New Insights for the Future Design of Composites Composed of Hydrochar and Zeolite for Developing Advanced Biofuels from Cranberry Pomace
Abstract
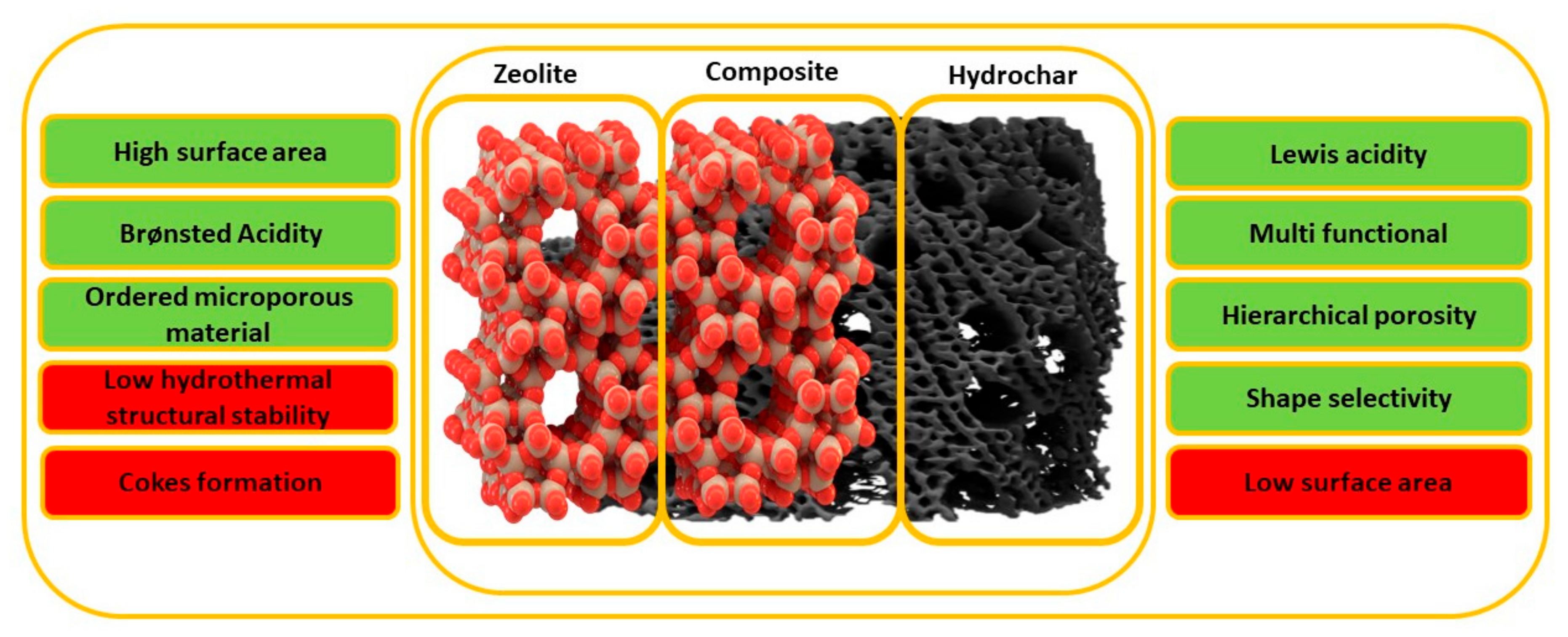
:1. Introduction
- Creating meso/macropores into the micropores structure of the zeolite;
- Increasing the number of accessible active sites for macromolecules;
- Enhancing the thermal stability of the zeolite;
- Creating 3D interconnected structure using activated hydrochar.
