Synthesis of Furfuryl Alcohol from Furfural: A Comparison between Batch and Continuous Flow Reactors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Charcaterizations
2.2. Hydrogenation of Furfural in Batch Reactor
2.3. Continuous Flow Reactor
3. Discussion-Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Espro, C.; Gumina, B.; Szumelda, T.; Paone, E.; Mauriello, F. Catalytic Transfer Hydrogenolysis as an effective tool for the reductive upgrading of cellulose, hemicellulose, lignin, and their derived molecules. Catalysts 2018, 8, 313. [Google Scholar] [CrossRef] [Green Version]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Bonacci, S.; Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.; Oliverio, M.; Procopio, A. Montmorillonite K10-Catalyzed Solvent-Free Conversion of Furfural into Cyclopentenones. Catalysts 2019, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, A.; Selishcheva, S.; Yakovlev, V. Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol. Catalysts 2018, 8, 595. [Google Scholar] [CrossRef] [Green Version]
- Morozov, E. Furfural Production, 2nd ed.; Forest Industry: Moscow, Russia, 1988; pp. 32–56. [Google Scholar]
- Liu, D.; Zemlyanov, D.; Wu, T.; Lobo-Lapidus, R.J.; Dumesic, J.A.; Miller, J.T.; Marshall, C.L. Deactivation mechanistic studies of copper chromite catalyst for selective hydrogenation of 2-furfuraldehyde. J. Catal. 2013, 299, 336–345. [Google Scholar] [CrossRef]
- Musci, J.J.; Merlo, A.B.; Casella, M.L. Aqueous phase hydrogenation of furfural using carbon-supported Ru and RuSn catalysts. Catal. Today 2017, 296, 43–50. [Google Scholar] [CrossRef]
- Vorotnikov, V.; Mpourmpakis, G.; Vlachos, D.G. DFT study of furfural conversion to furan, furfuryl alcohol, and 2-methylfuran on Pd(111). ACS Catal. 2012, 2, 2496–2504. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Mahajani, V.V. Kinetics of Liquid-Phase Hydrogenation of Furfuraldehyde to Furfuryl Alcohol over a Pt / C Catalyst. Ind. Eng. Chem. Resour. 2003, 42, 3881–3885. [Google Scholar] [CrossRef]
- Zhao, Y. Facile synthesis of Pd nanoparticles on SiO2 for hydrogenation of biomass-derived furfural. Environ. Chem. Lett. 2014, 12, 185–190. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Gulyaeva, T.I.; Nizovskii, A.I.; Kalinkin, A.V.; Bukhtiyarov, V.I.; Lavrenov, A.V.; Likholobov, V.A. Effect of the nature of carbon support on the formation of active sites in Pd/C and Ru/C catalysts for hydrogenation of furfural. Catal. Today 2015, 249, 145–152. [Google Scholar] [CrossRef]
