Numerical Analysis of Heat Transfer Mechanism of Thermal Runaway Propagation for Cylindrical Lithium-ion Cells in Battery Module
Abstract
:1. Introduction
2. Model Establishment
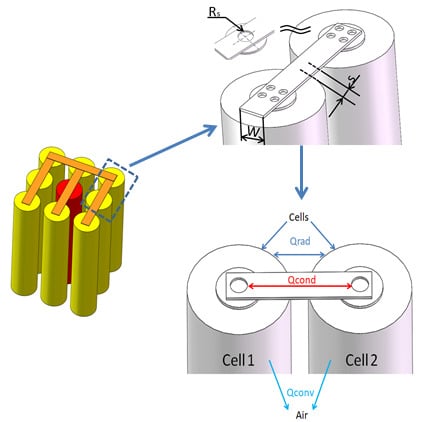
2.1. Battery Module Structure
2.2. Thermal Model
2.2.1. Electrochemical Model
2.2.2. Heat Transfer Model
2.2.3. Boundary Conditions of Heat Transfer
2.3. Model Verification
3. Results and Discussion
3.1. Effect of Cell Spacing
3.2. Effect of the Equivalent Radius of Solder Joints
3.3. Effect of the Width of the Cross-Section of the Electrode Tab
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fu, Y.; Lu, S.; Li, K.; Liu, C.; Cheng, X.; Zhang, H. An experimental study on burning behaviors of 18650 lithium ion batteries using a cone calorimeter. J. Power Sources 2015, 273, 216–222. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Feng, X.; Sun, J.; Ouyang, M.; Wang, F.; He, X.; Lu, L.; Peng, H. Characterization of penetration induced thermal runaway propagation process within a large format lithium ion battery module. J. Power Sources 2015, 275, 261–273. [Google Scholar] [CrossRef]
- Wilke, S.; Schweitzer, B.; Khateeb, S.; Al-Hallaj, S. Preventing thermal runaway propagation in lithium ion battery packs using a phase change composite material: An experimental study. J. Power Sources 2017, 340, 51–59. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Stoliarov, S.I.; Denlinger, M.; Masias, A.; Snyder, K. Heat release during thermally-induced failure of a lithium ion battery: Impact of cathode composition. Fire Saf. J. 2016, 85, 10–22. [Google Scholar] [CrossRef]
- Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Cui, H.; Hsu, H.; Gao, S.; Chen, T.; Li, Y.; et al. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database. Appl. Energy 2019, 246, 53–64. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Scheikl, S.; Planteu, R.; Voitic, G.; Wiltsche, H.; Stangl, C.; Fauler, G.; Thaler, A.; Hacker, V. Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes-impact of state of charge and overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Huang, X.; Cao, Z.; Chen, G. Thermally conductive separator with hierarchical nano/microstructures for improving thermal management of batteries. NANO Energy 2016, 22, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, D.; Chen, X.; Zhang, W.; Liu, J.; Yuen, R.; Wang, J. Investigation on the thermal hazards of 18650 lithium ion batteries by fire calorimeter. J. Therm. Anal. Calorim. 2015, 122, 755–763. [Google Scholar] [CrossRef]
- Inoue, T.; Mukai, K. Roles of positive or negative electrodes in the thermal runaway of lithium-ion batteries: Accelerating rate calorimetry analyses with an all–inclusive microcell. Electrochem. Commun. 2017, 77, 28–31. [Google Scholar] [CrossRef]
- Fu, Y.; Lu, S.; Shi, L.; Cheng, X.; Zhang, H. Ignition and combustion characteristics of lithium ion batteries under low atmospheric pressure. Energy 2018, 161, 38–45. [Google Scholar] [CrossRef]