2. Material and Methods
2.1. Feedstock and Chemicals
2.2. Hydrothermal Liquefaction Experiments
2.3. Characterization of Bioproducts
2.3.1. Py-GCMS Analysis
2.3.2. BET Analysis
3. Results and Discussion
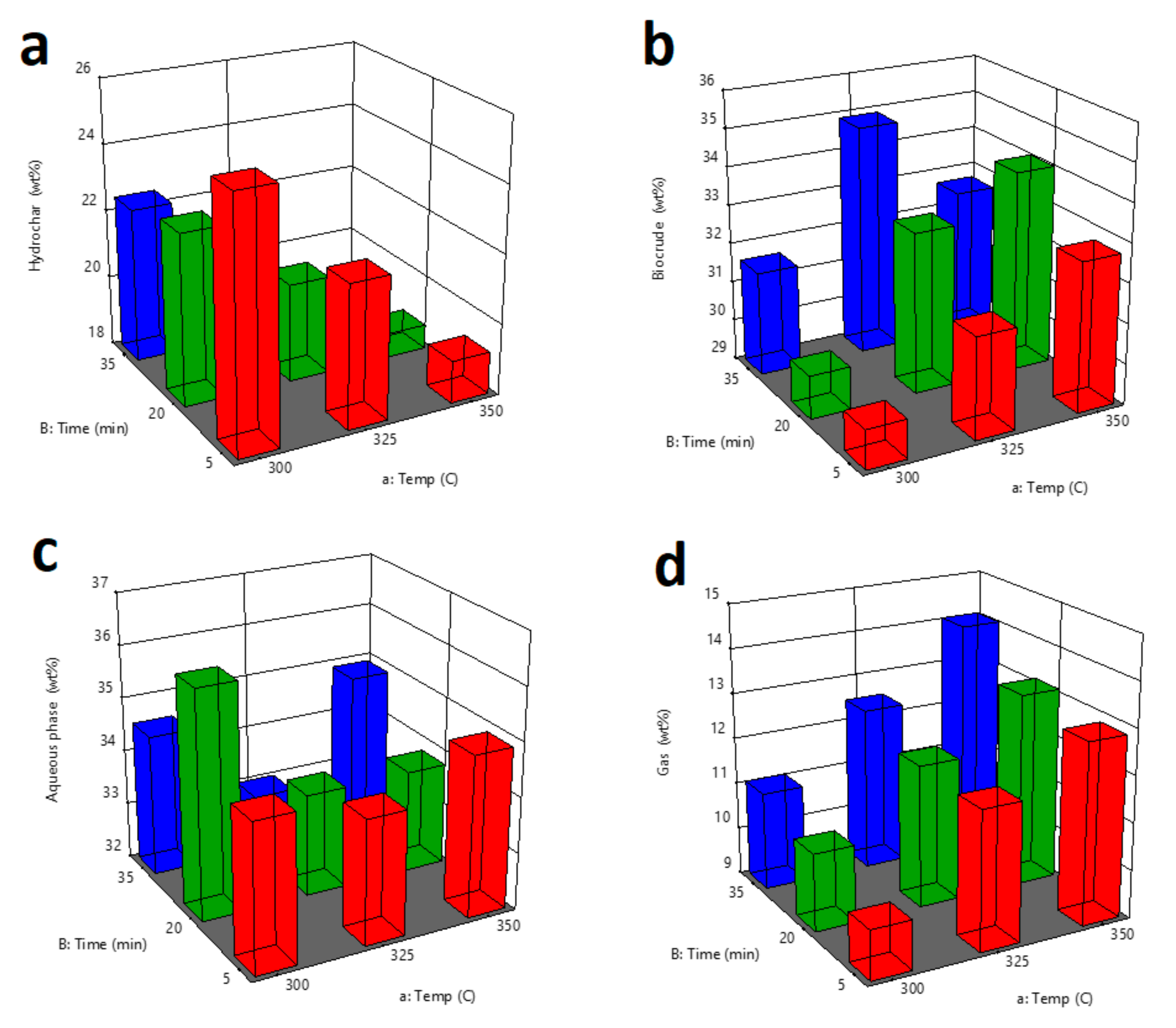
3.1. Optimization of Operating Parameters for Maximum Yield of Biocrude
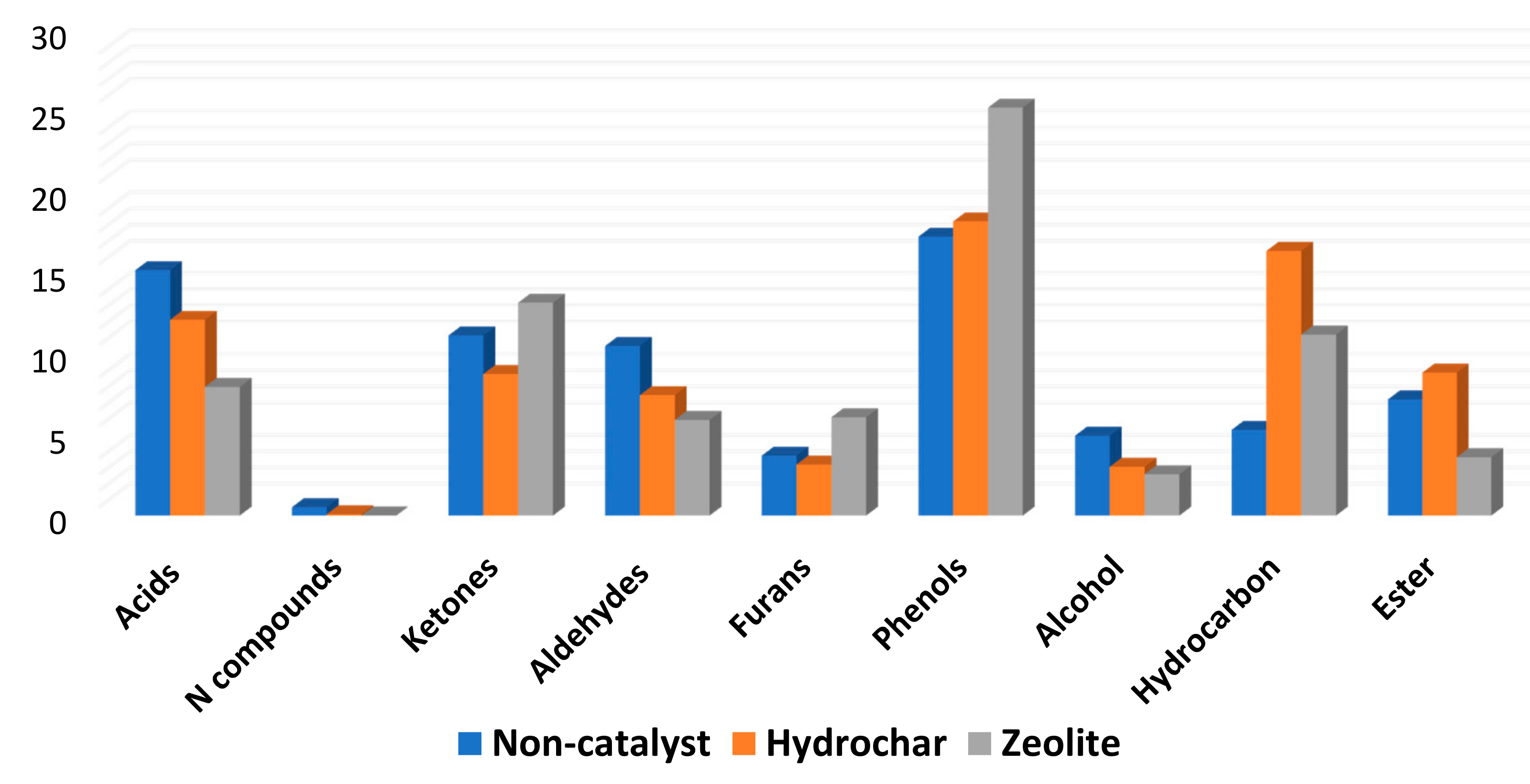
3.2. Chemicals Identified Based on Their Functional Groups
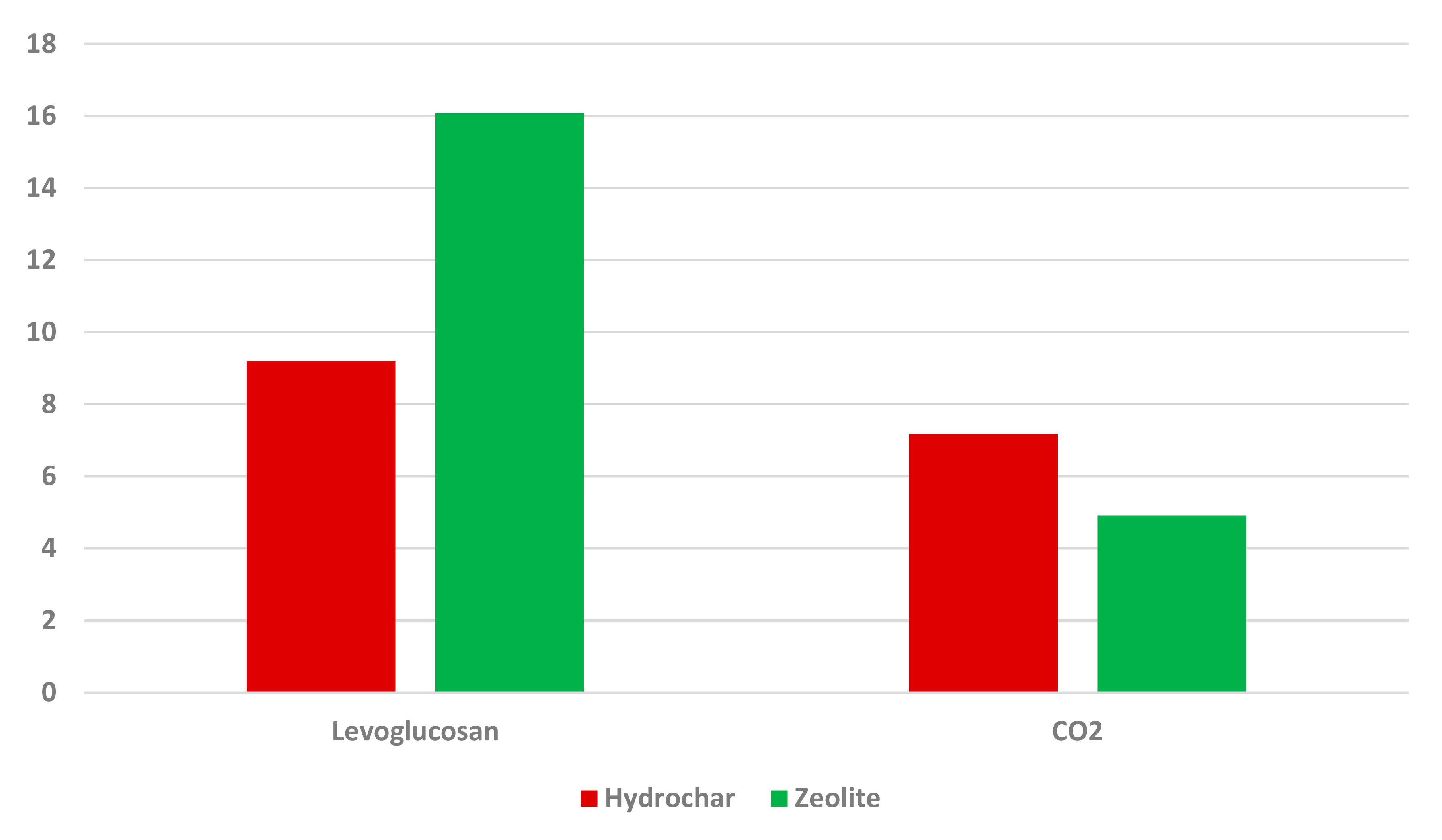
3.3. Decomposition Indicators in the HTL Process
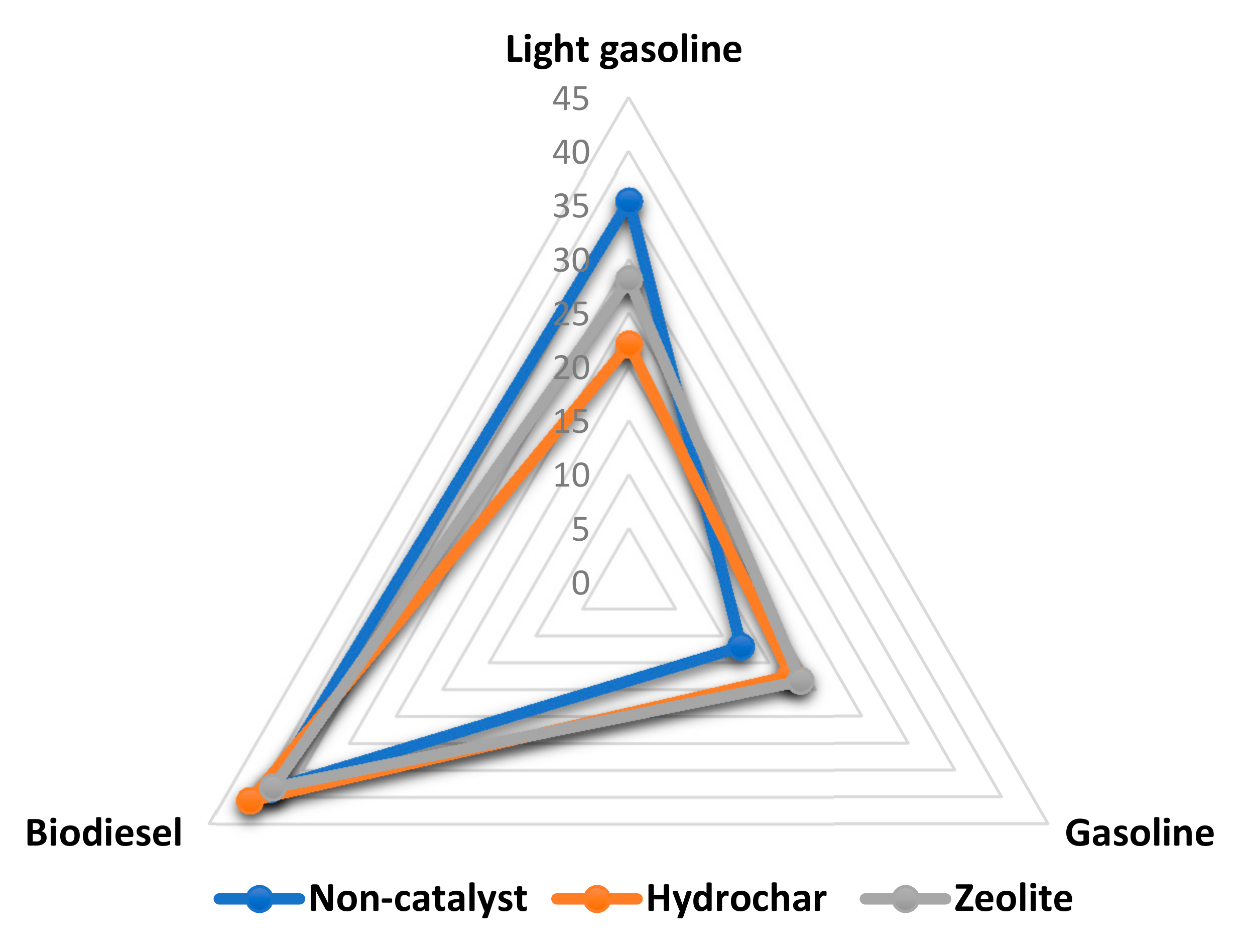
3.4. Hydrocarbon Fractions in the Biocrude
3.5. BET Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ross, K.A.; Ehret, D.; Godfrey, D.; Fukumoto, L.; Diarra, M. Characterization of Pilot Scale Processed Canadian Organic Cranberry (Vaccinium macrocarpon) and Blueberry (Vaccinium angustifolium) Juice Pressing Residues and Phenolic-Enriched Extractives. Int. J. Fruit Sci. 2017, 17, 202–232. [Google Scholar] [CrossRef]
- Struck, S.; Plaza, M.; Turner, C.; Rohm, H. Berry pomace-A review of processing and chemical analysis of its polyphenols. Int. J. Food Sci. Technol. 2016, 51, 1305–1318. [Google Scholar] [CrossRef]
- Ji, C.; Kong, C.X.; Mei, Z.L.; Li, J. A Review of the Anaerobic Digestion of Fruit and Vegetable Waste. Appl. Biochem. Biotechnol. 2017, 183, 906–922. [Google Scholar] [CrossRef] [PubMed]
