5. [Google Scholar] [CrossRef]
- Autonomous Cars’ Big Problem: The Energy Consumption of Edge Processing Reduces a Car’s Mileage with up to 30%. Available online: https://medium.com/@teraki/energy-consumption-required-by-edge-computing-reduces-a-autonomous-cars-mileage-with-up-to-30-46b6764ea1b7 (accessed on 4 March 2021).
- Baxter, J.A.; Merced, D.A.; Costinett, D.J.; Tolbert, L.M.; Ozpineci, B. Review of Electrical Architectures and Power Requirements for Automated Vehicles. In Proceedings of the 2018 IEEE Transportation and Electrification Conference and Expo (ITEC 2018), Long Beach, CA, USA, 13–15 June 2018; pp. 102–107. [Google Scholar]
- Islam, E.S.; Moawad, A.; Kim, N.; Rousseau, A. Vehicle Electrification Impacts on Energy Consumption for Different Connected-Autonomous Vehicle Scenario Runs. World Electr. Veh. J. 2019, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- Hardy, K.; Krasenbrink, A. EV-Smart Grid Interoperability Centers in Europe and the U.S.; Argonne National Laboratory: Lemont, IL, USA, 2011. [Google Scholar]
- Supporting Innovative Solutions for Smart Grids and Storage, Innovation and Networks Executive Agency INEA; European Commission: Brussels, Belgium, 2018.
- Uddin, K.; Dubarry, M.; Glick, M.B. The viability of vehicle-to-grid operations from a battery technology and policy perspective. Energy Policy 2018, 113, 342–347. [Google Scholar] [CrossRef]
- Peterson, S.B.; Apt, J.; Whitacre, J.F. Lithium-ion battery cell degradation resulting from realistic vehicle and vehicle-to-grid utilization. J. Power Sources 2010, 195, 2385–2392. [Google Scholar] [CrossRef]
- Thingvad, A.; Marinelli, M. Influence of V2G Frequency Services and Driving on Electric Vehicles Battery Degradation in the Nordic Countries. In Proceedings of the EVS 31 & EVTeC 2018, Kobe, Japan, 1–3 October 2018; p. 20189132. [Google Scholar]
- van Heuveln, K.; Ghotge, R.; Annema, J.A.; van Bergen, E.; van Wee, B.; Pesch, U. Factors influencing consumer acceptance of vehicle-to-grid by electric vehicle drivers in the Netherlands. Travel Behav. Soc. 2021, 24, 34–45. [Google Scholar] [CrossRef]
- Geske, J.; Schumann, D. Willing to participate in vehicle-to-grid (V2G)? Why not! Energy Policy 2018, 120, 392–401. [Google Scholar] [CrossRef]
- COM(2020) 798/3 Proposal for a New UN GTR on In-Vehicle Battery Durability for Electrified Vehicles; ECE-TRANS-WP.29-GRPE-2021-18e; UNECE: Geneva, Switzerland, 2021.
- Proposal for a Regulation of the European Parliament and of the Council Concerning Batteries and Waste Batteries, Repealing Directive 2006/66/EC and Amending Regulation (EU) No 2019/1020; European Commission: Brussels, Belgium, 2020.
- Betz, J.; Bieker, G.; Meister, P.; Placke, T.; Winter, M.; Schmuch, R. Theoretical versus Practical Energy: A Plea for More Transparency in the Energy Calculation of Different Rechargeable Battery Systems. Adv. Energy Mater. 2019, 9. [Google Scholar] [CrossRef]
- Li, J.; Murphy, E.; Winnick, J.; Kohl, P.A. Studies on the cycle life of commercial lithium ion batteries during rapid charge-discharge cycling. J. Power Sources 2001, 102, 294–301. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, C.; Eshetu, G.G.; Laruelle, S.; Grugeon, S.; Zaghib, K.; Mauger, A.; Guyomard, D.; Rojo, T.; Gisbert-trejo, N.; et al. From solid solution electrodes and the rocking-chair concept to today’s batteries. Angew. Chem. Int. Ed. 2020, 59, 534–538. [Google Scholar] [CrossRef]
- Dahn, J.R.; Zheng, T.; Liu, Y.; Xue, J.S. Mechanisms for lithium insertion in carbonaceous materials. Science 1995, 270, 590–593. [Google Scholar] [CrossRef]
- Lain, M.J.; Brandon, J.; Kendrick, E. Design strategies for high power vs. High energy lithium ion cells. Batteries 2019, 5, 64. [Google Scholar] [CrossRef] [Green Version]
- Raccichini, R.; Amores, M.; Hinds, G. Critical Review of the Use of Reference Electrodes in Li-Ion Batteries: A Diagnostic Perspective. Batteries 2019, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; McGraw Hill Professional: New York, NY, USA, 2002; ISBN 9780470402528. [Google Scholar]
- International Energy Agency. Global EV Outlook 2020; OECD: Paris, France, 2020. [CrossRef]
- McKerracher, C.; Izadi-Najafabadi, A.; Soulopoulos, N.; Doherty, D.; Frith, J.T.; Albanese, N.; Grant, A.; Berryman, I. Electric Vehicle Outlook 2019; IEA: Paris, France, 2019. Available online: https://www.iea.org/reports/global-ev-outlook-2019 (accessed on 3 March 2021).
- Logan, E.R.; Dahn, J.R. Electrolyte Design for Fast-Charging Li-Ion Batteries. Trends Chem. 2020, 2, 354–366. [Google Scholar] [CrossRef]
- Xu, C.; Märker, K.; Lee, J.; Mahadevegowda, A.; Reeves, P.J.; Day, S.J.; Groh, M.F.; Emge, S.P.; Ducati, C.; Layla Mehdi, B.; et al. Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries. Nat. Mater. 2021, 20, 84–92. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014. [Google Scholar] [CrossRef] [Green Version]
- Peled, E. The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems—The Solid Electrolyte Interphase Model. J. Electrochem. Soc. 1979, 126, 2047–2051. [Google Scholar] [CrossRef]
- Peled, E.; Menkin, S. Review-SEI: Past, present and future. J. Electrochem. Soc. 2017, 164, A1703–A1719. [Google Scholar] [CrossRef]
- Zhou, Y.; Su, M.; Yu, X.; Zhang, Y.; Wang, J.G.; Ren, X.; Cao, R.; Xu, W.; Baer, D.R.; Du, Y.; et al. Real-time mass spectrometric characterization of the solid–electrolyte interphase of a lithium-ion battery. Nat. Nanotechnol. 2020, 15, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Bennett, W.R. Fundamental Investigation of Si Anode in Li-Ion Cells. In Proceedings of the 2012 IEEE Energytech, Cleveland, OH, USA, 29–31 May 2012; pp. 1–5. [Google Scholar]
- Moretti, A.; Sharova, V.; Carvalho, D.V.; Boulineau, A.; Porcher, W.; de Meatza, I.; Passerini, S. A Comparison of Formation Methods for Graphite//LiFePO4 Cells. Batter. Supercaps 2019, 2, 240–247. [Google Scholar] [CrossRef] [Green Version]
- An, S.J.; Li, J.; Du, Z.; Daniel, C.; Wood, D.L. Fast formation cycling for lithium ion batteries. J. Power Sources 2017, 342, 846–852. [Google Scholar] [CrossRef]
- Armand, M.; Axmann, P.; Bresser, D.; Copley, M.; Edström, K.; Ekberg, C.; Guyomard, D.; Lestriez, B.; Novák, P.; Petranikova, M.; et al. Lithium-ion batteries—Current state of the art and anticipated developments. J. Power Sources 2020, 479. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef]
- Levi, M.D.; Aurbach, D. The mechanism of lithium intercalation in graphite film electrodes in aprotic media. Part 1. High resolution slow scan rate cyclic voltammetric studies and modeling. J. Electroanal. Chem. 1997, 421, 79–88. [Google Scholar] [CrossRef]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Fuller, T.F.; Doyle, M.; Newman, J. Simulation and Optimization of the Dual Lithium Ion Insertion Cell. J. Electrochem. Soc. 1994, 141, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wetjen, M.; Pritzl, D.; Jung, R.; Solchenbach, S.; Ghadimi, R.; Gasteiger, H.A. Differentiating the Degradation Phenomena in Silicon-Graphite Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2017, 164, A2840–A2852. [Google Scholar] [CrossRef]
