Physicochemical Properties of Biodiesel Synthesised from Grape Seed, Philippine Tung, Kesambi, and Palm Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.1.1. Palm (Elaeis guineensis)
2.1.2. Grape Seed (Vitis vinifera)
2.1.3. Philippine Tung (Reutealis trisperma)
2.1.4. Kesambi (Schleichera oleosa)
2.2. Equipment and Apparatus Setup
2.3. Biodiesel Production
2.3.1. Grape Seed Biodiesel
2.3.2. Philippine Tung Biodiesel
2.3.3. Kesambi Biodiesel
2.4. Biodiesel Characterization
2.5. Antioxidants
2.6. Blending
3. Results and Discussion
3.1. Fatty Acid Composition of Biodiesels
3.2. Biodiesel Characteristics
3.2.1. Oxidation Stability
3.2.2. Kinematic Viscosity
3.2.3. Flashpoint
3.2.4. Cloud Point (CP), Pour Point (PP), and Cold Filter Plugging Point (CFPP)
3.2.5. Calorific Value
3.2.6. Other Properties
3.3. Effects of Antioxidants on Oxidation Stability and Kinematic Viscosity of Biodiesels
3.3.1. Oxidation Stability
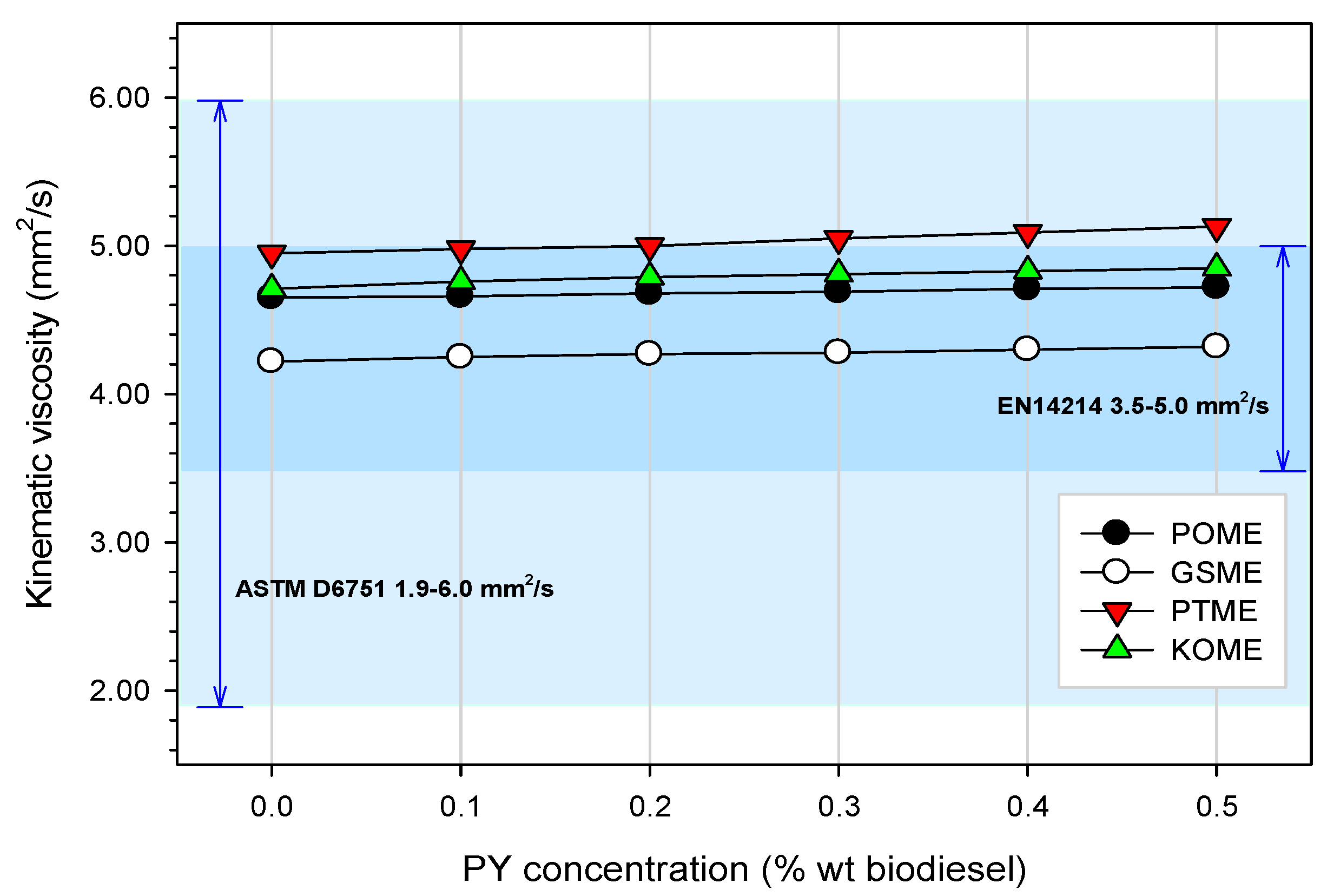
3.3.2. Kinematic Viscosity
3.4. Effect of Blending Ratio on Some Physicochemical Properties of Biodiesels
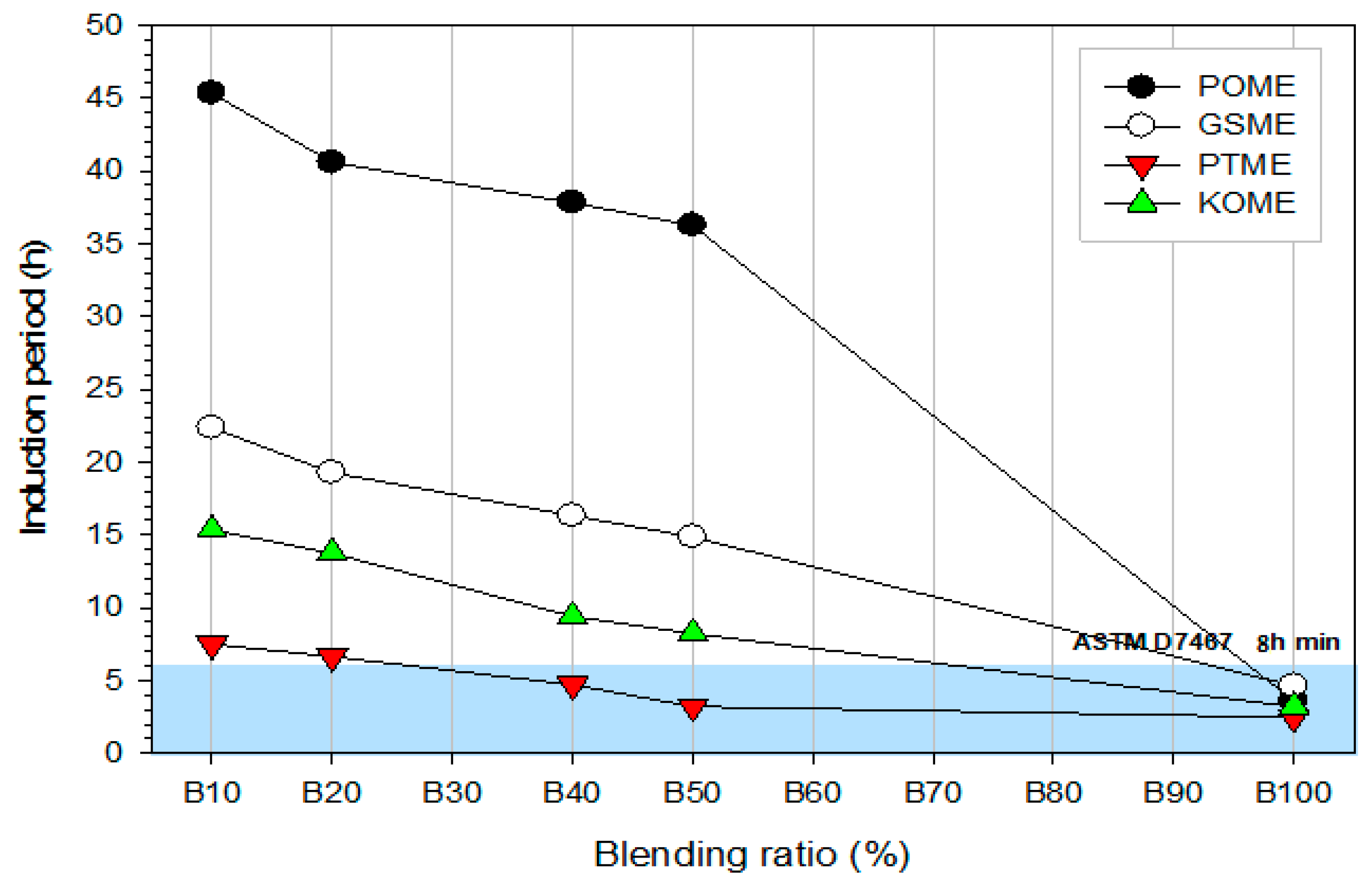
3.4.1. Oxidation stability
3.4.2. Kinematic Viscosity
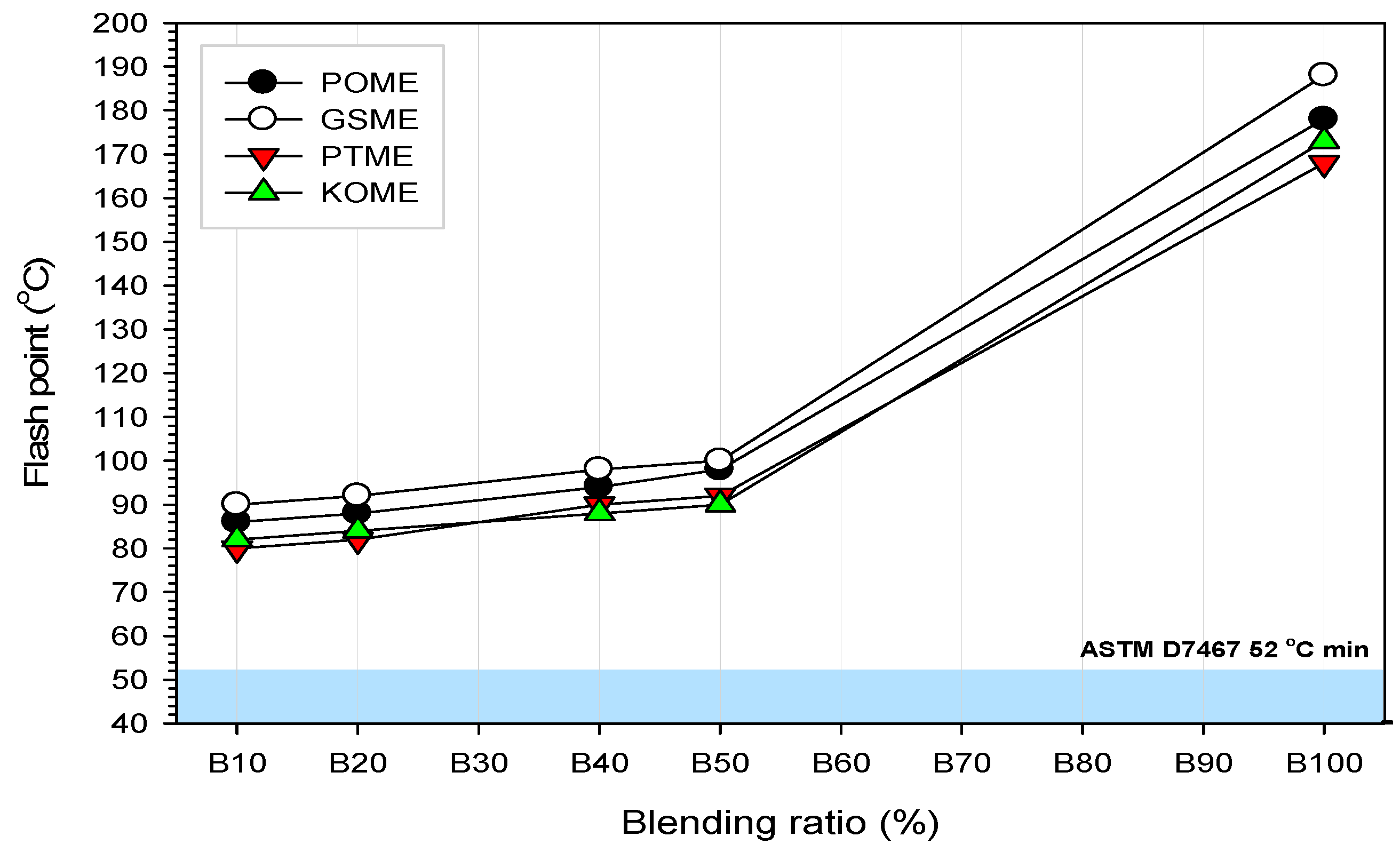
3.4.3. Flashpoint
4. Conclusions
- (a)
- From the FAME analysis, palm oil and kesambi oil biodiesels had similar profiles, containing a 1:1 mixture of unsaturated and saturated fatty acids, while grape seed oil and Philippine tung oil biodiesels mostly contained unsaturated fatty acids.
- (b)
- All tested pure biodiesels fulfilled the biodiesel standards for ASTM D6751, except for oxidation stability for PTME. However, POME did not fulfil the cold flow properties.
- (c)
- The addition of the phenolic antioxidant PY significantly increased the oxidative stability of the tested biodiesels.
- (d)
- All the biodiesel blends met the ASTM D7467 standards for blends of up to 20%. However, GSME and POME blends met the ASTM D7467 requirements up to 50%.