- Razaghi, A.; Karthikeyan, O.P.; Hao, H.T.N.; Heimann, K. Hydrolysis treatments of fruit and vegetable waste for production of biofuel precursors. Bioresour. Technol. 2016, 217, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, P.; Sumithra Devi, S.; Nand, K. Anaerobic digestion of fruit and vegetable processing wastes for biogas production. Bioresour. Technol. 1992, 40, 43–48. [Google Scholar] [CrossRef]
- Anouti, S.; Haarlemmer, G.; Déniel, M.; Roubaud, A. Analysis of Physicochemical Properties of Bio-Oil from Hydrothermal Liquefaction of Blackcurrant Pomace. Energy Fuels 2016, 30, 398–406. [Google Scholar] [CrossRef]
- Savage, P.E.; Duan, P.; Savage, P.E. Hydrothermal Liquefaction of a Microalga with Heterogeneous Catalysts Hydrothermal Liquefaction of a Microalga with Heterogeneous Catalysts. Ind. Eng. Chem. Res. 2016, 52–61. [Google Scholar] [CrossRef]
- Scarsella, M.; de Caprariis, B.; Damizia, M.; De Filippis, P. Heterogeneous catalysts for hydrothermal liquefaction of lignocellulosic biomass: A review. Biomass Bioenergy 2020, 140, 105662. [Google Scholar] [CrossRef]
- Xu, D.; Lin, G.; Guo, S.; Wang, S.; Guo, Y.; Jing, Z. Catalytic hydrothermal liquefaction of algae and upgrading of biocrude: A critical review. Renew. Sustain. Energy Rev. 2018, 97, 103–118. [Google Scholar] [CrossRef]
- Ennaert, T.; Van Aelst, J.; Dijkmans, J.; De Clercq, R.; Schutyser, W.; Dusselier, M.; Verboekend, D.; Sels, B.F. Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem. Soc. Rev. 2016, 45, 584–611. [Google Scholar] [CrossRef] [Green Version]