- Deng, Z.; Sun, Q.; He, Y. Experimental study on the trigger conditions of 18650 type lithium-ion battery thermal runaway fire extension. Fire Sci. Technol. 2018, 37, 690. [Google Scholar]
- Feng, X.; Sun, J.; Ouyang, M.; He, X.; Lu, L.; Han, X.; Fang, M.; Peng, H. Characterization of large format lithium ion battery exposed to extremely high temperature. J. Power Sources 2014, 272, 457. [Google Scholar] [CrossRef]
- Rong, L.; Zhang, H.; Fu, Y.; Cheng, X.D. Effect of Heating Temperature on Thermal Stability of Lithium-ion Battery. DEStech Trans. Environ. Energy Earth Sci. 2017. [Google Scholar] [CrossRef]
- Hofmann, A.; Uhlmann, N.; Ziebert, C.; Wiegand, O.; Schmidt, A.; Hanemann, T. Preventing Li-ion cell explosion during thermal runaway with reduced pressure. Appl. Therm. Eng. 2017, 124, 539–544. [Google Scholar] [CrossRef]
- Lopez, C.F.; Jeevarajan, J.A.; Mukherjee, P.P. Experimental analysis of thermal runaway and propagation in lithium-ion battery modules. J. Electrochem. Soc. 2015, 162, A1905–A1915. [Google Scholar] [CrossRef]
- Lamb, J.; Orendorff, C.J.; Steele, L.A.M.; Spangler, S.W. Failure propagation in multi-cell lithium ion batteries. J. Power Sources 2015, 283, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yang, H.; Li, Y.; Wang, G.; Wang, J. Thermal runaway and fire behaviors of large-scale lithium ion batteries with different heating methods. J. Hazard. Mater. 2019, 379, 120730. [Google Scholar] [CrossRef]
- Coman, P.T.; Darcy, E.C.; Veje, C.T.; White, R.E. Numerical analysis of heat propagation in a battery pack using a novel technology for triggering thermal runaway. Appl. Energy 2017, 203, 189–200. [Google Scholar] [CrossRef]
- Spotnitz, R.M.; Weaver, J.; Yeduvaka, G.; Doughty, D.H.; Roth, E.P. Simulation of abuse tolerance of lithium-ion battery packs. J. Power Sources 2007, 163, 1080–1086. [Google Scholar] [CrossRef]
- Larsson, F.; Anderson, J.; Andersson, P.; Mellander, B.E. Thermal modelling of cell-to-cell fire propagation and cascading thermal runaway failure effects for lithium-ion battery cells and modules using fire walls. J. Electrochem. Soc. 2016, 163, 2854–2865. [Google Scholar] [CrossRef]
- Feng, X.; Lu, L.; Ouyang, M.; Li, J.; He, X. A 3D thermal runaway propagation model for a large format lithium ion battery module. Energy 2016, 115, 194–208. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, W.; Luo, X.; Jiang, N. Analysis method of heat release rate of lithium-ion battery based on domino effect. J. Beijing Univ. Aeronaut. Astronaut. 2017, 43, 902. [Google Scholar]
- Kim, G.-H.; Pesaran, A.; Spotnitz, R. A three–dimensional thermal abuse model for lithium-ion cells. J. Power Sources 2007, 170, 476–489. [Google Scholar] [CrossRef]
- Mandal, B.K.; Padhi, A.K.; Shi, Z.; Chakraborty, S.; Filler, R. Thermal runaway inhibitors for lithium battery electrolytes. J. Power Sources 2006, 161, 1341–1345. [Google Scholar] [CrossRef]