- Fulajtárova, K.; Soták, T.; Hronec, M.; Vávra, I.; Dobroˇcka, E.; Omastová, M. Aqueous phase hydrogenation of furfural to furfuryl alcohol over Pd–Cu catalysts. Appl. Catal. A 2015, 502, 78–85. [Google Scholar] [CrossRef]
- Wu, J.; Shen, Y.; Liu, C.; Wang, H.; Geng, C.; Zhang, Z. Vapor phase hydrogenation of furfural to furfuryl alcohol over environmentally friendly Cu-Ca/SiO2 catalyst. Catal. Commun. 2005, 6, 633–637. [Google Scholar] [CrossRef]
- Villaverde, M.M.; Bertero, N.M.; Garetto, T.F.; Marchi, A.J. Selective liquid-phase hydrogenation of furfural to furfuryl alcohol over Cu-based catalysts. Catal. Today 2013, 213, 87–92. [Google Scholar] [CrossRef]
- Khromova, S.A.; Bykova, M.V.; Bulavchenko, O.A.; Ermakov, D.Y.; Saraev, A.A.; Kaichev, V.V.; Venderbosch, R.H.; Yakovlev, V.A. Furfural Hydrogenation to Furfuryl Alcohol over Bimetallic Ni–Cu Sol–Gel Catalyst: A Model Reaction for Conversion of Oxygenates in Pyrolysis Liquids. Top. Catal. 2016, 59, 1413–1423. [Google Scholar] [CrossRef]
- Sitthisa, S.; Sooknoi, T.; Ma, Y.; Balbuena, P.B.; Resasco, D.E. Kinetics and mechanism of hydrogenation of furfural on Cu/SiO2 catalysts. J. Catal. 2011, 277, 1–13. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Shilov, I.N.; Alekseeva, M.V.; Selishcheva, S.A.; Yakovlev, V.A. Study of the Composition Effect of Molybdenum-Modified Nickel–Copper Catalysts on Their Activity and Selectivity in the Hydrogenation of Furfural to Different Valuable Chemicals. Catal. Ind. 2018, 10, 228–236. [Google Scholar] [CrossRef]
- Audemar, M.; Ciotonea, C.; De Oliveira Vigier, K.; Royer, S.; Ungureanu, A.; Dragoi, B.; Dumitriu, E.; Jérôme, F. Selective Hydrogenation of Furfural to Furfuryl Alcohol in the Presence of a Recyclable Cobalt/SBA-15 Catalyst. ChemSusChem 2015, 8, 1885–1891. [Google Scholar] [CrossRef]
- Villaverde, M.M.; Garetto, T.F.; Marchi, A.J. Liquid-phase transfer hydrogenation of furfural to furfuryl alcohol on Cu–Mg–Al catalysts. Catal. Commun. 2015, 58, 6–10. [Google Scholar] [CrossRef]
- Srivastava, S.; Mohanty, P.; Parikh, J.K.; Dalai, A.K.; Amritphale, S.S.; Khare, A.K. Cr-free Co–Cu/SBA-15 catalysts for hydrogenation of biomass-derived α-, β-unsaturated aldehyde to alcohol. Chin. J. Catal. 2015, 36, 933–942. [Google Scholar] [CrossRef]
- Wei, S.; Cui, H.; Wang, J.; Zhuo, S.; Yi, W.; Wang, L.; Li, Z. Preparation and activity evaluation of NiMoB/-Al2O3 catalyst by liquid-phase furfural hydrogenation. Particuology 2011, 9, 69–74. [Google Scholar] [CrossRef]