- Nizamuddin, S.; Jadhav, A.; Qureshi, S.S.; Baloch, H.A.; Siddiqui, M.T.H.; Mubarak, N.M.; Griffin, G.; Madapusi, S.; Tanksale, A.; Ahamed, M.I. Synthesis and characterization of polylactide/rice husk hydrochar composite. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chiappero, M.; Norouzi, O.; Hu, M.; Demichelis, F.; Berruti, F.; Di Maria, F.; Mašek, O.; Fiore, S. Review of biochar role as additive in anaerobic digestion processes. Renew. Sustain. Energy Rev. 2020, 131. [Google Scholar] [CrossRef]
- Norouzi, O.; Salimi, P.; Di Maria, F.; Pourhosseini, S.E.M.; Safari, F. Synthesis and Design of Engineered Biochars as Electrode Materials in Energy Storage Systems; Springer: Singapore, 2019; ISBN 9789811337680. [Google Scholar]
- Pourhosseini, S.E.M.; Norouzi, O.; Salimi, P.; Naderi, H.R. Synthesis of a Novel Interconnected 3D Pore Network Algal Biochar Constituting Iron Nanoparticles Derived from a Harmful Marine Biomass as High-Performance Asymmetric Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2018, 6, 4746–4758. [Google Scholar] [CrossRef]
- Norouzi, O.; Kheradmand, A.; Jiang, Y.; Di Maria, F.; Masek, O. Superior activity of metal oxide biochar composite in hydrogen evolution under artificial solar irradiation: A promising alternative to conventional metal-based photocatalysts. Int. J. Hydrogen Energy 2019, 44, 28698–28708. [Google Scholar] [CrossRef]
- Norouzi, O.; Di Maria, F. Catalytic effect of functional and Fe composite biochars on biofuel and biochemical derived from the pyrolysis of green marine biomass. Fermentation 2018, 4, 96. [Google Scholar] [CrossRef] [Green Version]