- Study on the Review of the List of Critical Raw Materials; European Commission: Brussels, Belgium, 2017; ISBN 978-92-79-47937-3. [CrossRef]
- Liu, Q.; Du, C.; Shen, B.; Zuo, P.; Cheng, X.; Ma, Y.; Yin, G.; Gao, Y. Understanding of Undesirable Anode Lithium Plating Issues in Lithium-Ion Batteries. RSC Adv. 2016, 6, 88683–88700. [Google Scholar] [CrossRef]
- Tanim, T.R.; Dufek, E.J.; Dickerson, C.C.; Wood, S.M. Electrochemical quantification of lithium plating: Challenges and considerations. J. Electrochem. Soc. 2019, 166, A2689–A2696. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. The Effects of Defects on Localized Plating in Lithium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A1365–A1373. [Google Scholar] [CrossRef] [Green Version]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Campbell, I.D.; Marzook, M.; Marinescu, M.; Offer, G.J. How observable is lithium plating? Differential voltage analysis to identify and quantify lithium plating following fast charging of cold lithium-ion batteries. J. Electrochem. Soc. 2019, 166, A725–A739. [Google Scholar] [CrossRef]
- Landa-Medrano, I.; Eguia-Barrio, A.; Sananes-Israel, S.; Lijó-Pando, S.; Boyano, I.; Alcaide, F.; Urdampilleta, I.; De Meatza, I. In Situ Analysis of NMC∣Graphite Li-Ion Batteries by Means of Complementary Electrochemical Methods. J. Electrochem. Soc. 2020, 167, 090528. [Google Scholar] [CrossRef]
- Attia, P.M.; Das, S.; Harris, S.J.; Bazant, M.Z.; Chueh, W.C. Electrochemical kinetics of sei growth on Carbon Black: Part I. experiments. J. Electrochem. Soc. 2019, 166, E97–E106. [Google Scholar] [CrossRef] [Green Version]
- Wood, D.L.; Li, J.; An, S.J. Formation Challenges of Lithium-Ion Battery Manufacturing. Joule 2019, 3, 2884–2888. [Google Scholar] [CrossRef]
- Mao, C.; An, S.J.; Meyer, H.M.; Li, J.; Wood, M.; Ruther, R.E.; Wood, D.L. Balancing formation time and electrochemical performance of high energy lithium-ion batteries. J. Power Sources 2018, 402, 107–115. [Google Scholar] [CrossRef]
- Ma, R.; Shao, L.; Wu, K.; Shui, M.; Wang, D.; Pan, J.; Long, N.; Ren, Y.; Shu, J. Comparison of LiVPO4F to Li4Ti5O12 as anode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 8615–8627. [Google Scholar] [CrossRef]
- Chen, Z.; Belharouak, I.; Sun, Y.-K.; Amine, K. Titanium-Based Anode Materials for Safe Lithium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 959–969. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Wen, Y.; Xie, J.; Xia, Q.; Zhang, T.; Zeng, Z.; Du, X. High performance Li4Ti5O12 material as anode for lithium-ion batteries. Electrochim. Acta 2013, 113, 679–685. [Google Scholar] [CrossRef]
- Vujković, M.; Stojković, I.; Mitrić, M.; Mentus, S.; Cvjetićanin, N. Hydrothermal synthesis of Li4Ti5O12/C nanostructured composites: Morphology and electrochemical performance. Mater. Res. Bull. 2013, 48, 218–223. [Google Scholar] [CrossRef]
- Fu, R.; Zhou, X.; Fan, H.; Blaisdell, D.; Jagadale, A.; Zhang, X.; Xiong, R. Comparison of lithium-ion anode materials using an experimentally verified physics-based electrochemical model. Energies 2017, 10, 2174. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Xiao, J.; Nie, Z.; Zhang, J.-G. Lattice Mn3+ Behaviors in Li4Ti5O12/LiNi0.5Mn1.5O4 Full Cells. J. Electrochem. Soc. 2013, 160, A1264–A1268. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X. High Rate Capability of Nd-Doped Li4Ti5O12 as an Effective Anode Material for Lithium-Ion Battery. Int. J. Electrochem. Sci. 2013, 8, 7816–7824. [Google Scholar]
- Liu, H.; Zhu, Z.; Yan, Q.; Yu, S.; He, X.; Chen, Y.; Zhang, R.; Ma, L.; Liu, T.; Li, M.; et al. A disordered rock salt anode for fast-charging lithium-ion batteries. Nature 2020, 585, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, M.; Harvey, S.P.; Arca, E.; Stetson, C.; Teeter, G.; Ban, C.; Stradins, P. Surface SiO2 thickness controls uniform-to-localized transition in lithiation of silicon anodes for lithiumion batteries. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Swamy, T.; Chiang, Y.-M. Electrochemical Charge Transfer Reaction Kinetics at the Silicon-Liquid Electrolyte Interface. J. Electrochem. Soc. 2015, 162, A7129–A7134. [Google Scholar] [CrossRef]
- Li, P.; Zhao, G.; Zheng, X.; Xu, X.; Yao, C.; Sun, W.; Dou, S.X. Recent progress on silicon-based anode materials for practical lithium-ion battery applications. Energy Storage Mater. 2018, 15, 422–446. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Peng, X.; Zeng, W.; Jin, Z.; Tian, W.; Gao, B.; Zhou, Y.; Chu, P.K.; Huo, K. Highly Stretchable Conductive Glue for High-Performance Silicon Anodes in Advanced Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1704858. [Google Scholar] [CrossRef]
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-based anodes for lithium-ion batteries: Effectiveness of materials synthesis and electrode preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef] [Green Version]
- Jung, R.; Metzger, M.; Haering, D.; Solchenbach, S.; Marino, C.; Tsiouvaras, N.; Stinner, C.; Gasteiger, H.A. Consumption of Fluoroethylene Carbonate (FEC) on Si-C Composite Electrodes for Li-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1705–A1716. [Google Scholar] [CrossRef]
- Alaboina, P.K.; Cho, J.S.; Uddin, M.J.; Cho, S.-J. Mechanically prelithiated silicon nano alloy as highly engineered anode material. Electrochim. Acta 2017, 258, 623–630. [Google Scholar] [CrossRef]
- Li, X.; Colclasure, A.M.; Finegan, D.P.; Ren, D.; Shi, Y.; Feng, X.; Cao, L.; Yang, Y.; Smith, K. Degradation mechanisms of high capacity 18650 cells containing Si-graphite anode and nickel-rich NMC cathode. Electrochim. Acta 2019, 297, 1109–1120. [Google Scholar] [CrossRef]
- Pan, K.; Zou, F.; Canova, M.; Zhu, Y.; Kim, J.H. Systematic electrochemical characterizations of Si and SiO anodes for high-capacity Li-Ion batteries. J. Power Sources 2019, 413, 20–28. [Google Scholar] [CrossRef]
- Chae, S.; Choi, S.-H.; Namhyung, K.; Sung, J.; Cho, J. Integration of Graphite and Silicon Anodes for the Commercialization of High-Energy Lithium-Ion Batteries. Angew. Chem. 2019, 59, 110–135. [Google Scholar] [CrossRef] [Green Version]
- Feng, K.; Li, M.; Liu, W.; Kashkooli, A.G.; Xiao, X.; Cai, M.; Chen, Z. Silicon-Based Anodes for Lithium-Ion Batteries: From Fundamentals to Practical Applications. Small 2018, 14. [Google Scholar] [CrossRef]
- Andersson, R.; Hernández, G.; Edström, K.; Mindemark, J. Micro versus Nano: Impact of Particle Size on the Flow Characteristics of Silicon Anode Slurries. Energy Technol. 2020, 8, 2000056. [Google Scholar] [CrossRef]
- Jaumann, T.; Balach, J.; Langklotz, U.; Sauchuk, V.; Fritsch, M.; Michaelis, A.; Teltevskij, V.; Mikhailova, D.; Oswald, S.; Klose, M.; et al. Lifetime vs. rate capability: Understanding the role of FEC and VC in high-energy Li-ion batteries with nano-silicon anodes. Energy Storage Mater. 2017, 6, 26–35. [Google Scholar] [CrossRef]