- (e)
- All of the biodiesel blends exceeded the minimum flashpoint requirement for the ASTM standard, which means all of them are safe for use. Furthermore, GSME exhibited the highest flashpoint for all blend ratios.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mofijur, M.; Mahlia, T.M.I.; Silitonga, A.S.; Ong, H.C.; Silakhori, M.; Hasan, M.H.; Putra, N.; Rahman, S.M.A. Phase Change Materials (PCM) for Solar Energy Usages and Storage: An Overview. Energies 2019, 12, 3167. [Google Scholar] [CrossRef] [Green Version]
- Ong, H.C.; Milano, J.; Silitonga, A.S.; Hassan, M.H.; Shamsuddin, A.H.; Wang, C.-T.; Mahlia, T.M.I.; Siswantoro, J.; Kusumo, F.; Sutrisno, J. Biodiesel production from Calophyllum inophyllum-Ceiba pentandra oil mixture: Optimization and characterization. J. Clean. Prod. 2019, 219, 183–198. [Google Scholar] [CrossRef]
- Damanik, N.; Ong, H.C.; Tong, C.W.; Mahlia, T.M.I.; Silitonga, A.S. A review on the engine performance and exhaust emission characteristics of diesel engines fueled with biodiesel blends. Environ. Sci. Pollut. Res. 2018, 25, 15307–15325. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.; Techato, K.; Taweekun, J.; Rahman, M.; Rasul, M.; Mahlia, T.M.I.; Ashrafur, S. An overview of recent developments in biomass pyrolysis technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef] [Green Version]
- Leong, K.Y.; Ku Ahmad, K.Z.; Ong, H.C.; Ghazali, M.J.; Baharum, A. Synthesis and thermal conductivity characteristic of hybrid nanofluids – A review. Renew. Sustain. Energy Rev. 2017, 75, 868–878. [Google Scholar] [CrossRef]
- Ismail, S.M.; Moghavvemi, M.; Mahlia, T.M.I. Characterization of PV panel and global optimization of its model parameters using genetic algorithm. Energy Convers. Manag. 2013, 73, 10–25. [Google Scholar] [CrossRef]
- Amin, M.; Putra, N.; Kosasih, E.A.; Prawiro, E.; Luanto, R.A.; Mahlia, T.M.I. Thermal properties of beeswax/graphene phase change material as energy storage for building applications. Appl. Therm. Eng. 2017, 112, 273–280. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C.; Naghavi, M.S.; Sadeghinezhad, E.; Akhiani, A.R. Preparation and characterization of palmitic acid/graphene nanoplatelets composite with remarkable thermal conductivity as a novel shape-stabilized phase change material. Appl. Therm. Eng. 2013, 61, 633–640. [Google Scholar] [CrossRef]
- Latibari, S.T.; Mehrali, M.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol–gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Tan, S.X.; Lim, S.; Ong, H.C.; Pang, Y.L. State of the art review on development of ultrasound-assisted catalytic transesterification process for biodiesel production. Fuel 2019, 235, 886–907. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Silitonga, A.S.; Chen, W.H.; Kusumo, F.; Dharma, S.; Sebayang, A.H. Optimization of biodiesel production by microwave irradiation-assisted transesterification for waste cooking oil-Calophyllum inophyllum oil via response surface methodology. Energy Convers. Manag. 2018, 158, 400–415. [Google Scholar] [CrossRef]
- Hasan, M.H.; Mahlia, T.M.I.; Nur, H. A review on energy scenario and sustainable energy in Indonesia. Renew. Sust. Energy Rev. 2012, 16, 2316–2328. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Dharma, S.; Kusumo, F.; Siswantoro, J.; Milano, J.; Daud, K.; Mahlia, T.M.I.; et al. Evaluation of the engine performance and exhaust emissions of biodiesel-bioethanol-diesel blends using kernel-based extreme learning machine. Energy 2018, 159, 1075–1087. [Google Scholar] [CrossRef]
- Al, H.; Eze, V.; Harvey, A. Production of biodiesel from waste shark liver oil for biofuel applications. Renew. Energy 2020, 145, 99–105. [Google Scholar]
- Anwar, M.; Rasul, M.G.; Ashwath, N.; Rahman, M.M. Optimisation of Second-Generation Biodiesel Production from Australian Native Stone Fruit Oil Using Response Surface Method. Energies 2018, 11, 2566. [Google Scholar] [CrossRef] [Green Version]