- Norouzi, O.; Di Maria, F.; Dutta, A. Biochar-based composites as electrode active materials in hybrid supercapacitors with particular focus on surface topography and morphology. J. Energy Storage 2020, 29, 101291. [Google Scholar] [CrossRef]
- Pico, J.; Xu, K.; Guo, M.; Mohamedshah, Z.; Ferruzzi, M.G.; Martinez, M.M. Manufacturing the ultimate green banana flour: Impact of drying and extrusion on phenolic profile and starch bioaccessibility. Food Chem. 2019, 297, 124990. [Google Scholar] [CrossRef]
- Heidari, M.; Norouzi, O.; Salaudeen, S.A.; Acharya, B.; Dutta, A. Prediction of hydrothermal carbonization with respect to the biomass components and severity factor. Energy Fuels 2019. [Google Scholar] [CrossRef]
- Kumar, H.; Muppaneni, T.; Ponnusamy, S.; Sudasinghe, N.; Pegallapati, A.; Selvaratnam, T.; Seger, M.; Dungan, B.; Nirmalakhandan, N.; Schaub, T.; et al. Temperature effect on hydrothermal liquefaction of Nannochloropsis gaditana and Chlorella sp. Appl. Energy 2016, 165, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhao, Q.; Lin, Y.; Hu, Y.; Wang, H.; Zhang, G. Characterization of Aqueous Products Obtained from Hydrothermal Liquefaction of Rice Straw: Focus on Product Comparison via Microwave-Assisted and Conventional Heating. Energy Fuels 2018, 32, 510–516. [Google Scholar] [CrossRef]