- Garcia-Olmo, J.; Yepez, A.; Balu, A.M.; Prinsen, P.; Garcia, A.; Mazière, A.; Len, C.; Luque, R. Activity of continuous flow synthesized Pd-based nanocatalysts in the flow hydroconversion of furfural. Tetrahedron 2017, 73, 5599–5604. [Google Scholar] [CrossRef]
- Wang, Y.; Prinsen, P.; Triantafyllidis, K.S.; Karakoulia, S.A.; Yepez, A.; Len, C.; Luque, R. Batch versus continuous flow performance of supported mono- and bimetallic nickel catalysts for catalytic transfer hydrogenation of furfural in isopropanol. ChemCatChem. 2018, 10, 3459–3468. [Google Scholar] [CrossRef]
- Wang, Y.; Prinsen, P.; Triantafyllidis, K.S.; Karakoulia, S.A.; Trikalitis, P.N.; Yepez, A.; Len, C.; Luque, R. Comparative study of supported monometallic catalysts in the liquid-phase hydrogenation of furfural: Batch and continuous flow. ACS Sustainable Chem. Eng. 2018, 6, 9831–9844. [Google Scholar] [CrossRef]
- Selishcheva, S.A.; Smirnov, A.A.; Fedorov, A.V.; Ermakov, D.Y.; Gulyaeva, Y.K.; Yakovlev, V.A. Production of Furfuryl Alcohol in the Presence of Copper-Containing Catalysts in the Selective Hydrogenation of Furfural. Catal. Ind. 2019, 11, 216–223. [Google Scholar] [CrossRef]
- Wang, Y.; Deyang, P.; Rodriguez-Padrón, D.; Len, C. Recent Advances in Catalytic Hydrogenation of Furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef] [Green Version]