- Chia, S.R.; Ong, H.C.; Chew, K.W.; Show, P.L.; Phang, S.-M.; Ling, T.C.; Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Sustainable approaches for algae utilisation in bioenergy production. Renew. Energy 2018, 129, 838–852. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.-H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Hossain, N.; Zaini, J.; Mahlia, T.; Azad, A.K. Elemental, morphological and thermal analysis of mixed microalgae species from drain water. Renew. Energy 2019, 131, 617–624. [Google Scholar] [CrossRef]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sust. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Alleman, T.L.; Fouts, L.; McCormick, R.L. Quality analysis of wintertime B6–B20 biodiesel blend samples collected in the United States. Fuel Process. Technol. 2011, 92, 1297–1304. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Mahlia, T.M.I.; Kusumo, F.; Dharma, S.; Sebayang, A.H.; Sembiring, R.W.; Shamsuddin, A.H. Intensification of Reutealis trisperma biodiesel production using infrared radiation: Simulation, optimisation and validation. Renew. Energy 2019, 133, 520–527. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Yusaf, T.; Kusumo, F.; Mahlia, T.M.I. Synthesis and optimization of Hevea brasiliensis and Ricinus communis as feedstock for biodiesel production: A comparative study. Ind. Crops Prod. 2016, 85, 274–286. [Google Scholar] [CrossRef]
- Muthiya, J.S.; Pachamuthu, S. Electrochemical NOx reduction and oxidation of HC and PM emissions from biodiesel fuelled diesel engines using electrochemically activated cell. Int. J. Green Energy 2018, 15, 314–324. [Google Scholar] [CrossRef]
- Armas, O.; Gomez, M.A.; Barrientos, E.J.; Boehman, A.L. Estimation of Opacity Tendency of Ethanol- and Biodiesel-Diesel Blends by Means of the Smoke Point Technique. Energy Fuels 2011, 25, 3283–3288. [Google Scholar] [CrossRef]
- Lu, X.C.; Ma, J.J.; Ji, L.B.; Huang, Z. Simultaneous reduction of NOx emission and smoke opacity of biodiesel-fueled engines by port injection of ethanol. Fuel 2008, 87, 1289–1296. [Google Scholar] [CrossRef]
- Olutoye, M.A.; Hameed, B.H. Production of biodiesel fuel by transesterification of different vegetable oils with methanol using Al2O3 modified MgZnO catalyst. Bioresour. Technol. 2013, 132, 103–108. [Google Scholar] [CrossRef]
- Silitonga, A.; Shamsuddin, A.; Mahlia, T.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.; Masjuki, H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.H.; Lim, S.; Pang, Y.L. Investigation of carbon-based solid acid catalyst from Jatropha curcas biomass in biodiesel production. Energy Convers. Manag. 2017, 144, 10–17. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Mazaheri, H.; Ong, H.C.; Masjuki, H.H.; Amini, Z.; Harrison, M.D.; Wang, C.-T.; Kusumo, F.; Alwi, A. Rice bran oil based biodiesel production using calcium oxide catalyst derived from Chicoreus brunneus shell. Energy 2018, 144, 10–19. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Yusaf, T. Engine performance and emissions using Jatropha curcas, Ceiba pentandra and Calophyllum inophyllum biodiesel in a CI diesel engine. Energy 2014, 69, 427–445. [Google Scholar] [CrossRef]
- Mofijur, M.; Atabani, A.E.; Masjuki, H.H.; Kalam, M.A.; Masum, B.M. A study on the effects of promising edible and non-edible biodiesel feedstocks on engine performance and emissions production: A comparative evaluation. Renew. Sustain. Energy Rev. 2013, 23, 391–404. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Kalam, M.; Masjuki, H.; Bhuiya, M.; Mofijur, M. An experimental investigation into biodiesel stability by means of oxidation and property determination. Energy 2012, 44, 616–622. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Luo, Y.-M. Oxidation stability of biodiesel derived from free fatty acids associated with kinetics of antioxidants. Fuel Process. Technol. 2011, 92, 1387–1393. [Google Scholar] [CrossRef]