- Taghavi, S.; Norouzi, O.; Tavasoli, A.; Di Maria, F.; Signoretto, M.; Menegazzo, F.; Di Michele, A. Catalytic conversion of Venice lagoon brown marine algae for producing hydrogen-rich gas and valuable biochemical using algal biochar and Ni/SBA-15 catalyst. Int. J. Hydrogen Energy 2018, 43, 19918–19929. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, S.; Wu, L.; Wang, S.; Li, C.Z. One-pot conversion of biomass-derived xylose and furfural into levulinate esters via acid catalysis. Chem. Commun. 2017, 53, 2938–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef] [PubMed]
- Saba, A.; Lopez, B.; Lynam, J.G.; Reza, M.T. Hydrothermal Liquefaction of Loblolly Pine: Effects of Various Wastes on Produced Biocrude. ACS Omega 2018, 3, 3051–3059. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Kim, K.H.; Brown, R.C. Catalytic pyrolysis of individual components of lignocellulosic biomass. Green Chem. 2014, 16, 727–735. [Google Scholar] [CrossRef]
- Adam, J.; Blazsó, M.; Mészáros, E.; Stöcker, M.; Nilsen, M.H.; Bouzga, A.; Hustad, J.E.; Grønli, M.; Øye, G. Pyrolysis of biomass in the presence of Al-MCM-41 type catalysts. Fuel 2005, 84, 1494–1502. [Google Scholar] [CrossRef]
- Pan, X.; Fan, Z.; Chen, W.E.I.; Ding, Y.; Luo, H.; Bao, X. Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles. Nat. Mater. 2007, 507–511. [Google Scholar] [CrossRef]
- Yang, L.; Tsilomelekis, G.; Caratzoulas, S.; Vlachos, D.G. Mechanism of Brønsted Acid-Catalyzed Glucose Dehydration. ChemSusChem 2015, 8, 1334–1341. [Google Scholar] [CrossRef]
