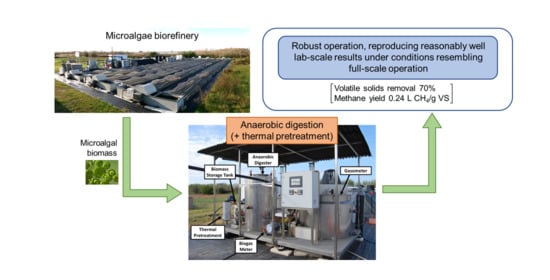
Scaling-Up the Anaerobic Digestion of Pretreated Microalgal Biomass within a Water Resource Recovery Facility
Abstract
:1. Introduction
2. Materials and Methods
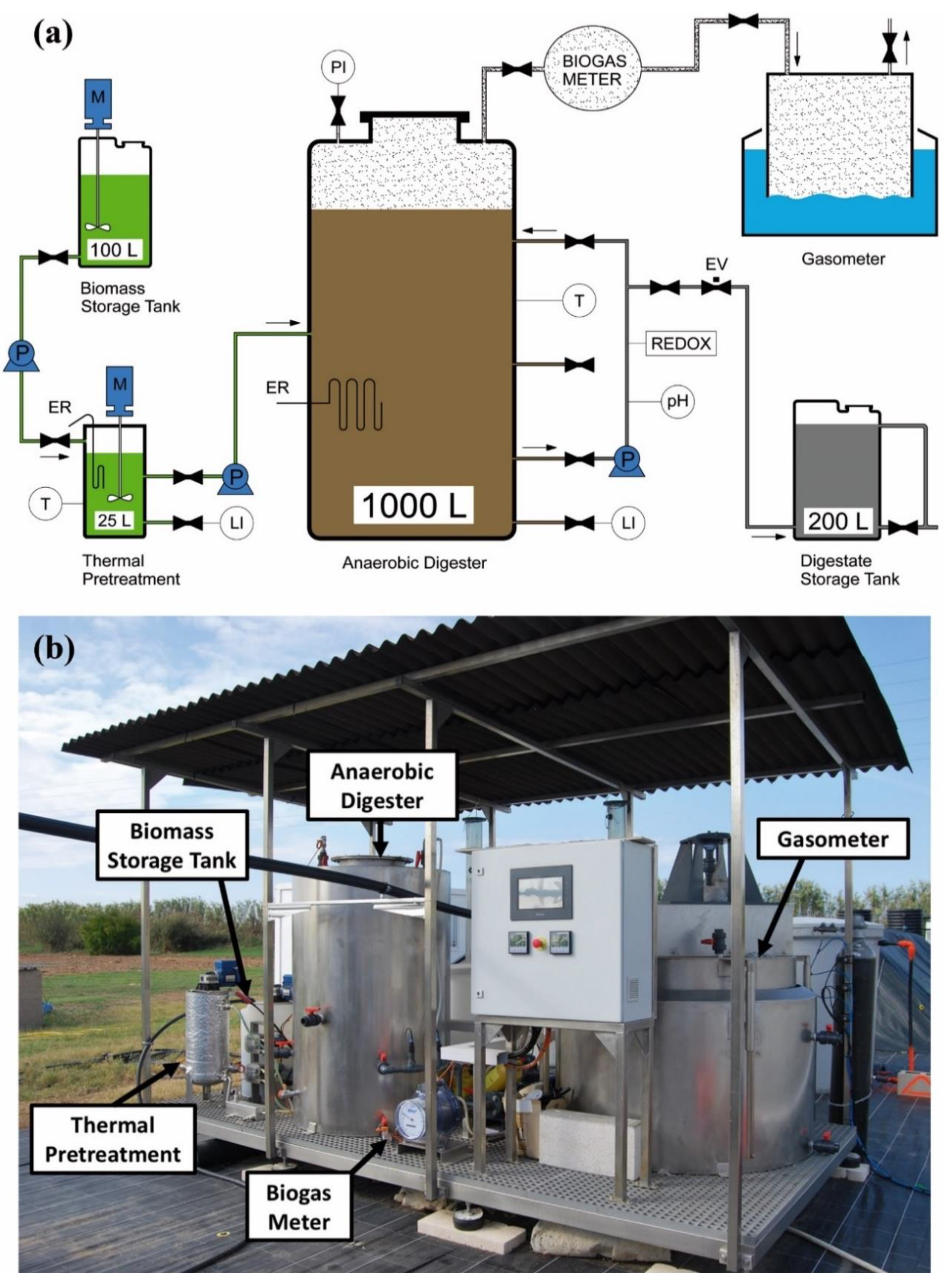
2.1. Demonstration-Scale Set-Up
2.1.1. Microalgal Biomass Production and Harvesting
2.1.2. Thermal Pretreatment and Anaerobic Digestion
2.2. Analytical Methods
2.3. Determination of Parameters
3. Results
3.1. Microalgal Biomass Production and Harvesting
3.2. Thermal Pretreatment of Microalgal Biomass
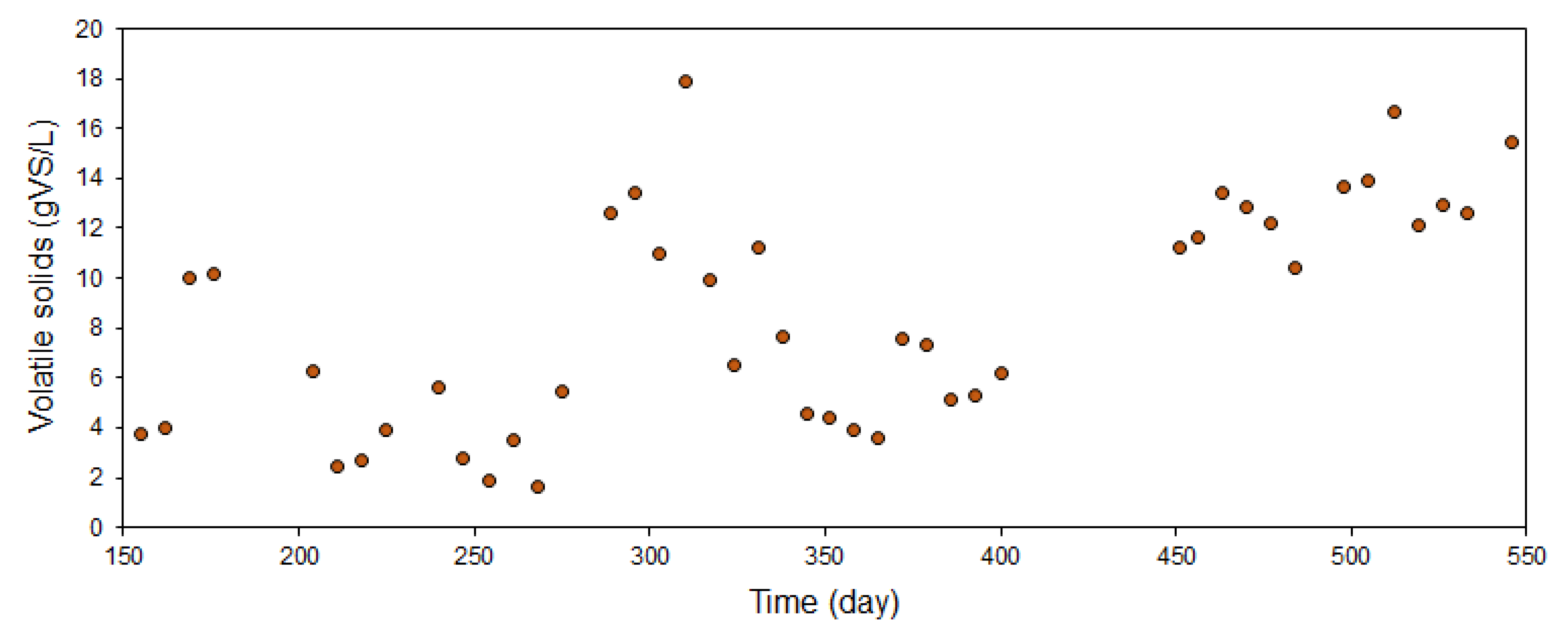
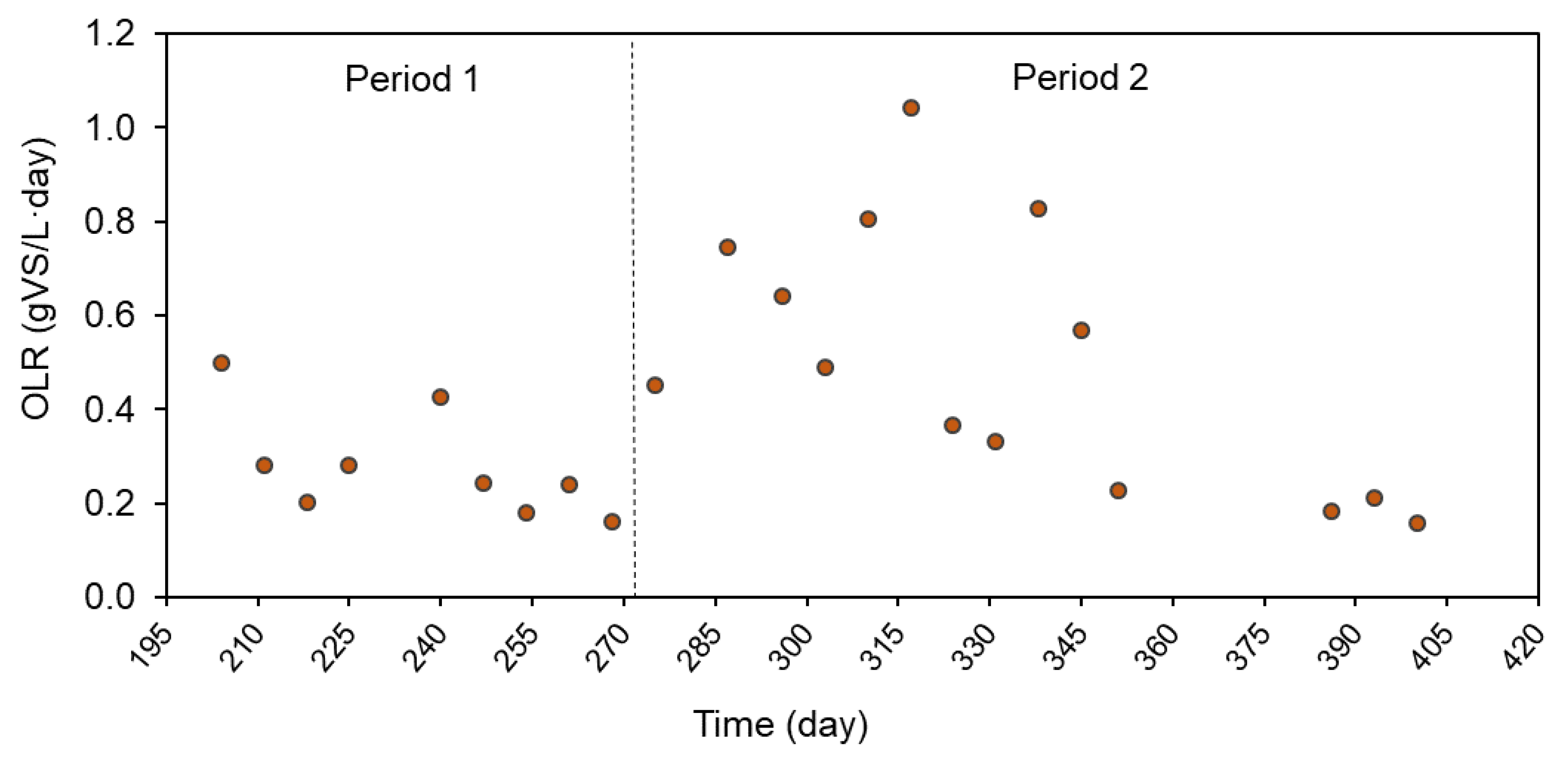
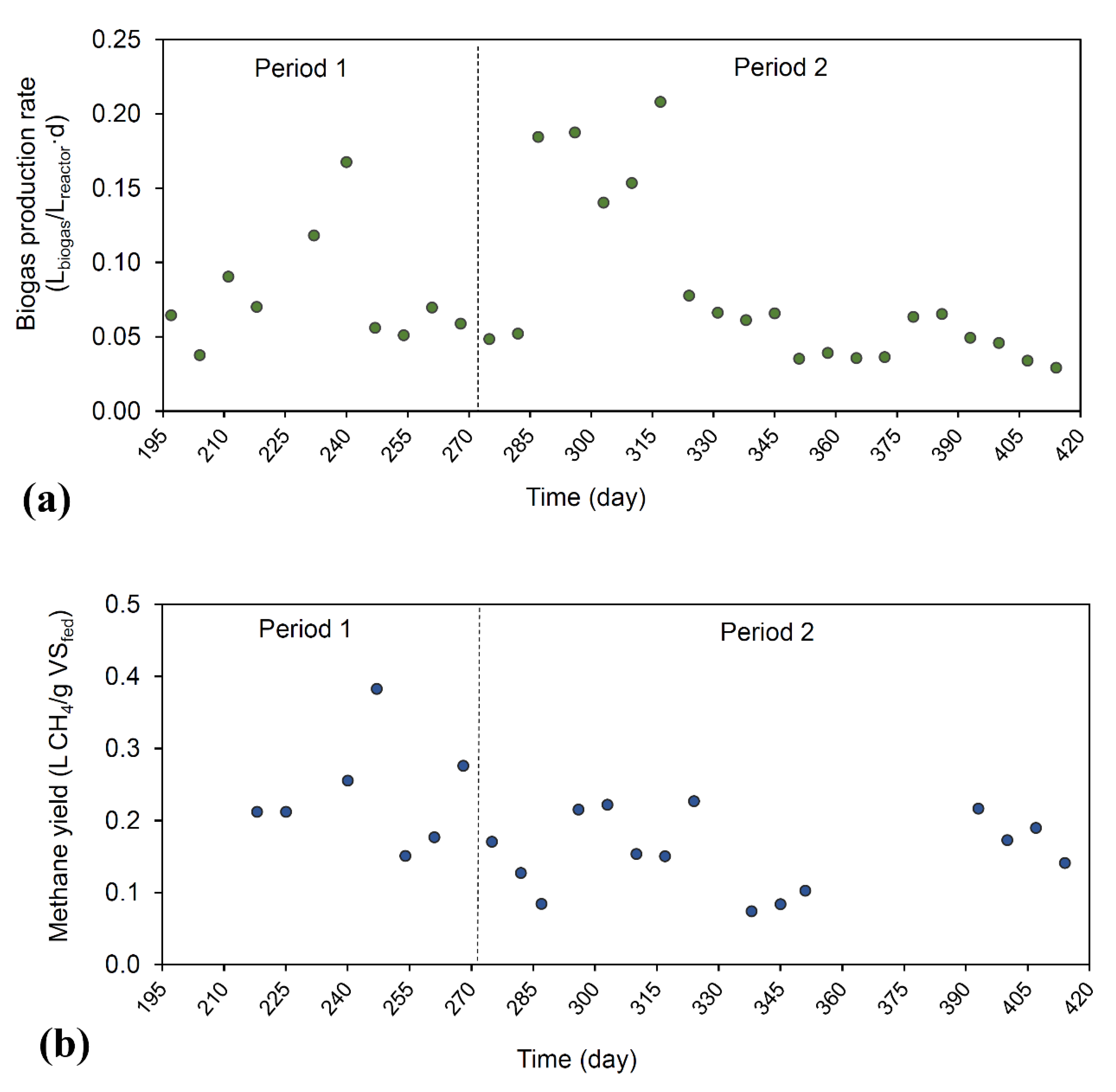
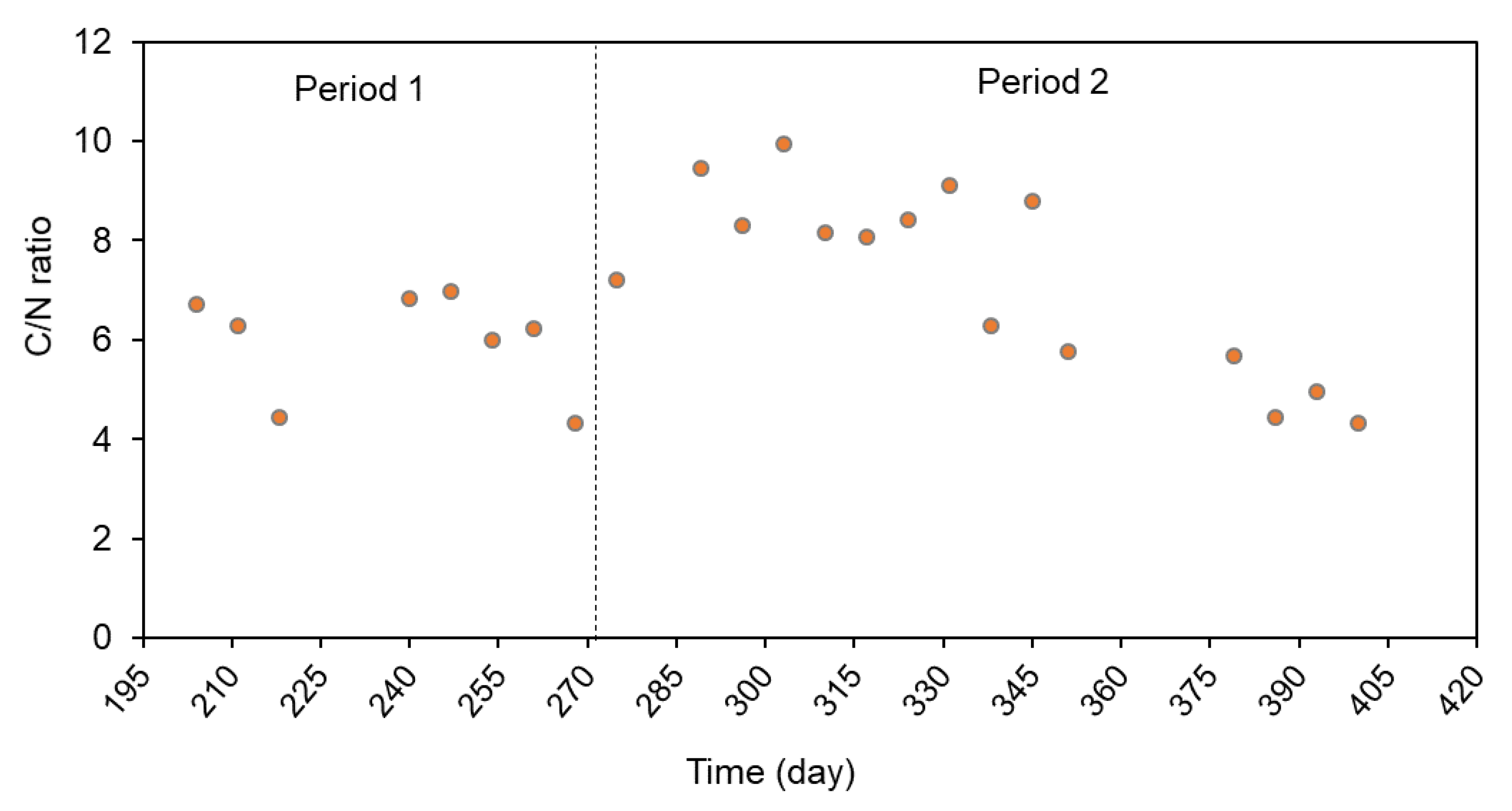