- Rawat, D.S.; Joshi, G.; Lamba, B.Y.; Tiwari, A.K.; Kumar, P. The effect of binary antioxidant proportions on antioxidant synergy and oxidation stability of Jatropha and Karanja biodiesels. Energy 2015, 84, 643–655. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Khurana, D. Long-term storage oxidation stability of Karanja biodiesel with the use of antioxidants. Fuel Process. Technol. 2013, 106, 447–452. [Google Scholar] [CrossRef]
- Santos, N.A.; Cordeiro, A.M.T.M.; Damasceno, S.S.; Aguiar, R.T.; Rosenhaim, R.; Filho, J.R.C.; Santos, I.M.G.; Maia, A.S.; Souza, A.G. Commercial antioxidants and thermal stability evaluations. Fuel 2012, 97, 638–643. [Google Scholar] [CrossRef]
- Singh, R.; Ibrahim, M.; Esa, N.; Iliyana, M. Composting of waste from palm oil mill: A sustainable waste management practice. Rev. Environ. Sci. Biotechnol. 2010, 9, 331–344. [Google Scholar] [CrossRef]
- Aslanpour, M.; Baneh, H.D.; Tehranifar, A.; Shoor, M. Evaluating the absorption rate of macro and microelements in the leaf of grape sefid bidaneh cv. under drought conditions. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 2019, 10, 515–525. [Google Scholar]
- Perl, A.; Eshdat, Y. Biotechnology in Agriculture and Forestry; Pua, E.C., Davey, M.R., Eds.; Springer: Heidelberg, Germany, 2007; pp. 189–208. [Google Scholar]
- Kim, H.; Kim, S.-G.; Choi, Y.; Jeong, H.-S.; Lee, J. Changes in Tocopherols, Tocotrienols, and Fatty Acid Contents in Grape Seed Oils during Oxidation. J. Am. Oil Chem. Soc. 2008, 85, 487–489. [Google Scholar] [CrossRef]
- Pranowo, D.; Herman, M. Kemiri Minyak as a Conservation Plant and Source of Renewable Energy (Kemiri Minyak Sebagai Tanaman Konservasi dan Sumber Energi Terbarukan); Balittri: Sukabumi, Indonesia, 2011. [Google Scholar]
- Martín, C.; Moure, A.; Martín, G.; Carrillo, E.; Domínguez, H.; Parajó, J.C. Fractional characterisation of jatropha, neem, moringa, trisperma, castor and candlenut seeds as potential feedstocks for biodiesel production in Cuba. Biomass Bioenergy 2010, 34, 533–538. [Google Scholar] [CrossRef]
- Gautam, M.; Vikas, B.; Tandon, R. Sexual System in Schleichera oleosa (Lour.) Oken (Sapindaceae). J. Plant Reprod. Biol. 2009, 1, 73–80. [Google Scholar]
- Sharma, Y.C.; Singh, B. An ideal feedstock, kusum (Schleichera triguga) for preparation of biodiesel: Optimization of parameters. Fuel 2010, 89, 1470–1474. [Google Scholar] [CrossRef]
- Srinivas, K.; Baboo, R.V.C. Antiulcer activity of Schleichera oleosa (Lour.)Oken. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 567–569. [Google Scholar]
- Sudradjat, R.; Endro, P.; Hendra, D.; Setiawan, D. Biodiesel Manufacturing from Kesambi Seed; Bogor Argicultural University: Bogor, Indonesia, 2010. [Google Scholar]
- Anwar, M.; Rasul, M.G.; Ashwath, N. Production optimization and quality assessment of papaya (Carica papaya) biodiesel with response surface methodology. Energy Convers. Manag. 2018, 156, 103–112. [Google Scholar] [CrossRef]
- Bhuiya, M.M.K.; Rasul, M.; Khan, M.; Ashwath, N.; Mofijur, M. Comparison of oil extraction between screw press and solvent (n-hexane) extraction technique from beauty leaf (Calophyllum inophyllum L.) feedstock. Ind. Crops Prod. 2020, 144, 112024. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Atabani, A.E.; Fattah, I.M.R.; Mobarak, H.M. Comparative evaluation of performance and emission characteristics of Moringa oleifera and Palm oil based biodiesel in a diesel engine. Ind. Crops Prod. 2014, 53, 78–84. [Google Scholar] [CrossRef]
- Varatharajan, K.; Cheralathan, M.; Velraj, R. Mitigation of NOx emissions from a jatropha biodiesel fuelled DI diesel engine using antioxidant additives. Fuel 2011, 90, 2721–2725. [Google Scholar] [CrossRef]
- Varatharajan, K.; Pushparani, D. Screening of antioxidant additives for biodiesel fuels. Renew. Sustain. Energy Rev. 2018, 82, 2017–2028. [Google Scholar] [CrossRef]