- Pourhosseini, S.E.M.; Norouzi, O.; Naderi, H.R. Study of micro/macro ordered porous carbon with olive-shaped structure derived from Cladophora glomerata macroalgae as efficient working electrodes of supercapacitors. Biomass Bioenergy 2017, 107, 287–298. [Google Scholar] [CrossRef]
| Parameters | Cranberry | ± |
|---|---|---|
| Moisture content (%) | 13.00 | 0.53 |
| Particle size (D 4.3) | 286.85 | 2.28 |
| Protein content (%) | 4.29 | 0.02 |
| TDF_IDF (%) | 72.71 | 0.81 |
| TDF_SDFP (%) | 11.97 | 0.22 |
| Total starch (%) | no | |
| Reducing sugars (%) | 6.34 | 0.01 |
| EPP (mg/100 g) | 1210.11 | 143.43 |
| HPP (mg/100) | 2000.41 | 210.44 |
| NEPA anthocyanidins (mg/100 g) | 161.52 | 10.31 |
| DPPH EPP (IC50) | 2.52 | 0.21 |
| DPPH HPP (IC50) | 0.56 | 0.01 |
| DPPH NEPA (IC50) | 0.16 | 0.01 |
| Carbon (%) | 50.69 | 0.09 |
| Hydrogen (%) | 6.88 | 0.03 |
| Nitrogen (%) | 0.92 | 0.03 |
| Oxygen (%) | 45.86 | 0.12 |
| Calcium (%) | 0.05 | 0.01 |
| Magnesium (%) | 0.04 | 0.002 |
| Potassium (%) | 0.24 | 0.03 |
| Run | a:Temp | B:Time | Hydrochar | Biocrude | Aqueous Phase | Gas |
|---|---|---|---|---|---|---|
| C | min | wt% | wt% | wt% | wt% | |
| 1 | 325 | 35 | 19.47 | 34.90 | 33.03 | 12.60 |
| 2 | 325 | 20 | 20.98 | 33.09 | 33.80 | 12.13 |
| 3 | 350 | 35 | 18.16 | 33.01 | 34.82 | 14.01 |
| 4 | 350 | 20 | 18.69 | 34.19 | 34.02 | 13.10 |
| 5 | 350 | 5 | 19.21 | 32.80 | 35.11 | 12.88 |
| 6 | 325 | 20 | 20.80 | 33.15 | 33.98 | 12.07 |
| 7 | 325 | 35 | 19.40 | 34.98 | 33.07 | 12.55 |
| 8 | 325 | 5 | 22.14 | 31.59 | 34.29 | 11.98 |
| 9 | 350 | 5 | 19.28 | 32.87 | 34.85 | 13.00 |