- Audemar, M.; Ramdani, W.; Junhui, T.; Ifrim, A.R.; Ungureanu, A.; Jérôme, F.; Royer, S.; De Oliveira Vigier, K. Selective hydrogenation of xylose to xylitol over Co/SiO2 catalysts. ChemCatChem 2020. [Google Scholar] [CrossRef]
- Taylor, M.J.; Jiang, L.; Reichert, J.; Papageorgiou, A.C.; Beaumont, S.K.; Wilson, K.; Lee, A.F.; Barth, J.V.; Kyriakou, G. Catalytic Hydrogenation and Hydrodeoxygenation of Furfural over Pt(111): A Model System for the Rational Design and Operation of Practical Biomass Conversion Catalysts. J. Phys. Chem. C Nanomater. Interfaces 2017, 121, 8490–8497. [Google Scholar] [CrossRef]

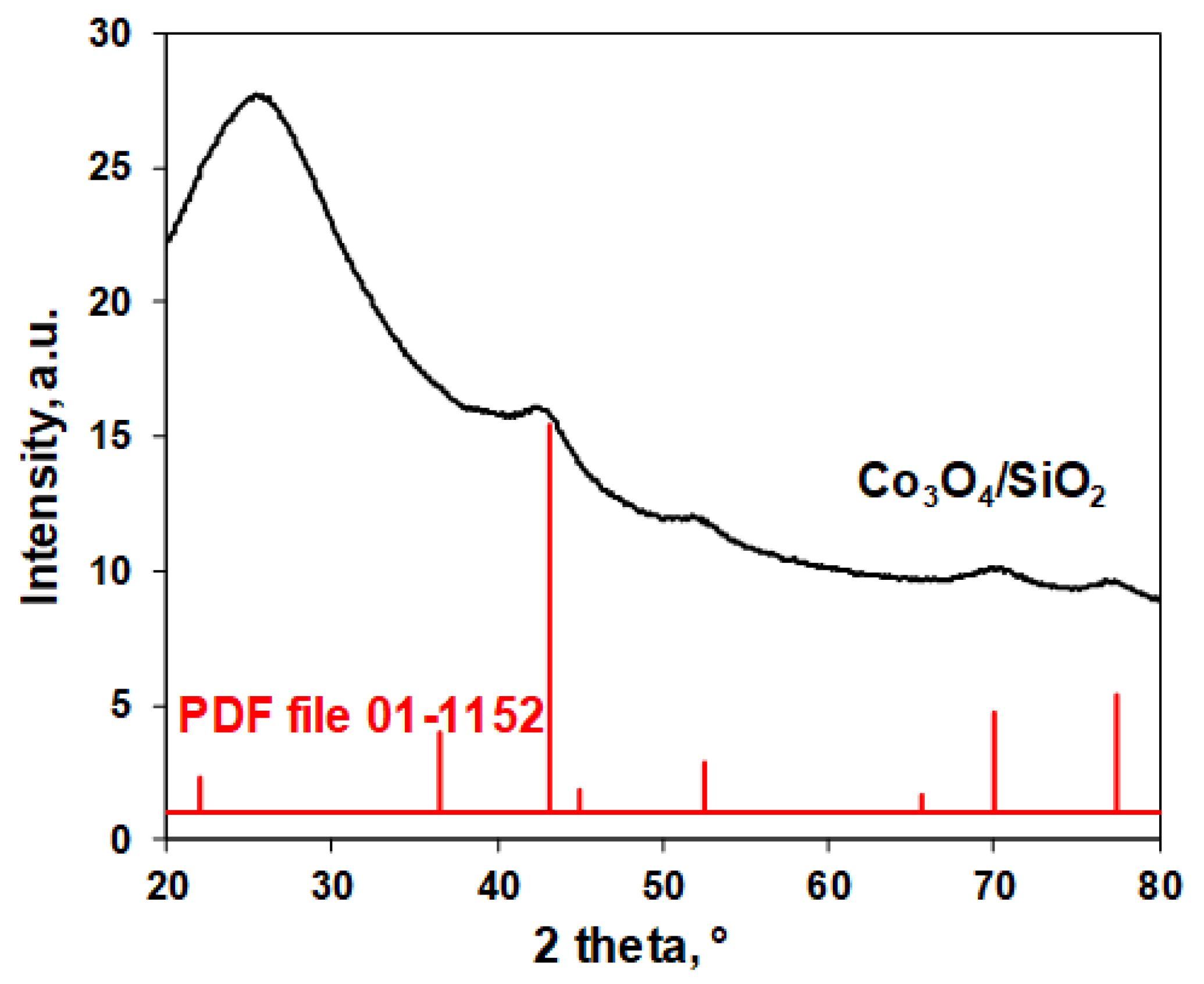

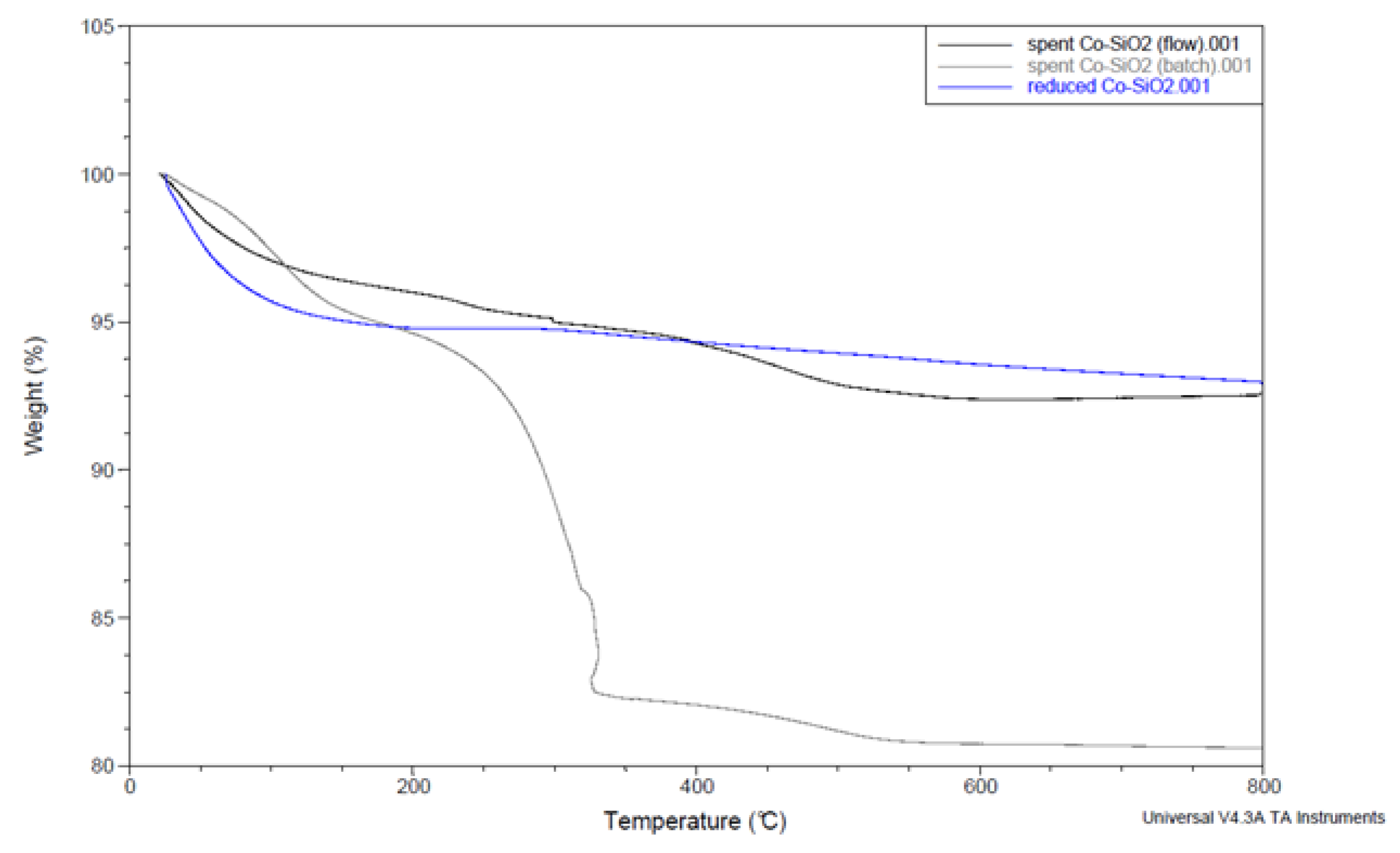
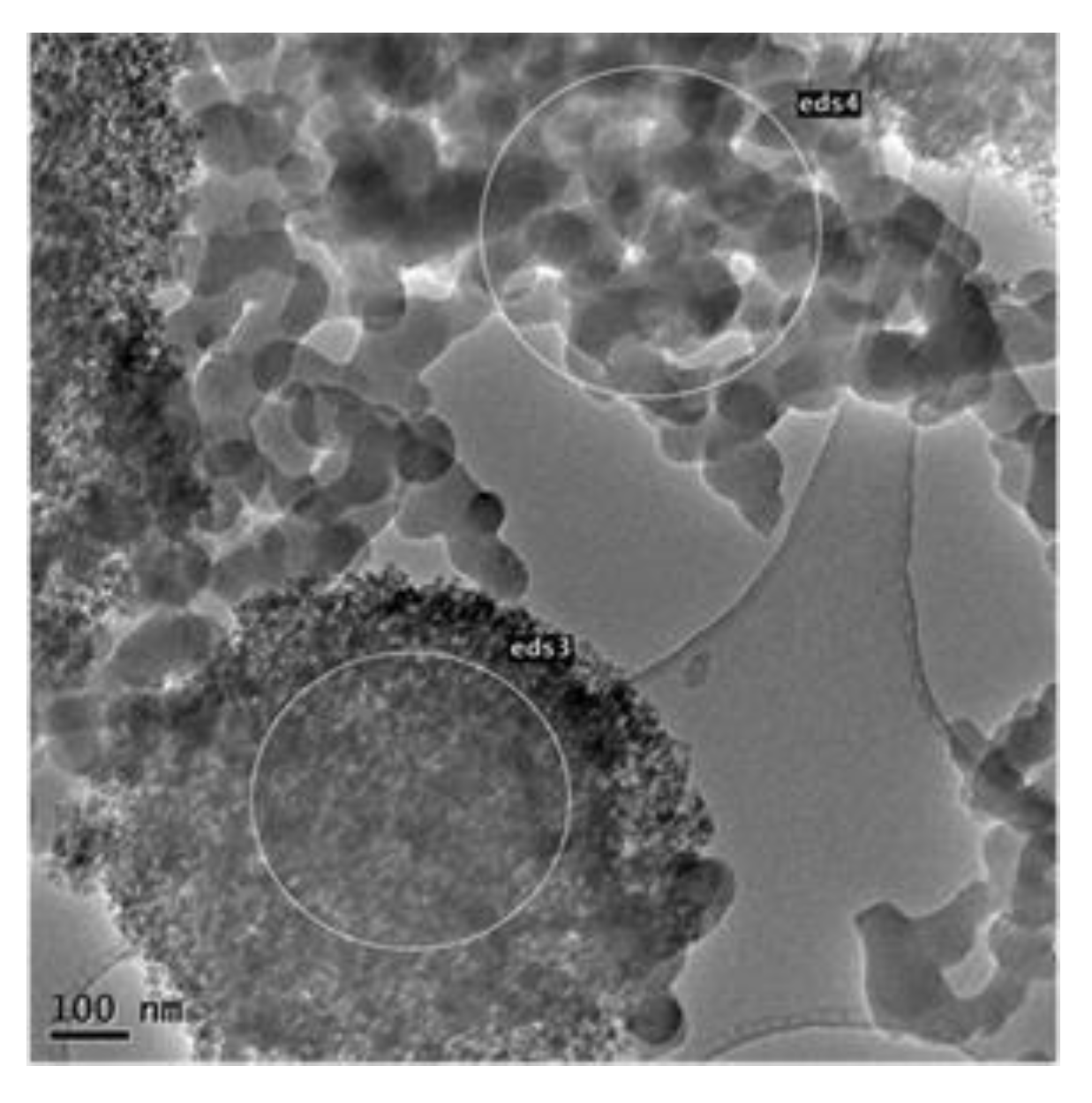


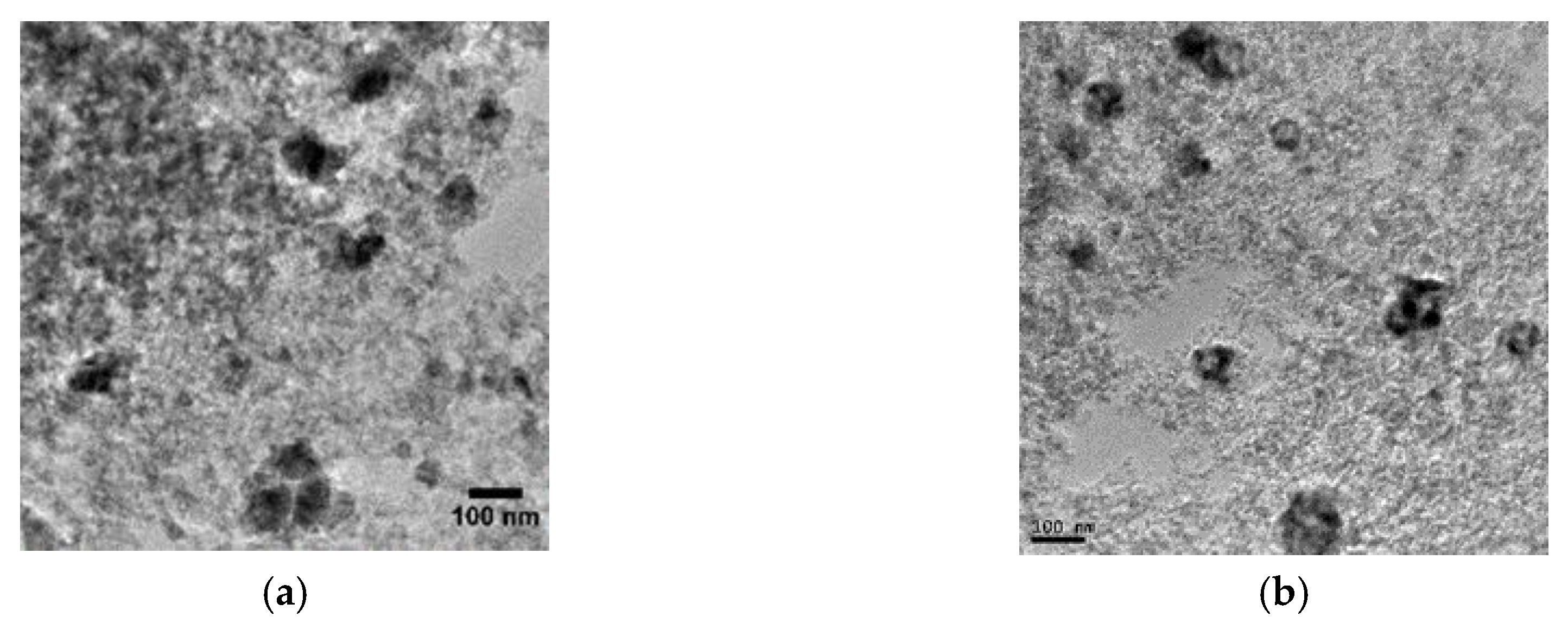
| Characteristics | Co3O4/SiO2 | Co/SiO2 |
|---|---|---|
| XRD phase | Poorly crystalized Co3O4 | n.d.[a] |
| Dpart./[b] nm | n.d. [a] | Aggregates 10 to > 100 nm Crystals < 20 nm |
| SBET/[c] m2·g−1 | 185 | 169 |
| Vp/[c] m3·g−1 | 0.71 | 0.63 |
| Dp/[c] nm | 15.0 | 14.7 |
| Reaction Time (h) | PH2 (bar) | Temperature (°C) | Conversion (%) | Selectivity to FOL (%) | STY (g·L−1·h−1) | |
|---|---|---|---|---|---|---|
| entry 1 | 1 | 20 | 150 | 100 | 100 | 13.2 |