- Yang, Z.; Hollebone, B.P.; Wang, Z.; Yang, C.; Landriault, M. Factors affecting oxidation stability of commercially available biodiesel products. Fuel Process. Technol. 2013, 106, 366–375. [Google Scholar] [CrossRef]
- Fernández, C.M.; Ramos, M.J.; Pérez, Á.; Rodríguez, J.F. Production of biodiesel from winery waste: Extraction, refining and transesterification of grape seed oil. Bioresour. Technol. 2010, 101, 7019–7024. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Chen, J.-H.; Chang, C.-Y.; Chang, C.-C. Biodiesel production from tung (Vernicia montana) oil and its blending properties in different fatty acid compositions. Bioresour. Technol. 2010, 101, 9521–9526. [Google Scholar] [CrossRef] [PubMed]
- Bouaid, A.; Martinez, M.; Aracil, J. Production of biodiesel from bioethanol and Brassica carinata oil: Oxidation stability study. Bioresour. Technol. 2009, 100, 2234–2239. [Google Scholar] [CrossRef]
- Islam, A.; Taufiq-Yap, Y.H.; Ravindra, P.; Teo, S.H.; Sivasangar, S.; Chan, E.-S. Biodiesel synthesis over millimetric γ-Al2O3/KI catalyst. Energy 2015, 89, 965–973. [Google Scholar] [CrossRef]
- Tesfa, B.; Mishra, R.; Gu, F.; Powles, N. Prediction models for density and viscosity of biodiesel and their effects on fuel supply system in CI engines. Renew. Energy 2010, 35, 2752–2760. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, J., Jr.; Cardoso, F.; Lachter, E.; Estevão, L.M.; Lima, E.; Nascimento, R.V. Correlating chemical structure and physical properties of vegetable oil esters. J. Am. Oil Chem. Soc. 2006, 83, 353–357. [Google Scholar] [CrossRef]
- Refaat, A.A. Correlation between the chemical structure of biodiesel and its physical properties. Int. J. Environ. Sci. Technol. 2009, 6, 677–694. [Google Scholar] [CrossRef] [Green Version]
- Boog, J.H.F.; Silveira, E.L.C.; de Caland, L.B.; Tubino, M. Determining the residual alcohol in biodiesel through its flashpoint. Fuel 2011, 90, 905–907. [Google Scholar] [CrossRef]
- Berman, P.; Nizri, S.; Wiesman, Z. Castor oil biodiesel and its blends as alternative fuel. Biomass Bioenergy 2011, 35, 2861–2866. [Google Scholar] [CrossRef]
- Yaliwal, V.S.; Banapurmath, N.R.; Hosmath, R.S.; Khandal, S.V.; Budzianowski, W.M. Utilization of hydrogen in low calorific value producer gas derived from municipal solid waste and biodiesel for diesel engine power generation application. Renew. Energy 2016, 99, 1253–1261. [Google Scholar] [CrossRef]
- Nehdi, I.A.; Sbihi, H.; Tan, C.P.; Al-Resayes, S.I. Garden cress (Lepidium sativum Linn.) seed oil as a potential feedstock for biodiesel production. Bioresour. Technol. 2012, 126, 193–197. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, Z.; Xu, G.; Lian, X.; Yang, C.; Zhao, W.; Ma, P.; Lin, H.; Han, S. Effect of poly-alpha-olefin pour point depressant on cold flow properties of waste cooking oil biodiesel blends. Fuel 2016, 184, 110–117. [Google Scholar] [CrossRef]
- Pereira, G.G.; Morales, A.; Marmesat, S.; Ruiz-Méndez, M.V.; Barrera-Arellano, D.; Dobarganes, M.C. Effect of temperature on the oxidation of soybean biodiesel. Grasas Aceites 2015, 66, e072. [Google Scholar] [CrossRef] [Green Version]
- Lapuerta, M.; Rodríguez-Fernández, J.; Ramos, Á.; Álvarez, B. Effect of the test temperature and anti-oxidant addition on the oxidation stability of commercial biodiesel fuels. Fuel 2012, 93, 391–396. [Google Scholar] [CrossRef]
- Hasan, M.; Rahman, M. Performance and emission characteristics of biodiesel–diesel blend and environmental and economic impacts of biodiesel production: A review. Renew. Sustain. Energy Rev. 2017, 74, 938–948. [Google Scholar] [CrossRef] [Green Version]


| Properties | Test Method | Equipment |
|---|---|---|
| Flashpoint | ASTM D93 | NPM 440 automatic (Normalab, France) |
| Cloud point | ASTM D2500 | NTE 450 automatic (Normalab, France) |
| Pour point | ASTM D97 | NTE 450 automatic (Normalab, France) |
| Cold filter plugging point (CFPP) | ASTM D6371 | NTL 450 automatic (Normalab, France) |
| Kinematic viscosity at 40 °C | ASTM D445 | SVM 3000 (Anton Paar, UK) |