| 10 | 350 | 20 | 18.77 | 34.12 | 33.78 | 13.33 |
| 11 | 350 | 35 | 18.11 | 32.55 | 35.15 | 14.19 |
| 12 | 300 | 5 | 25.22 | 30.00 | 34.71 | 10.07 |
| 13 | 300 | 35 | 22.56 | 31.68 | 34.62 | 11.14 |
| 14 | 300 | 20 | 23.01 | 30.11 | 36.19 | 10.69 |
| Morphological Characteristic | Units | Hydrochar | Commercial Zeolite |
|---|---|---|---|
| BET surface area | m2/g | 10 | 603 |
| Total pore volume | cm3/g | 0.45 | 0.646 |
| Micropore volume | cm3/g | 0.001 | 0.384 |
| Mesopore volume | cm3/g | 0.54 | 0.123 |
| Micropore surface area | m2/g | 0.03 | 466 |
| Mesopore surface area | m2/g | 9 | 137 |
| Average pore diameter | Nm | 25 | 1.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norouzi, O.; Heidari, M.; M. Martinez, M.; Dutta, A. New Insights for the Future Design of Composites Composed of Hydrochar and Zeolite for Developing Advanced Biofuels from Cranberry Pomace. Energies 2020, 13, 6600. https://doi.org/10.3390/en13246600
Norouzi O, Heidari M, M. Martinez M, Dutta A. New Insights for the Future Design of Composites Composed of Hydrochar and Zeolite for Developing Advanced Biofuels from Cranberry Pomace. Energies. 2020; 13(24):6600. https://doi.org/10.3390/en13246600
Chicago/Turabian StyleNorouzi, Omid, Mohammad Heidari, Mario M. Martinez, and Animesh Dutta. 2020. "New Insights for the Future Design of Composites Composed of Hydrochar and Zeolite for Developing Advanced Biofuels from Cranberry Pomace" Energies 13, no. 24: 6600. https://doi.org/10.3390/en13246600