3.3. Anaerobic Digestion Performance and Biogas Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Union. Council Directive 91/271/EEC of 21 May 1991 concerning urban wastewater treatment. Off. J. Eur. Commun. 1991, 135, 40–52. [Google Scholar]
- Union. European Parliament and Council Directive 2000/60/EC of 23 of October 2000 establishing a framework for the Community action in the field of water policy. Off. J. Eur. Commun. 2000, 22, 35–38. [Google Scholar]
- Statistical Office of the European Union (Eurostat). Resident Population Connected to Wastewater Collection and Treatment Systems Reported to the “OECD/Eurostat Joint Questionnaire—Inland Waters-2012”; Statistical Office of the European Union (Eurostat): Luxembourg, 2017. [Google Scholar]
- Lens, P.N.L.; Zeeman, G.; Lettinga, G. Decentralised Sanitation and Reuse–Concepts, Systems and Implementation; IWA Publishing: London, UK, 2001; ISBN 9781900222471. [Google Scholar]
- Von Sperling, M. Comparison among the most frequently used systems for wastewater treatment in developing countries. Water Sci. Technol. 1996, 33, 59–62. [Google Scholar] [CrossRef]
- Von Sperling, M. Wastewater Characteristics, Treatment and Disposal, 1st ed.; IWA Publishing: London, UK, 2007; Volume 1, ISBN 1 84339 161 9. [Google Scholar]
- De Godos, I.; Arbib, Z.; Lara, E.; Rogalla, F. Evaluation of High Rate Algae Ponds for treatment of anaerobically digested wastewater: Effect of CO2 addition and modification of dilution rate. Bioresour. Technol. 2016, 220, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Santiago, A.F.; Calijuri, M.L.; Assemany, P.P.; Calijuri, M.D.C.; Reis, A.J.D. Dos Algal biomass production and wastewater treatment in high rate algal ponds receiving disinfected effluent. Environ. Technol. 