- Mazouzi, D.; Karkar, Z.; Hernandez, C.R.; Manero, P.J.; Guyomard, D.; Roué, L.; Lestriez, B. Critical roles of binders and formulation at multiscales of silicon-based composite electrodes. J. Power Sources 2015, 280, 533–549. [Google Scholar] [CrossRef]
- Chae, S.; Ko, M.; Kim, K.; Ahn, K.; Cho, J. Confronting Issues of the Practical Implementation of Si Anode in High-Energy Lithium-Ion Batteries. Joule 2017, 1, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.; Bok, T.; Kim, S.; Park, S. Fundamental Understanding of Nanostructured Si Electrodes: Preparation and Characterization. ChemNanoMat 2018, 4, 319–337. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, Y.; Xian, X.; Liu, N.; Li, W. Fabrication of Porous Si@C Composites with Core-Shell Structure and Their Electrochemical Performance for Li-ion Batteries. Batteries 2019, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Louli, A.J.; Li, J.; Trussler, S.; Fell, C.R.; Dahn, J.R. Volume, Pressure and Thickness Evolution of Li-Ion Pouch Cells with Silicon-Composite Negative Electrodes. J. Electrochem. Soc. 2017, 164, A2689–A2696. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, L.; Zhan, J.; Du, N.; Liu, W.; Ma, J.; Su, L.; Wang, L. Recycling silicon-based industrial waste as sustainable sources of Si/SiO2 composites for high-performance Li-ion battery anodes. J. Power Sources 2019. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Liu, Y.; Emani, S.; Shaw, L.L. Synthesis and performance of nanostructured silicon/graphite composites with a thin carbon shell and engineered voids. Electrochim. Acta 2017, 258, 274–283. [Google Scholar] [CrossRef]
- Suh, S.S.; Yoon, W.Y.; Kim, D.H.; Kwon, S.U.; Kim, J.H.; Kim, Y.U.; Jeong, C.U.; Chan, Y.Y.; Kang, S.H.; Lee, J.K. Electrochemical behavior of SiOx anodes with variation of oxygen ratio for Li-ion batteries. Electrochim. Acta 2014, 148, 111–117. [Google Scholar] [CrossRef]
- Wang, J.; Bao, W.; Ma, L.; Tan, G.; Su, Y.; Chen, S.; Wu, F.; Lu, J.; Amine, K. Scalable Preparation of Ternary Hierarchical Silicon Oxide-Nickel-Graphite Composites for Lithium-Ion Batteries. ChemSusChem 2015, 8, 4073–4080. [Google Scholar] [CrossRef]
- Tan, T.; Lee, P.-K.; Yu, D.Y.W. Probing the Reversibility of Silicon Monoxide Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2018, 166, A5210–A5214. [Google Scholar] [CrossRef]
- Nguyen, T.T.D.; Abada, S.; Lecocq, A.; Bernard, J.; Petit, M.; Marlair, G.; Grugeon, S.; Laruelle, S. Understanding the thermal runaway of ni-rich lithium-ion batteries. World Electr. Veh. J. 2019, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, N.E.; Shevchenko, E.V.; Park, J.; Lee, E.; Xie, J.; Chen, B.; Zhong, Y.; Filatov, A.S.; Anderson, J.S. Synthesis, modular composition, and electrochemical properties of lamellar iron sulfides. J. Mater. Chem. A 2020, 8, 15834–15844. [Google Scholar] [CrossRef]
- Sun, D.; Wang, L.; Li, Y.; Yang, Y.; Zhou, X.; Ma, G.; Lei, Z. Confined Metal Sulfides Nanoparticles into Porous Carbon Nanosheets with Surface-Controlled Reactions for Fast and Stable Lithium-Ion Batteries. ChemElectroChem 2019, 6, 4464–4470. [Google Scholar] [CrossRef]
- Wang, J.; He, H.; Wu, Z.; Liang, J.; Han, L.; Xin, H.L.; Guo, X.; Zhu, Y.; Wang, D. Controllable construction of flower-like FeS/Fe2O3 composite for lithium storage. J. Power Sources 2018, 392, 193–199. [Google Scholar] [CrossRef]
- Lv, J.; Du, J.; Jia, H.; Ma, J.; Zheng, S.; Nie, Y.; Song, K.; Bai, L. Hierarchical carbon-coated Fe1-xS/mesocarbon microbeads composite as high-performance lithium-ion batteries anode. Ceram. Int. 2020, 46, 9485–9491. [Google Scholar] [CrossRef]
- Fei, L.; Williams, B.P.; Yoo, S.H.; Carlin, J.M.; Joo, Y.L. A general approach to fabricate free-standing metal sulfide@carbon nanofiber networks as lithium ion battery anodes. Chem. Commun. 2016, 52, 1501–1504. [Google Scholar] [CrossRef]
- Li, L.; Gao, C.; Kovalchuk, A.; Peng, Z.; Ruan, G.; Yang, Y.; Fei, H.; Zhong, Q.; Li, Y.; Tour, J.M. Sandwich structured graphene-wrapped FeS-graphene nanoribbons with improved cycling stability for lithium ion batteries. Nano Res. 2016, 9, 2904–2911. [Google Scholar] [CrossRef]
- Jin, H.; Xin, S.; Chuang, C.; Li, W.; Wang, H.; Zhu, J.; Xie, H.; Zhang, T.; Wan, Y.; Qi, Z.; et al. Black phosphorus composites with engineered interfaces for high-rate high-capacity lithium storage. Science 2020, 370, 192–197. [Google Scholar] [CrossRef]
- Tokur, M.; Aydin, A.; Cetinkaya, T.; Akbulut, H. Shoring Up the Lithium Ion Batteries with Multi-Component Silicon Yolk-Shell Anodes for Grid-Scale Storage Systems: Experimental and Computational Mechanical Studies. J. Electrochem. Soc. 2017, 164, A2238–A2250. [Google Scholar] [CrossRef]
- Asenbauer, J.; Hoefling, A.; Indris, S.; Tübke, J.; Passerini, S.; Bresser, D. Mechanistic Insights into the Lithiation and Delithiation of Iron-Doped Zinc Oxide: The Nucleation Site Model. ACS Appl. Mater. Interfaces 2020, 12, 8206–8218. [Google Scholar] [CrossRef]
- Hu, M.; Pang, X.; Zhou, Z. Review Recent progress in high-voltage lithium ion batteries. J. Power Sources 2013, 237, 229–242. [Google Scholar] [CrossRef]
- Xu, C.; Reeves, P.J.; Jacquet, Q.; Grey, C.P. Phase Behavior during Electrochemical Cycling of Ni-Rich Cathode Materials for Li-Ion Batteries. Adv. Energy Mater. 2020, 11, 2003404. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, H.; Wang, B.; Tuo, K.; Wang, P.; Wang, S.; Liang, W.; Lu, H.; Li, S. Structural evolution of nickel-rich layered cathode material LiNi0.8Co0.1Mn0.1O2 at different current rates. Ionics (Kiel) 2021, 27, 517–526. [Google Scholar] [CrossRef]
- Wu, Y.; Li, M.; Wahyudi, W.; Sheng, G.; Miao, X.; Anthopoulos, T.D.; Huang, K.W.; Li, Y.; Lai, Z. Performance and Stability Improvement of Layered NCM Lithium-Ion Batteries at High Voltage by a Microporous Al2O3 Sol-Gel Coating. ACS Omega 2019, 4, 13972–13980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, B.; Biendicho, J.J.; Hull, S.; Smith, R.I.; West, A.R. In-Situ Neutron Studies of Electrodes for Li-Ion Batteries Using a Deuterated Electrolyte: LiCoO2 as a Case Study. J. Electrochem. Soc. 2018, 165, A793–A801. [Google Scholar] [CrossRef] [Green Version]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef]
- Shaari, H.R.; Sethuprakhash, V. Review of electrochemical performance of LINIO2 and their derivatives as cathode material for lithium-ion batteries. J. Teknol. Sci. Eng. 2014, 70, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Julien, C.M.; Mauger, A. Lithium-Ion Batteries; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.L.; Deng, Y.P.; Chen, Z. Ni-Rich/Co-Poor Layered Cathode for Automotive Li-Ion Batteries: Promises and Challenges. Adv. Energy Mater. 2020, 10, 1–28. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, X.; Lv, C.; Liu, T.; Xia, Y.; Shang, L.; Waterhouse, G.I.N.; Yang, D.; Zhang, T. Multishelled Ni-Rich Li(NixCoyMnz)O2 Hollow Fibers with Low Cation Mixing as High-Performance Cathode Materials for Li-Ion Batteries. Adv. Sci. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Park, K.J.; Hwang, J.Y.; Ryu, H.H.; Maglia, F.; Kim, S.J.; Lamp, P.; Yoon, C.S.; Sun, Y.K. Degradation Mechanism of Ni-Enriched NCA Cathode for Lithium Batteries: Are Microcracks Really Critical? ACS Energy Lett. 2019, 4, 1394–1400. [Google Scholar] [CrossRef] [Green Version]