- Liu, R.; Chen, J.; Xun, J.; Jiao, K.; Du, Q. Numerical investigation of thermal behaviors in lithium-ion battery stack discharge. Appl. Energy 2014, 132, 288–297. [Google Scholar] [CrossRef]
- Kshetrimayum, K.S.; Yoon, Y.-G.; Gye, H.-R.; Lee, C.J. Preventing Heat Propagation and Thermal Runaway in Electric Vehicle Battery Modules using Integrated PCM and Micro–channel Plate Cooling System. Appl. Therm. Eng. 2019, 159, 113797. [Google Scholar] [CrossRef]
- Wu, P.; Jan, R.; FENG, X.; Zhang, M.; Lu, L.; He, X.; Ouyang, M. Thermal Runaway Propagation within Module Consists of Large Format Li-Ion Cells; SAE-China Congress 2015; Selected Papers 2016; Springer: Singapore, 2015. [Google Scholar]
- Yang, J.; Yan, H.; Tian, C.; Li, J. Exploration on Automatic Welding Methods for Battery Pack of New Energy Automobiles. Adv. Mater. Res. 2014, 986, 923–926. [Google Scholar] [CrossRef]
- Yang, J.; Tian, C.; Hu, H.; Yan, H. Research on the Orthogonal Test of Spot Welding Parameters of power battery packs. Appl. Mech. Mater. 2015, 716, 1118–1122. [Google Scholar] [CrossRef]
- Feng, X.; He, X.; Ouyang, M.; Lu, L.; Wu, P.; Kulp, C.; Prasser, S. Thermal runaway propagation model for designing a safer battery pack with 25 Ah LiNixCoyMnzO2 large format lithium ion battery. Appl. Energy 2015, 154, 74–91. [Google Scholar] [CrossRef]
- Ma, Y.; Teng, H.; Thelliez, M. Electro–Thermal Modeling of a Lithium-ion Battery System. SAE Int. J. Eng. 2010, 3, 306–317. [Google Scholar] [CrossRef]
- Lee, K.J.; Smith, K.; Pesaran, A.; Kim, G.H. Three dimensional thermal-, electrical-, and electrochemical-coupled model for cylindrical wound large format lithium-ion batteries. J. Power Sources 2013, 241, 20–32. [Google Scholar] [CrossRef]
- Lai, Y.; Wu, W.; Chen, K.; Wang, S.; Xin, C. A compact and lightweight liquid-cooled thermal management solution for cylindrical lithium-ion power battery pack. Int. J. Heat Mass Transf. 2019, 144, 118581. [Google Scholar] [CrossRef]
- Hatchard, T.D.; MacNeil, D.D.; Basu, A.; Dahn, J.R. Thermal model of cylindrical and prismatic lithium-ion cells. J. Electrochem. Soc. 2001, 148, 755–761. [Google Scholar] [CrossRef]
- Dong, T.; Peng, P.; Jiang, F. Numerical modeling and analysis of the thermal behavior of NCM lithium-ion batteries subjected to very high C-rate discharge/charge operations. Int. J. Heat Mass Transf. 2018, 117, 261–272. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Lorén, A.; Mellander, B.E. Characteristics of lithium-ion batteries during fire tests. J. Power Sources 2014, 271, 414–420. [Google Scholar] [CrossRef]
- Kizilel, R.; Sabbah, R.; Selman, J.R.; Al-Hallaj, S. An alternative cooling system to enhance the safety of Li–ion battery packs. J. Power Sources 2009, 194, 1105–1112. [Google Scholar] [CrossRef]






| 2.57 × 105 (J kg−1) | 0.15 | ||
| 1.714 × 106 (J kg−1) | 0.75 | ||
| 7.9 × 105 (J kg−1) | 0.44 | ||
| 1.55 × 105 (J kg−1) | 1 | ||
| 1.5 × 106 (J kg−1) | 1 | ||
| 2.25 × 1015 (s−1) | 0.033 | ||
| 2.5 × 1013 (s−1) | 1 | ||