| entry 2 | 1.5 | 20 | 150 | 100 | 65 | 5.9 |
| entry 3 | 1 | 40 | 150 | 100 | 85 | 11.4 |
| entry 4 | 1 | 20 | 180 | 100 | 80 | 10.8 |
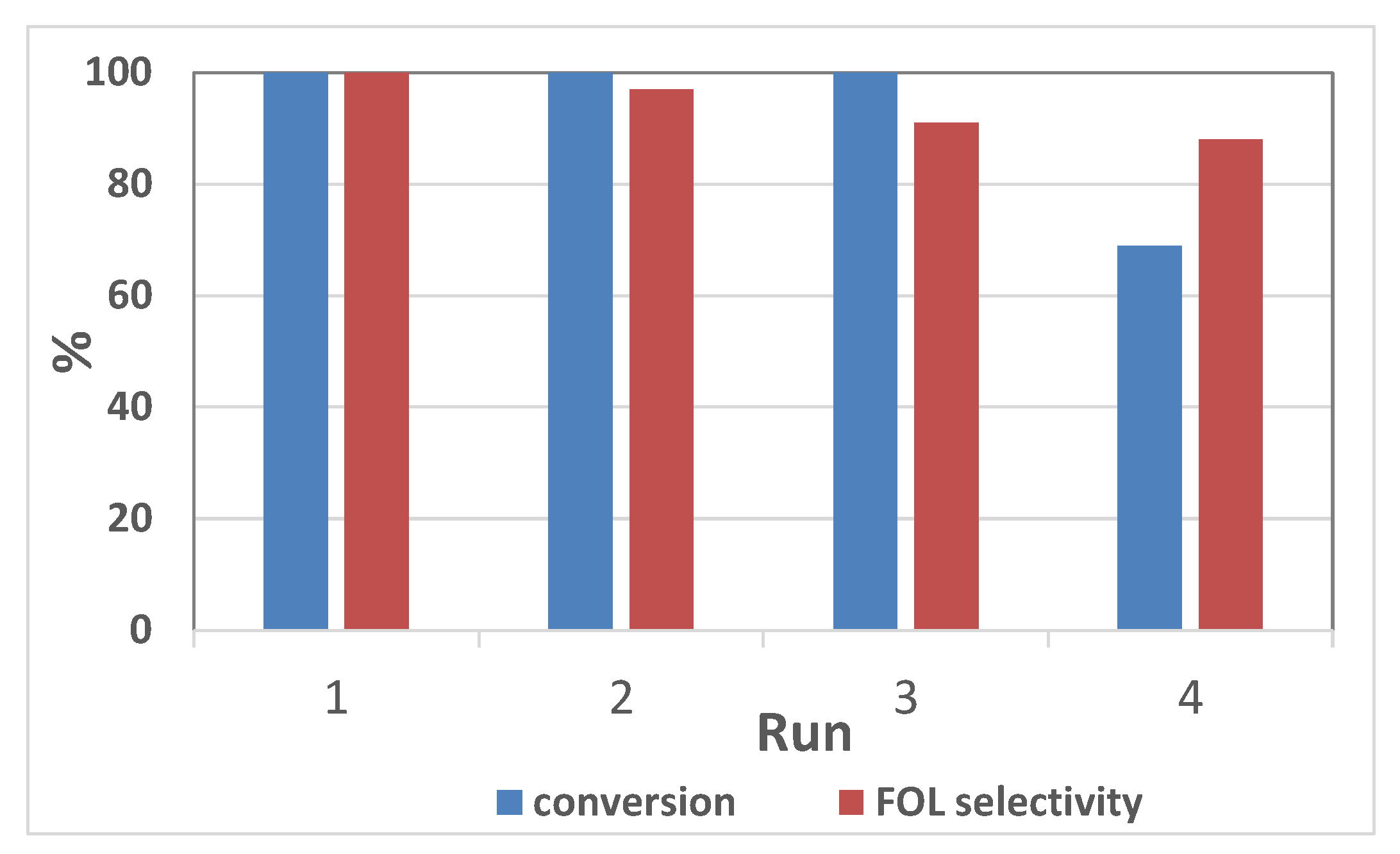
| Reactor | PH2 (bar) | Conversion (%) | Selectivity to FOL (%) | STY (g·L−1·h−1) |
|---|---|---|---|---|
| Batch 2 | 20 | 100 | 100 | 13.2 |
| Flow 3 | 20 | 52 | 50 | 16.6 |
| 40 | 81 | 77 | 26.6 | |
| 60 | 100 | 92 | 30.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Audemar, M.; Wang, Y.; Zhao, D.; Royer, S.; Jérôme, F.; Len, C.; De Oliveira Vigier, K. Synthesis of Furfuryl Alcohol from Furfural: A Comparison between Batch and Continuous Flow Reactors. Energies 2020, 13, 1002. https://doi.org/10.3390/en13041002
Audemar M, Wang Y, Zhao D, Royer S, Jérôme F, Len C, De Oliveira Vigier K. Synthesis of Furfuryl Alcohol from Furfural: A Comparison between Batch and Continuous Flow Reactors. Energies. 2020; 13(4):1002. https://doi.org/10.3390/en13041002
Chicago/Turabian StyleAudemar, Maïté, Yantao Wang, Deyang Zhao, Sébastien Royer, François Jérôme, Christophe Len, and Karine De Oliveira Vigier. 2020. "Synthesis of Furfuryl Alcohol from Furfural: A Comparison between Batch and Continuous Flow Reactors" Energies 13, no. 4: 1002. https://doi.org/10.3390/en13041002