- Takanashi, S.; Abe, Y. Improvement of the electrochemical performance of an NCA positive-electrode material of lithium ion battery by forming an Al-rich surface layer. Ceram. Int. 2017, 43, 9246–9252. [Google Scholar] [CrossRef]
- Li, H.; Zhou, P.; Liu, F.; Li, H.; Cheng, F.; Chen, J. Stabilizing nickel-rich layered oxide cathodes by magnesium doping for rechargeable lithium-ion batteries. Chem. Sci. 2019, 10, 1374–1379. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.K.; Lee, B.R.; Noh, H.J.; Wu, H.; Myung, S.T.; Amine, K. A novel concentration-gradient Li[Ni0.83Co0.07Mn0.10]O2 cathode material for high-energy lithium-ion batteries. J. Mater. Chem. 2011, 21, 10108–10112. [Google Scholar] [CrossRef]
- Boulineau, A.; Croguennec, L.; Delmas, C.; Weill, F. Structure of Li2MnO3 with different degrees of defects. Solid State Ion. 2010, 180, 1652–1659. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.H.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112–3125. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.H.; Johnson, C.S.; Vaughey, J.T.; Hackney, S.A. Comments on the structural complexity of lithium-rich Li1+xM1−xO2 electrodes (M = Mn, Ni, Co) for lithium batteries. Electrochem. Commun. 2006, 8, 1531–1538. [Google Scholar] [CrossRef]
- Yang, J.; Hou, M.; Haller, S.; Wang, Y.; Wang, C.; Xia, Y. Improving the Cycling Performance of the Layered Ni-Rich Oxide Cathode by Introducing Low-Content Li2MnO3. Electrochim. Acta 2016, 189, 101–110. [Google Scholar] [CrossRef]
- Johnson, C.S.; Kim, J.S.; Lefief, C.; Li, N.; Vaughey, J.T.; Thackeray, M.M. The significance of the Li2MnO3 component in “composite” xLi 2MnO3·(1-x)LiMn 0.5Ni 0.5O2 electrodes. Electrochem. Commun. 2004, 6, 1085–1091. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Li, B. Recent progress in Li-rich layered oxides as cathode materials for Li-ion batteries. RSC Adv. 2014, 4, 63268–63284. [Google Scholar] [CrossRef]
- Lu, Z.; Beaulieu, L.Y.; Donaberger, R.A.; Thomas, C.L.; Dahn, J.R. Synthesis, Structure, and Electrochemical Behavior of Li[Ni[sub x]Li[sub 1/3−2x/3]Mn[sub 2/3−x/3]]O[sub 2]. J. Electrochem. Soc. 2002, 149, A778. [Google Scholar] [CrossRef]
- Yu, X.; Lyu, Y.; Gu, L.; Wu, H.; Bak, S.M.; Zhou, Y.; Amine, K.; Ehrlich, S.N.; Li, H.; Nam, K.W.; et al. Understanding the rate capability of high-energy-density Li-rich layered Li1.2Ni0.15Co0.1Mn0.55O2 cathode materials. Adv. Energy Mater. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Johnson, C.S.; Korte, S.D.; Vaughey, J.T.; Thackeray, M.M.; Bofinger, T.E.; Shao-Horn, Y.; Hackney, S.A. Structural and electrochemical analysis of layered compounds from Li2MnO3. J. Power Sources 1999, 81–82, 491–495. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Holzapfel, M.; Novák, P.; Johnson, C.S.; Kang, S.H.; Thackeray, M.M.; Bruce, P.G. Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode Li[Ni0.2Li0.2Mn0.6]O2. J. Am. Chem. Soc. 2006, 128, 8694–8698. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, L.; Wang, Z.; Tu, W.; Yang, X.; Lin, Y.; Liao, Y.; Xu, M.; Li, W. Insight into the capacity fading of layered lithium-rich oxides and its suppression: Via a film-forming electrolyte additive. RSC Adv. 2018, 8, 25794–25801. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Croy, J.R.; Thackeray, M.M. Comments on stabilizing layered manganese oxide electrodes for Li batteries. Electrochem. Commun. 2013, 36, 103–106. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Robertson, A.D.; Bruce, P.G. Overcharging manganese oxides: Extracting lithium beyond Mn4+. J. Power Sources 2005, 146, 275–280. [Google Scholar] [CrossRef]
- Assat, G.; Tarascon, J.M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 2018, 3, 373–386. [Google Scholar] [CrossRef]
- Zhang, P.; Zhai, X.; Huang, H.; Zhou, J.; Li, X.; He, Y.; Guo, Z. Suppression of structural phase transformation of Li-rich Mn-based layered cathode materials with Na ion substitution strategy. Electrochim. Acta 2020, 349, 136402. [Google Scholar] [CrossRef]
- Guo, H.; Xia, Y.; Zhao, H.; Yin, C.; Jia, K.; Zhao, F.; Liu, Z. Stabilization effects of Al doping for enhanced cycling performances of Li-rich layered oxides. Ceram. Int. 2017, 43, 13845–13852. [Google Scholar] [CrossRef]
- Qiao, Q.Q.; Qin, L.; Li, G.R.; Wang, Y.L.; Gao, X.P. Sn-stabilized Li-rich layered Li(Li0.17Ni0.25Mn0.58)O2 oxide as a cathode for advanced lithium-ion batteries. J. Mater. Chem. A 2015, 3, 17627–17634. [Google Scholar] [CrossRef]
- Park, K.; Kim, J.; Park, J.H.; Hwang, Y.; Han, D. Synchronous phase transition and carbon coating on the surface of Li-rich layered oxide cathode materials for rechargeable Li-ion batteries. J. Power Sources 2018, 105–110. [Google Scholar] [CrossRef]
- Lei, Y.; Ni, J.; Hu, Z.; Wang, Z.; Gui, F.; Li, B.; Ming, P.; Zhang, C.; Elias, Y.; Aurbach, D.; et al. Surface Modification of Li-Rich Mn-Based Layered Oxide Cathodes: Challenges, Materials, Methods, and Characterization. Adv. Energy Mater. 2020, 10, 1–27. [Google Scholar] [CrossRef]
- Kang, S.H.; Thackeray, M.M. Enhancing the rate capability of high capacity xLi2MnO3 (1-x)LiMO2 (M = Mn, Ni, Co) electrodes by Li-Ni-PO4 treatment. Electrochem. Commun. 2009, 11, 748–751. [Google Scholar] [CrossRef]
- Ue, M.; Sakaushi, K.; Uosaki, K. Basic knowledge in battery research bridging the gap between academia and industry. Mater. Horiz. 2020. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Mohanty, D.; Daniel, C.; Polzin, B.J.; Croy, J.R.; Trask, S.E.; Wood, D.L. Correlation of Electrolyte Volume and Electrochemical Performance in Lithium-Ion Pouch Cells with Graphite Anodes and NMC532 Cathodes. J. Electrochem. Soc. 2017, 164, A1195–A1202. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Meyer, H.M.; Trask, S.E.; Polzin, B.J.; Wood, D.L. Electrolyte Volume Effects on Electrochemical Performance and Solid Electrolyte Interphase in Si-Graphite/NMC Lithium-Ion Pouch Cells. ACS Appl. Mater. Interfaces 2017, 9, 18799–18808. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Shibata, T.; Yoshimoto, N.; Ishikawa, M. Anodic behavior of aluminum in organic solutions with different electrolytic salts for lithium ion batteries. Electrochim. Acta 2002, 47, 2787–2793. [Google Scholar] [CrossRef]
- Solchenbach, S.; Metzger, M.; Egawa, M.; Beyer, H.; Gasteiger, H.A. Quantification of PF 5 and POF 3 from Side Reactions of LiPF 6 in Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A3022–A3028. [Google Scholar] [CrossRef] [Green Version]
- Brückner, L.; Frank, J.; Elwert, T. Industrial recycling of lithium-ion batteries—A critical review of metallurgical process routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Li, L.; Zhou, S.; Han, H.; Li, H.; Nie, J.; Armand, M.; Zhou, Z.; Huang, X. Transport and Electrochemical Properties and Spectral Features of Non-Aqueous Electrolytes Containing LiFSI in Linear Carbonate Solvents. J. Electrochem. Soc. 2011, 158, A74. [Google Scholar] [CrossRef]