| Density at 15 °C | ASTM D1298 | DM 40 (Mettler Toledo) |
| Oxidation stability | EN 14112 | 873 Rancimat (Metrohm, Switzerland) |
| Carbon residue (100% sample) | ASTM D4530 | NMC 440 automatic (Normalab, France) |
| Copper corrosion | ASTM D130 | Seta copper corrosion bath 11300-0 (Stanhope-Seta, UK) |
| Sulfur content (S500 grade) | ASTM D5453 | Multi EA 5000 elemental analyzer (analytikjena, UK) |
| Water content | EN ISO 12937 | 831 KF coulometer (Metrohm ion analysis) |
| Moisture | EN ISO 12937 | 831 KF coulometer (Metrohm ion analysis) |
| Calorific value (Gross) | - | Parr 6200 calorimeter (Parr instrument, US) |
| Antioxidant | CAS Number | Molecular Formula | Molecular Weight (g/mol) | Melting Point (°C) | Boiling Point (°C) | Formula | Reference |
|---|---|---|---|---|---|---|---|
| Pyrogallol | 87-66-1 | C6h6o3 | 126.11 | 131–134 | 309 | C6H6O3 | [54] |
| Fatty Acid Name | Composition (wt. %) | ||||
|---|---|---|---|---|---|
| Palm Oil | Grape Seed Oil | Philippine Tung Oil | Kesambi Oil | ||
| Lauric acid | C12:0 | 0.3 | - | 0.1 | - |
| Myristic acid | C14:0 | 1.4 | - | 0.1 | - |
| Palmitic acid | C16:0 | 47.2 | 6.8 | 14.7 | 11.7 |
| Palmitoleic acid | C16:1 | - | 0.1 | 0.5 | 1.1 |
| Stearic acid | C18:0 | 4.1 | 3.8 | 6.6 | 3.9 |
| Oleic acid | C18:1 | 36.4 | 18.5 | 31.3 | 45.4 |
| Linoleic acid | C18:2 | 10.5 | 70.2 | 38.2 | 7.2 |
| Linolenic acid | C18:3 | - | 0.3 | 0.3 | 0.4 |
| α-elaeostearic acid | C18:3 | - | - | 2.3 | - |
| Arachidic acid | C20:0 | - | 0.2 | 0.2 | 30.2 |
| Gondoic acid | C20:1 | - | - | 0.2 | - |
| Behenic acid | C22:0 | - | - | 1.1 | - |
| Erucic acid | C22:1 | - | - | 4.3 | - |
| Lignoceric acid | C24:0 | - | - | 0.1 | - |
| Total unsaturated fatty acids | 46.9 | 89.1 | 77.1 | 54.1 | |
| Total Saturated fatty acids | 53.0 | 10.8 | 22.9 | 45.8 | |
| Properties | Unit | POME | GSME | PTME | KOME | ASTM D6751 | EN 14214 |
|---|---|---|---|---|---|---|---|
| Flashpoint | °C | 178 | 188 | 168 | 173 | 130 minimum | 120 minimum |
| Cloud point | °C | 10 | −5 | 2 | 5 | −3 to 12 | - |
| Pour point | °C | 3 | −5 | 1 | 4 | −15 to 16 | - |
| Cold filter plugging point (CFPP) | °C | 11 | 0 | 0 | 4 | - | +5 maximum |
| Kinematic viscosity at 40 °C | mm2/s | 4.65 | 4.22 | 4.95 | 4.71 | 1.9 to 6.0 | 3.5 to 5.0 |
| Density at 15 °C | kg/m3 | 876.9 | 886.7 | 889.6 | 875.6 | - | 860–900 |
| Oxidation stability | h | 3.57 | 4.62 | 2.47 | 3.21 | 3 minimum | 8 minimum |
| Carbon residue (100% sample) | % m/m | 0 | 0 | 0 | 0 | 0.050 maximum | 0.3 maximum |
| Copper corrosion | - | 1a | 1a | 1a | 1a | 3 maximum | class 1 |
| Sulfur content (S500 grade) | ppm | 3.74 | 2.64 | 5.02 | 3.83 | 500 maximum | - |
| Water content | ppm | 472.4 | 246.7 | 356.2 | Nil | 500 maximum | 500 maximum |
| Moisture | wt.% | 0.0475 | 0.025 | 0.035 | Nil | - | 0.05 maximum |
| Caloric value (Gross) | MJ/kg | 44.80 | 38.89 | 40.24 | 42.27 | - | - |
| Properties | Unit | Test Method | ASTM D7467 [66] |
|---|---|---|---|
| Oxidation stability | h | EN 15751 | 6 min |
| Kinematic viscosity at 40 °C | mm2/s | ASTM D445 | 1.9–4.1 |
| Flashpoint | °C | ASTM D93 | 52 min |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, H.C.; Mofijur, M.; Silitonga, A.S.; Gumilang, D.; Kusumo, F.; Mahlia, T.M.I. Physicochemical Properties of Biodiesel Synthesised from Grape Seed, Philippine Tung, Kesambi, and Palm Oils. Energies 2020, 13, 1319. https://doi.org/10.3390/en13061319
Ong HC, Mofijur M, Silitonga AS, Gumilang D, Kusumo F, Mahlia TMI. Physicochemical Properties of Biodiesel Synthesised from Grape Seed, Philippine Tung, Kesambi, and Palm Oils. Energies. 2020; 13(6):1319. https://doi.org/10.3390/en13061319
Chicago/Turabian StyleOng, Hwai Chyuan, M. Mofijur, A.S. Silitonga, D. Gumilang, Fitranto Kusumo, and T.M.I. Mahlia. 2020. "Physicochemical Properties of Biodiesel Synthesised from Grape Seed, Philippine Tung, Kesambi, and Palm Oils" Energies 13, no. 6: 1319. https://doi.org/10.3390/en13061319