| 2.55 × 1014 (s−1) | 1 | ||
| 5.14 × 1025 (s−1) | 1 | ||
| 1.917 × 1025 (s−1) | 1 | ||
| 1.3508 × 105 (J mol−1) | 1 | ||
| 1.3508 × 105 (J mol−1) | 1 | ||
| 1.5888 × 105 (J mol−1) | 1.39 × 103 (kg m−3) | ||
| 2.74 × 105 (J mol−1) | 1.5 × 103 (kg m−3) | ||
| 2.86 × 105 (J mol−1) | 5 × 102 (kg m−3) |
| Heat Exchange Quantities of Each Cell | ||||||
|---|---|---|---|---|---|---|
| S = 2 mm | C1 | C2 | C4 | C5 | C7 | C8 |
| Qcond/J | 581 | 1423 | −92 | −4231 | 82 | 1904 |
| Qconv/J | −394 | −779 | −457 | −8734 | −226 | −965 |
| Qrad/J | 492 | 220 | 1209 | −10,830 | 552 | 127 |
| S = 3 mm | C1 | C2 | C4 | C5 | C7 | C8 |
| Qcond/J | 499 | 1380 | −71 | −4004 | 62 | 1803 |
| Qconv/J | −333 | −689 | −377 | −8724 | −192 | −848 |
| Qrad/J | 460 | 179 | 1069 | −10,999 | 511 | 100 |
| S = 4 mm | C1 | C2 | C4 | C5 | C7 | C8 |
| Qcond/J | 444 | 1314 | −61 | −3799 | 51 | 1701 |
| Qconv/J | −301 | −647 | −338 | −8740 | −176 | −792 |
| Qrad/J | 423 | 133 | 950 | −11,152 | 468 | 60 |
| Rs =1 mm | C1 | C2 | C4 | C5 | C7 | C8 |
| Qcond/J | 278 | 585 | 9 | −2055 | 38 | 792 |
| Qconv/J | −253 | −359 | −369 | −8895 | −173 | −436 |
| Qrad/J | 432 | 273 | 929 | −11,117 | 458 | 236 |
| Rs = 3 mm | C1 | C2 | C4 | C5 | C7 | C8 |
| Qcond/J | 380 | 1011 | −41 | −3044 | 41 | 1287 |
| Qconv/J | −282 | −526 | −348 | −8848 | −174 | −626 |
| Qrad/J | 427 | 194 | 942 | −11,128 | 465 | 145 |
| Rs = 5 mm | C1 | C2 | C4 | C5 | C7 | C8 |
| Qcond/J | 444 | 1314 | −6 | −3800 | 51 | 1701 |
| Qconv/J | −301 | −647 | −338 | −8740 | −175 | −792 |
| Qrad/J | 424 | 133 | 950 | −11,154 | 468 | 60 |
| W = 2 mm | C1 | C2 | C4 | C5 | C7 | C8 |
| Qcond/J | 33 | 230 | −22 | −578 | 9 | 260 |
| Qconv/J | −164 | −216 | −339 | −8907 | −156 | −228 |
| Qrad/J | 452 | 338 | 928 | −11,137 | 456 | 332 |
| W = 6 mm | C1 | C2 | C4 | C5 | C7 | C8 |
| Qcond/J | 156 | 496 | −27 | −1486 | 22 | 625 |
| Qconv/J | −209 | −321 | −351 | −8903 | −166 | −368 |
| Qrad/J | 443 | 290 | 931 | −11,122 | 458 | 267 |
| W = 10 mm | C1 | C2 | C4 | C5 | C7 | C8 |
| Qcond/J | 278 | 584 | 9 | −2055 | 38 | 792 |
| Qconv/J | −253 | −359 | −369 | −8895 | −173 | −436 |
| Qrad/J | 432 | 273 | 929 | −11,117 | 458 | 236 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Song, A.; Wang, S.; Cheng, J.; Tao, C. Numerical Analysis of Heat Transfer Mechanism of Thermal Runaway Propagation for Cylindrical Lithium-ion Cells in Battery Module. Energies 2020, 13, 1010. https://doi.org/10.3390/en13041010
Tang Z, Song A, Wang S, Cheng J, Tao C. Numerical Analysis of Heat Transfer Mechanism of Thermal Runaway Propagation for Cylindrical Lithium-ion Cells in Battery Module. Energies. 2020; 13(4):1010. https://doi.org/10.3390/en13041010
Chicago/Turabian StyleTang, Zhiguo, Anqi Song, Shoucheng Wang, Jianping Cheng, and Changfa Tao. 2020. "Numerical Analysis of Heat Transfer Mechanism of Thermal Runaway Propagation for Cylindrical Lithium-ion Cells in Battery Module" Energies 13, no. 4: 1010. https://doi.org/10.3390/en13041010