- Alvarado, J.; Schroeder, M.A.; Zhang, M.; Borodin, O.; Gobrogge, E.; Olguin, M.; Ding, M.S.; Gobet, M.; Greenbaum, S.; Meng, Y.S.; et al. A carbonate-free, sulfone-based electrolyte for high-voltage Li-ion batteries. Mater. Today 2018, 21, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Hernández, G.; Naylor, A.J.; Chien, Y.C.; Brandell, D.; Mindemark, J.; Edström, K. Elimination of fluorination: The influence of fluorine-free electrolytes on the performance of LiNi1/3Mn1/3Co1/3O2/silicon-graphite li-ion battery cells. ACS Sustain. Chem. Eng. 2020, 8, 10041–10052. [Google Scholar] [CrossRef]
- Wang, C.; Meng, Y.S.; Xu, K. Perspective—Fluorinating Interphases. J. Electrochem. Soc. 2019, 166, A5184–A5186. [Google Scholar] [CrossRef]
- Xia, J.; Petibon, R.; Xiong, D.; Ma, L.; Dahn, J.R. Enabling linear alkyl carbonate electrolytes for high voltage Li-ion cells. J. Power Sources 2016, 328, 124–135. [Google Scholar] [CrossRef]
- Klett, M.; Gilbert, J.A.; Trask, S.E.; Polzin, B.J.; Jansen, A.N.; Dees, D.W.; Abraham, D.P. Electrode Behavior RE-Visited: Monitoring Potential Windows, Capacity Loss, and Impedance Changes in Li1.03(Ni0.5Co0.2Mn0.3)0.97O2 /Silicon-Graphite Full Cells. J. Electrochem. Soc. 2016, 163, A875–A887. [Google Scholar] [CrossRef]
- Dupré, N.; Moreau, P.; De Vito, E.; Quazuguel, L.; Boniface, M.; Bordes, A.; Rudisch, C.; Bayle-Guillemaud, P.; Guyomard, D. Multiprobe Study of the Solid Electrolyte Interphase on Silicon-Based Electrodes in Full-Cell Configuration. Chem. Mater. 2016, 28, 2557–2572. [Google Scholar] [CrossRef] [Green Version]
- Delpuech, N.; Dupre, N.; Moreau, P.; Bridel, J.S.; Gaubicher, J.; Lestriez, B.; Guyomard, D. Mechanism of Silicon Electrode Aging upon Cycling in Full Lithium-Ion Batteries. ChemSusChem 2016, 9, 841–848. [Google Scholar] [CrossRef]
- Lewandowski, A.; Świderska-Mocek, A. Ionic liquids as electrolytes for Li-ion batteries-An overview of electrochemical studies. J. Power Sources 2009, 194, 601–609. [Google Scholar] [CrossRef]
- Balducci, A.; Jeong, S.S.; Kim, G.T.; Passerini, S.; Winter, M.; Schmuck, M.; Appetecchi, G.B.; Marcilla, R.; Mecerreyes, D.; Barsukov, V.; et al. Development of safe, green and high performance ionic liquids-based batteries (ILLIBATT project). J. Power Sources 2011, 196, 9719–9730. [Google Scholar] [CrossRef]
- Brutti, S.; Simonetti, E.; De Francesco, M.; Sarra, A.; Paolone, A.; Palumbo, O.; Fantini, S.; Lin, R.; Falgayrat, A.; Choi, H.; et al. Ionic liquid electrolytes for high-voltage, lithium-ion batteries. J. Power Sources 2020, 479, 228791. [Google Scholar] [CrossRef]
- Cao, X.; He, X.; Wang, J.; Liu, H.; Röser, S.; Rad, B.R.; Evertz, M.; Streipert, B.; Li, J.; Wagner, R.; et al. High Voltage LiNi0.5Mn1.5O4/Li4Ti5O12 Lithium Ion Cells at Elevated Temperatures: Carbonate-versus Ionic Liquid-Based Electrolytes. ACS Appl. Mater. Interfaces 2016, 8, 25971–25978. [Google Scholar] [CrossRef]
- Choi, N.S.; Yew, K.H.; Lee, K.Y.; Sung, M.; Kim, H.; Kim, S.S. Effect of fluoroethylene carbonate additive on interfacial properties of silicon thin-film electrode. J. Power Sources 2006, 161, 1254–1259. [Google Scholar] [CrossRef]
- Jin, Y.; Kneusels, N.J.H.; Marbella, L.E.; Castillo-Martínez, E.; Magusin, P.C.M.M.; Weatherup, R.S.; Jónsson, E.; Liu, T.; Paul, S.; Grey, C.P. Understanding Fluoroethylene Carbonate and Vinylene Carbonate Based Electrolytes for Si Anodes in Lithium Ion Batteries with NMR Spectroscopy. J. Am. Chem. Soc. 2018, 140, 9854–9867. [Google Scholar] [CrossRef] [Green Version]
- Haregewoin, A.M.; Wotango, A.S.; Hwang, B.J. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives. Energy Environ. Sci. 2016, 9, 1955–1988. [Google Scholar] [CrossRef]
- Xu, K.; Ding, S.P.; Jow, T.R. Toward Reliable Values of Electrochemical Stability Limits for Electrolytes. J. Electrochem. Soc. 1999, 146, 4172–4178. [Google Scholar] [CrossRef]
- Xia, J.; Sinha, N.N.; Chen, L.P.; Kim, G.Y.; Xiong, D.J.; Dahn, J.R. Study of Methylene Methanedisulfonate as an Additive for Li-Ion Cells. J. Electrochem. Soc. 2014, 161, A84–A88. [Google Scholar] [CrossRef]
- Sinha, N.N.; Burns, J.C.; Dahn, J.R. Comparative Study of Tris(trimethylsilyl) Phosphate and Tris(trimethylsilyl) Phosphite as Electrolyte Additives for Li-Ion Cells. J. Electrochem. Soc. 2014, 161, A1084–A1089. [Google Scholar] [CrossRef]
- Yim, T.; Kim, S.H.; Woo, S.G.; Lee, K.; Song, J.H.; Cho, W.; Kim, K.J.; Kim, J.S.; Kim, Y.J. 1,3-Propanesultone as an effective functional additive to enhance the electrochemical performance of over-lithiated layered oxides. RSC Adv. 2014, 4, 19172–19176. [Google Scholar] [CrossRef]
- Pires, J.; Timperman, L.; Castets, A.; Peña, J.S.; Dumont, E.; Levasseur, S.; Dedryvère, R.; Tessier, C.; Anouti, M. Role of propane sultone as an additive to improve the performance of a lithium-rich cathode material at a high potential. RSC Adv. 2015, 5, 42088–42094. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Yang, J.; Nuli, Y. Artificial Interface Deriving from Sacrificial Tris(trimethylsilyl) phosphate Additive for Lithium Rich Cathode Materials. Electrochim. Acta 2014, 117, 99–104. [Google Scholar] [CrossRef]
- Rong, H.; Xu, M.; Xing, L.; Li, W. Enhanced cyclability of LiNi0.5Mn1.5O4 cathode in carbonate based electrolyte with incorporation of tris(trimethylsilyl)phosphate (TMSP). J. Power Sources 2014, 261, 148–155. [Google Scholar] [CrossRef]
- Guéguen, A.; Bolli, C.; Mendez, M.A.; Berg, E.J. Elucidating the Reactivity of Tris(trimethylsilyl)phosphite and Tris(trimethylsilyl)phosphate Additives in Carbonate Electrolytes—A Comparative Online Electrochemical Mass Spectrometry Study. ACS Appl. Energy Mater. 2020, 3, 290–299. [Google Scholar] [CrossRef]
- Qian, Y.; Hu, S.; Zou, X.; Deng, Z.; Xu, Y.; Cao, Z.; Kang, Y.; Deng, Y.; Shi, Q.; Xu, K.; et al. How electrolyte additives work in Li-ion batteries. Energy Storage Mater. 2019, 20, 208–215. [Google Scholar] [CrossRef]
- Ma, L.; Xia, J.; Dahn, J.R. Ternary Electrolyte Additive Mixtures for Li-Ion Cells that Promote Long Lifetime and Less Reactivity with Charged Electrodes at Elevated Temperatures. J. Electrochem. Soc. 2015, 162, A1170–A1174. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Banerjee, A.; Chen, Z.; Meng, Y.S. From nanoscale interface characterization to sustainable energy storage using all-solid-state batteries. Nat. Nanotechnol. 2020, 15, 170–180. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li+ -conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10039. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Chi, M.; Liang, C.; Dudney, N.J. Solid electrolyte: The key for high-voltage lithium batteries. Adv. Energy Mater. 2015, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 1–16. [Google Scholar] [CrossRef]
- Ko, S.; Yamada, Y.; Lander, L.; Yamada, A. Stability of conductive carbon additives in 5 V-class Li-ion batteries. Carbon N. Y. 2020, 158, 766–771. [Google Scholar] [CrossRef]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage-the transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Chou, S.; Liu, H.K.; Dou, S.X. Functional membrane separators for next-generation high-energy rechargeable batteries. Natl. Sci. Rev. 2017, 4, 917–933. [Google Scholar] [CrossRef] [Green Version]
- Wood, M.; Li, J.; Ruther, R.E.; Du, Z.; Self, E.C.; Meyer, H.M.; Daniel, C.; Belharouak, I.; Wood, D.L. Chemical stability and long-term cell performance of low-cobalt, Ni-Rich cathodes prepared by aqueous processing for high-energy Li-Ion batteries. Energy Storage Mater. 2020, 24, 188–197. [Google Scholar] [CrossRef]
- Yamada, M.; Watanabe, T.; Gunji, T.; Wu, J.; Matsumoto, F. Review of the Design of Current Collectors for Improving the Battery Performance in Lithium-Ion and Post-Lithium-Ion Batteries. Electrochem 2020, 1, 124–159. [Google Scholar] [CrossRef]
- Knoche, T.; Reinhart, G. Electrolyte Filling of Large-Scale Lithium-Ion Batteries: Challenges for Production Technology and Possible Approaches. Appl. Mech. Mater. 2015, 794, 11–18. [Google Scholar] [CrossRef]
- Pathan, T.S.; Rashid, M.; Walker, M.; Widanage, W.D.; Kendrick, E. Active formation of Li-ion batteries and its effect on cycle life. J. Phys. Energy 2019, 1, 044003. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, C.; Biendicho, J.J.; Han, X.; Liang, Z.; Du, R.; Li, M.; Li, J.; Arbiol, J.; Llorca, J.; et al. ZnSe/N-doped carbon nanoreactor with multiple adsorption sites for stable lithium-sulfur batteries. ACS Nano 2020, 14, 15492–15504. [Google Scholar] [CrossRef]
- Zhang, C.; Biendicho, J.J.; Zhang, T.; Du, R.; Li, J.; Yang, X.; Arbiol, J.; Zhou, Y.; Morante, J.R.; Cabot, A. Combined High Catalytic Activity and Efficient Polar Tubular Nanostructure in Urchin-Like Metallic NiCo2Se4 for High-Performance Lithium-Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Lai, W.H.; Yang, H.L.; Zhu, Y.F.; Lei, Y.J.; Zhao, L.; Peng, J.; Wang, Y.X.; Chou, S.L.; Liu, H.K. Efficient separators with fast Li-ion transfer and high polysulfide entrapment for superior lithium-sulfur batteries. Chem. Eng. J. 2021, 408, 127348. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Wu, J.; Zhou, M.; Liu, W.; Zhou, H. Advanced electrolyte design for stable lithium metal anode: From liquid to solid. Nano Energy 2021, 80, 105516. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, D.; Tang, P.; Zhang, C.; Jacas Biendicho, J.; Zhang, Y.; Llorca, J.; Wang, X.; Li, J.; Heggen, M.; et al. Atomically dispersed Fe in a C2N Based Catalyst as a Sulfur Host for Efficient Lithium–Sulfur Batteries. Adv. Energy Mater. 2020, 11, 2003507. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, C.; Chi, Z.; Ke, F.; Yang, Y.; Wang, A.; Wang, W.; Miao, L. How Far Away Are Lithium-Sulfur Batteries from Commercialization? Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef]
- Oxis January 2020. OXIS Energy Is Close to Achieving 500 Wh/kg and Is Targeting 600 Wh/kg with Solid State Lithium Sulfur Technology. [Press Release]. Available online: https://oxisenergy.com/https-oxisenergy-com-wp-content-uploads-2020-01-500-and-600-whkg-pressor-pdf/ (accessed on 29 March 2021).
- Solid-State Lithium Ion Batteries—The Challenges. Available online: https://www.intertek.com/blog/2019-05-21-lion/ (accessed on 29 March 2021).
- Zhang, W.; Liu, Y.; Guo, Z. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 2019, 5, 7412–7422. [Google Scholar] [CrossRef] [Green Version]
- McDowall, J.; Biensan, P.; Broussely, M. Industrial lithium ion battery safety—What are the tradeoffs? In Proceedings of the International Telecommunications Energy Conference (INTELEC), Rome, Italy, 30 September–4 October 2007; pp. 701–707. [Google Scholar]
- Ruiz, V.; Pfrang, A.; Kriston, A.; Omar, N.; Van den Bossche, P.; Boon-Brett, L. A review of international abuse testing standards and regulations for lithium ion batteries in electric and hybrid electric vehicles. Renew. Sustain. Energy Rev. 2018, 81, 1427–1452. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.A.; Varma, A.; Pol, V.G. Mechanistic elucidation of thermal runaway in potassium-ion batteries. J. Power Sources 2018, 375, 131–137. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Wang, Y.; Chen, J.; Zhou, H.; Huang, Y. Macroporous free-standing nano-sulfur/reduced graphene oxide paper as stable cathode for lithium-sulfur battery. Nano Energy 2015, 11, 678–686. [Google Scholar] [CrossRef]
- Cleaver, T.; Kovacik, P.; Marinescu, M.; Zhang, T.; Offer, G. Perspective—Commercializing Lithium Sulfur Batteries: Are We Doing the Right Research? J. Electrochem. Soc. 2018, 165, A6029–A6033. [Google Scholar] [CrossRef]
- Chung, S.-H.; Han, P.; Singhal, R.; Kalra, V.; Manthiram, A. Electrochemically Stable Rechargeable Lithium-Sulfur Batteries with a Microporous Carbon Nanofiber Filter for Polysulfide. Adv. Energy Mater. 2015, 5, 1500738. [Google Scholar] [CrossRef]
- Ma, G.; Huang, F.; Wen, Z.; Wang, Q.; Hong, X.; Jin, J.; Wu, X. Enhanced performance of lithium sulfur batteries with conductive polymer modified separators. J. Mater. Chem. A 2016, 4, 16968–16974. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Zhang, Z.; Liu, Z.; Huang, C.; Jin, Y. Graphdiyne-Modified Polyimide Separator: A Polysulfide-Immobilizing Net Hinders the Shuttling of Polysulfides in Lithium-Sulfur Battery. ACS Appl. Mater. Interfaces 2019, 11, 35738–35745. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 1–11. [Google Scholar] [CrossRef]
- Judez, X.; Zhang, H.; Li, C.; González-Marcos, J.A.; Zhou, Z.; Armand, M.; Rodriguez-Martinez, L.M. Lithium Bis(fluorosulfonyl)imide/Poly(ethylene oxide) Polymer Electrolyte for All Solid-State Li-S Cell. J. Phys. Chem. Lett. 2017, 8, 1956–1960. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Li, H.; Wu, H. Bin Recent Progress of Hybrid Solid-State Electrolytes for Lithium Batteries. Chem. A Eur. J. 2018, 24, 18293–18306. [Google Scholar] [CrossRef]
- Zeng, W.; Cheng, M.M.C.; Ng, S.K.Y. Effects of transition metal cation additives on the passivation of lithium metal anode in Li-S batteries. Electrochim. Acta 2019, 319, 511–517. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.R.; Gao, X.P. Lanthanum Nitrate As Electrolyte Additive To Stabilize the Surface Morphology of Lithium Anode for Lithium-Sulfur Battery. ACS Appl. Mater. Interfaces 2016, 8, 7783–7789. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fang, R.; Xie, D.; Zhang, W.; Huang, H.; Xia, Y.; Wang, X.; Xia, X.; Tu, J. Revisiting Scientific Issues for Industrial Applications of Lithium-Sulfur Batteries. Energy Environ. Mater. 2018, 1, 196–208. [Google Scholar] [CrossRef] [Green Version]
- Professor John Kilner: Solid State Batteries Are Essential for a Safe—MSE Supplies LLC. Available online: https://www.msesupplies.com/blogs/news/professor-john-kilner-solid-state-batteries-are-essential-for-a-safe-energy-storage-system (accessed on 29 March 2021).
- Solid-State Batteries-FutureBridge. Available online: https://www.futurebridge.com/blog/solid-state-batteries/ (accessed on 29 March 2021).
- Liao, Z.; Zhang, S.; Li, K.; Zhang, G.; Habetler, T.G. A survey of methods for monitoring and detecting thermal runaway of lithium-ion batteries. J. Power Sources 2019, 436, 226879. [Google Scholar] [CrossRef]
- Koch, S.; Birke, K.; Kuhn, R. Fast Thermal Runaway Detection for Lithium-Ion Cells in Large Scale Traction Batteries. Batteries 2018, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Cai, T.; Stefanopoulou, A.G.; Siegel, J.B. Early Detection for Li-Ion Batteries Thermal Runaway Based on Gas Sensing. ECS Trans. 2019, 89, 85–97. [Google Scholar] [CrossRef]
- Parekh, M.H.; Li, B.; Palanisamy, M.; Adams, T.E.; Tomar, V.; Pol, V.G. In Situ Thermal Runaway Detection in Lithium-Ion Batteries with an Integrated Internal Sensor. ACS Appl. Energy Mater. 2020, 3, 7997–8008. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Yung, W.K.C.; Pecht, M. Ultrasonic Health Monitoring of Lithium-Ion Batteries. Electronics 2019, 8, 751. [Google Scholar] [CrossRef] [Green Version]
- Raijmakers, L.H.J.; Danilov, D.L.; Van Lammeren, J.P.M.; Lammers, M.J.G.; Notten, P.H.L. Sensorless battery temperature measurements based on electrochemical impedance spectroscopy. J. Power Sources 2014, 247, 539–544. [Google Scholar] [CrossRef]
- Srinivasan, R.; Demirev, P.A.; Carkhuff, B.G. Rapid monitoring of impedance phase shifts in lithium-ion batteries for hazard prevention. J. Power Sources 2018, 405, 30–36. [Google Scholar] [CrossRef]
- Carkhuff, B.G.; Demirev, P.A.; Srinivasan, R. Impedance-Based Battery Management System for Safety Monitoring of Lithium-Ion Batteries. IEEE Trans. Ind. Electron. 2018, 65, 6497–6504. [Google Scholar] [CrossRef]
- Robinson, J.B.; Owen, R.E.; Kok, M.D.R.; Maier, M.; Majasan, J.; Braglia, M.; Stocker, R.; Amietszajew, T.; Roberts, A.J.; Bhagat, R.; et al. Identifying Defects in Li-Ion Cells Using Ultrasound Acoustic Measurements. J. Electrochem. Soc. 2020, 167, 120530. [Google Scholar] [CrossRef]
- Klink, J.; Grabow, J.; Orazov, N.; Benger, R.; Börger, A.; Ahlberg Tidblad, A.; Wenzl, H.; Beck, H.P. Thermal fault detection by changes in electrical behaviour in lithium-ion cells. J. Power Sources 2021, 490, 229572. [Google Scholar] [CrossRef]
- UNECE. Global Technical Regulation No. 20 Electric Vehicle Safety (EVS); UNECE: Geneva, Switzerland, 2018. [Google Scholar]
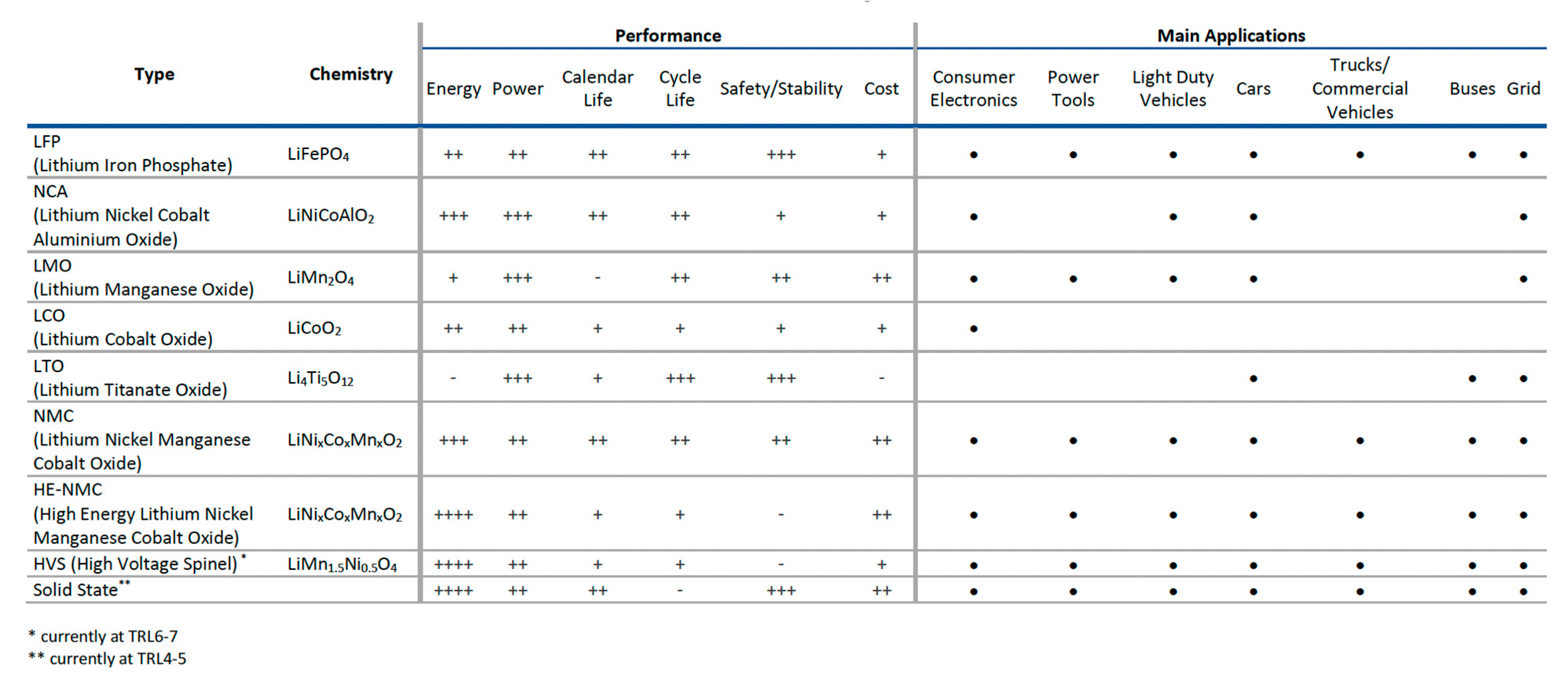
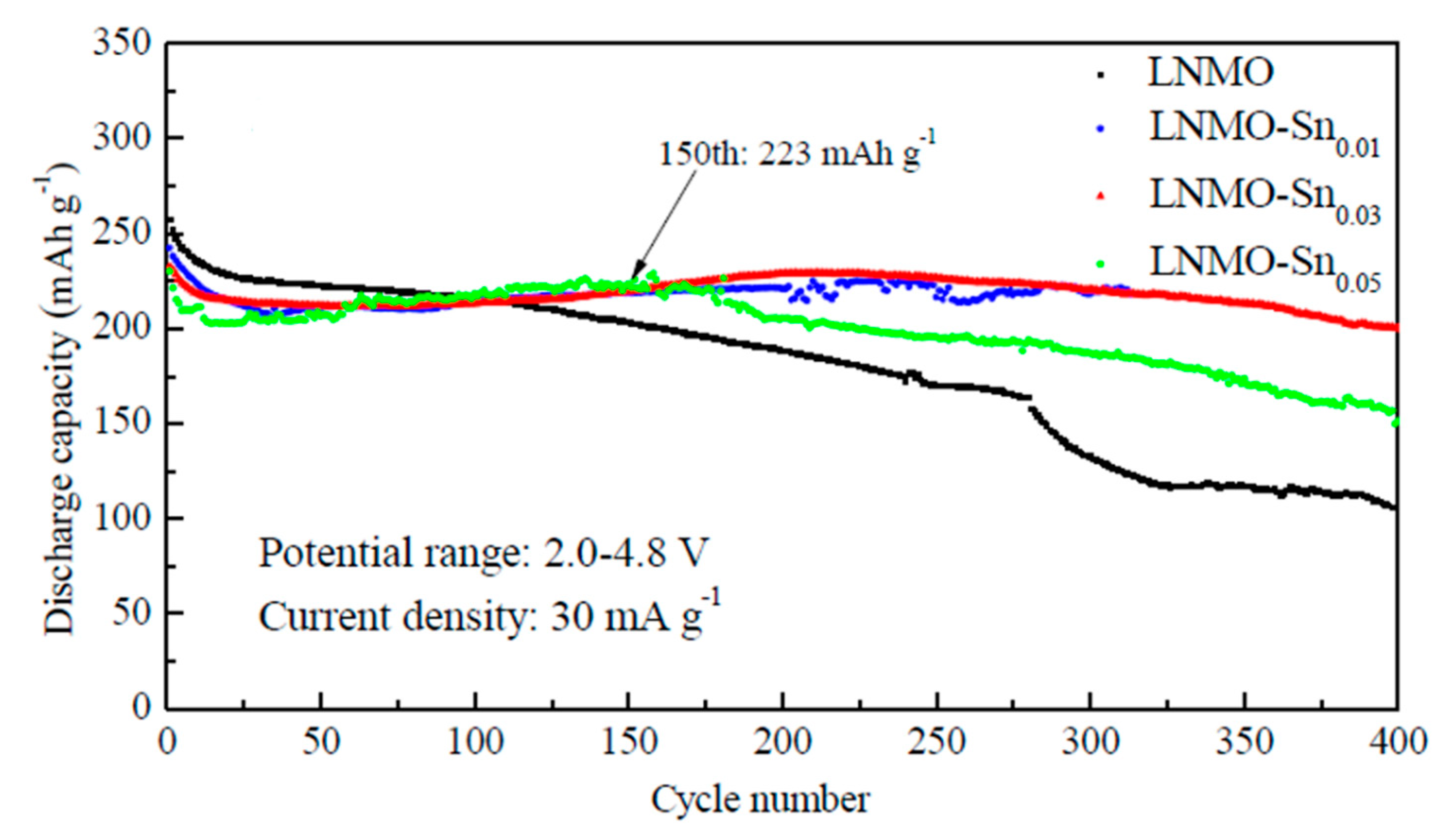
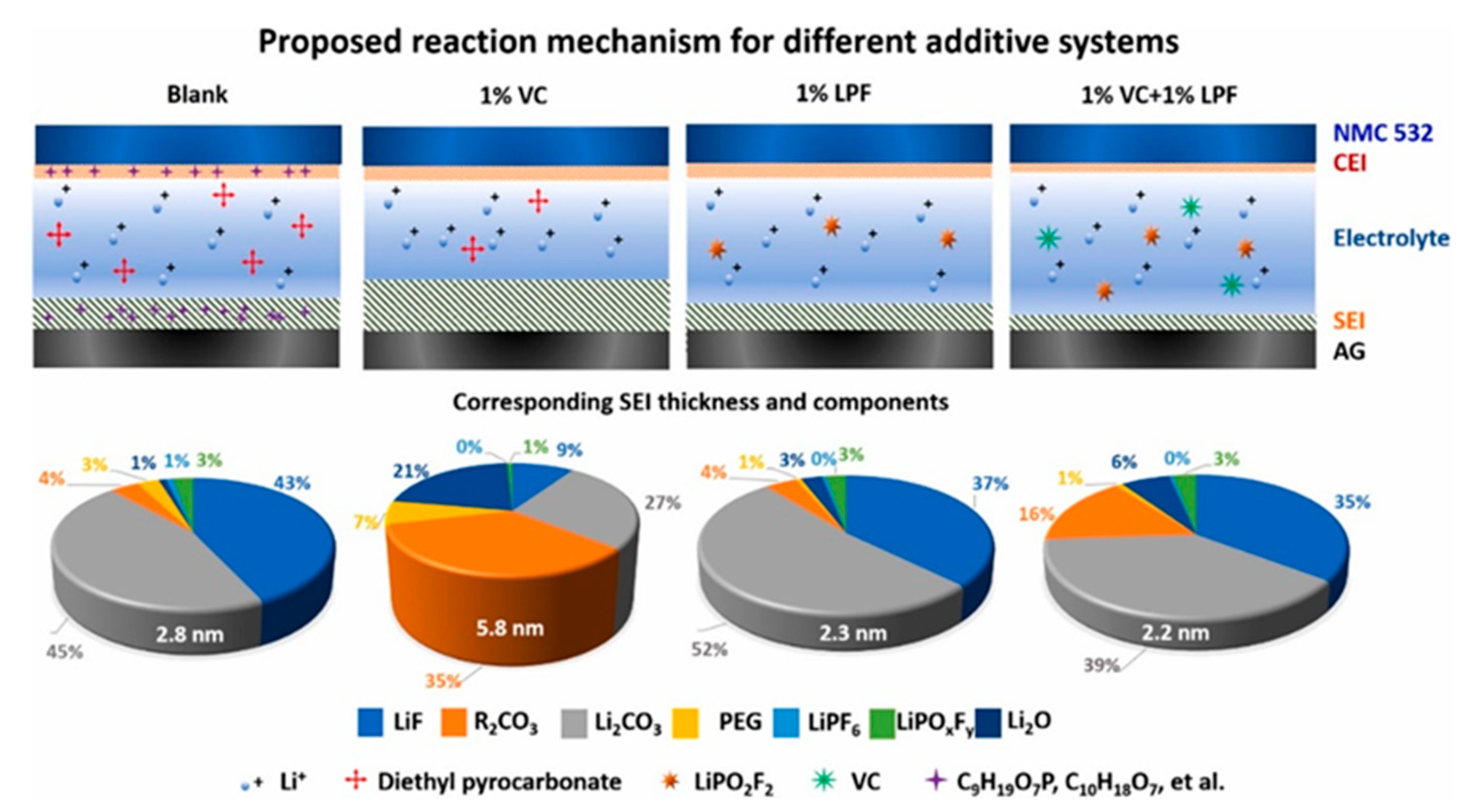
| Demand | Battery Requirements |
|---|---|
| Reducing range anxiety. | Increasing energy density. |
| Convenience of charging. | Fast charging. |
| Sustainable EV. | Reduce size of battery by higher density + fast charging. Reduction of critical raw materials (Novel active materials that do not rely on critical elements and materials). Increase the recycling potential of the components. |
| Climatisation. | Wider operating temperature window. |
| Autonomous driving. | Increase energy density to maintain same range performance or higher energy throughput (cycle life). |
| Connected vehicles. | |
| Comfort and infotainment. | |
| Temporary energy storage applications, e.g., vehicle-to-grid, vehicle-to-vehicle. | Energy throughput not reflected by mileage and calendar life (cycle life). |
| Reduced cost. | Reduction of critical raw materials use, reduction of battery size. |
| Increased safety. | Non-flammable materials, high stability of components. |
| Material | Energy Density | Cycle Life | Power Capability | Cost | Safety | Env. Friend. | Readiness |
|---|---|---|---|---|---|---|---|
| Graphite | 3 | 5 | 4 | 5 | 5 | 4 | 5 |
| LTO | 2 | 5 | 5 | 3 | 4 | 4 | 5 |
| Li3V2O5 | 3 | 4 | 3 | 3 | 3 | 4 | 2 |
| Si | 5 | 2 | 2 | 3 | 5 | 5 | 3 |
| SiOx | 4 | 3 | 3 | 4 | 5 | 5 | 4 |
| Sulphides | 4 | 2 | 2 | 2 | 3 | 5 | 2 |
| P | 4 | 2 | 2 | 3 | 1 | 1 | 2 |
| Ge | 4 | 2 | 2 | 1 | 4 | 3 | 1 |
| Zn0.9Fe0.1O | 3 | 3 | 1 | 4 | 4 | 4 | 1 |
| Material | Energy Density | Cycle Life | Power Capability | Cost | Safety | Env. Friend. | Readiness |
|---|---|---|---|---|---|---|---|
| LiFePO4 | 2 | 4 | 4 | 4 | 4 | 4 | 4 |
| LiMn2O4 | 3 | 3 | 3 | 4 | 3 | 4 | 3 |
| NCM811 | 3 | 2 | 3 | 2 | 3 | 1 | 2 |
| Li1+x(Mn, Ni)1−xO2 | 4 | 1 | 2 | 3 | 2 | 3 | 1 |
| Li-Ion Liquid Battery | Solid-State Battery | ||
|---|---|---|---|
| Advantages | Challenges | Advantages | Challenges |
| Low processing cost. | Shelf time is reduced by self-discharge. | High thermal stability. | Ceramic and glass separators are brittle and break due to pressure/stress. |
| Flexible separators can withstand high mechanical stress. | Flammable electrolyte causes a safety concern. | Less self-discharge. | Ceramic and glass are harder to manufacture in large quantities. Manufacturing process may emit toxic gases. |
| High ionic conductivity at room temperature. | Solid-electrolyte Interfacial Layer impacts the life cycle. | High ionic conductivity over a broad range of temperature. | |
| Limited choice of cathode material due to liquid electrolyte reaction. | Non-volatile electrolyte | ||
| Poor thermal stability. | Safer and non-flammable electrolyte options available | ||
| Overcharge sensitivity issue. | High energy density and tolerance. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tidblad, A.A.; Edström, K.; Hernández, G.; de Meatza, I.; Landa-Medrano, I.; Jacas Biendicho, J.; Trilla, L.; Buysse, M.; Ierides, M.; Horno, B.P.; et al. Future Material Developments for Electric Vehicle Battery Cells Answering Growing Demands from an End-User Perspective. Energies 2021, 14, 4223. https://doi.org/10.3390/en14144223
Tidblad AA, Edström K, Hernández G, de Meatza I, Landa-Medrano I, Jacas Biendicho J, Trilla L, Buysse M, Ierides M, Horno BP, et al. Future Material Developments for Electric Vehicle Battery Cells Answering Growing Demands from an End-User Perspective. Energies. 2021; 14(14):4223. https://doi.org/10.3390/en14144223
Chicago/Turabian StyleTidblad, Annika Ahlberg, Kristina Edström, Guiomar Hernández, Iratxe de Meatza, Imanol Landa-Medrano, Jordi Jacas Biendicho, Lluís Trilla, Maarten Buysse, Marcos Ierides, Beatriz Perez Horno, and et al. 2021. "Future Material Developments for Electric Vehicle Battery Cells Answering Growing Demands from an End-User Perspective" Energies 14, no. 14: 4223. https://doi.org/10.3390/en14144223