2017, 34, 1877–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arashiro, L.T.; Ferrer, I.; Rousseau, D.P.L.; Van Hulle, S.W.H.; Garfí, M. The effect of primary treatment of wastewater in high rate algal pond systems: Biomass and bioenergy recovery. Bioresour. Technol. 2019, 280, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Park, J.B.K.; Craggs, R.J. Algal production in wastewater treatment high rate algal ponds for potential biofuel use. Water Sci. Technol. 2011, 63, 2403–2410. [Google Scholar] [CrossRef]
- Passos, F.; Gutiérrez, R.; Uggetti, E.; Garfí, M.; García, J.; Ferrer, I. Towards energy neutral microalgae-based wastewater treatment plants. Algal Res. 2017, 28, 235–243. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [Green Version]
- Vernès, L.; Li, Y.; Chemat, F.; Abert-Vian, M. Plant Based “Green Chemistry 2.0.”. In Green Chemistry and Sustainable Technology; Li, Y., Chemat, F., Eds.; Green Chemistry and Sustainable Technology; Springer: Singapore, 2019; pp. 15–50. ISBN 978-981-13-3809-0. [Google Scholar]
- Arashiro, L.T.; Ferrer, I.; Pániker, C.C.; Gómez-Pinchetti, J.L.; Rousseau, D.P.L.; Van Hulle, S.W.H.; Garfí, M. Natural pigments and biogas recovery from microalgae grown in wastewater. ACS Sust. Chem. Eng. 2020, 8, 10691–10701. [Google Scholar] [CrossRef]
- Jankowska, E.; Sahu, A.K.; Oleskowicz-Popiel, P. Biogas from microalgae: Review on microalgae’ s cultivation, harvesting and pretreatment for anaerobic digestion. Renew. Sust. Energy Rev. 2017, 75, 692–709. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 1–21. [Google Scholar] [CrossRef]
- Marín, D.; Ortíz, A.; Díez-Montero, R.; Uggetti, E.; García, J.; Lebrero, R.; Muñoz, R. Influence of liquid-to-biogas ratio and alkalinity on the biogas upgrading performance in a demo scale algal-bacterial photobioreactor. Bioresour. Technol. 2019, 280, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Collet, P.; Lardon, L.; Hélias, A.; Steyer, J.-P.; Bernard, O. Life-Cycle Assessment of Microalgal-Based Biofuel, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444641922. [Google Scholar]
- Wang, M.; Park, C. Investigation of anaerobic digestion of Chlorella sp. and Micractinium sp. grown in high-nitrogen wastewater and their co-digestion with waste activated sludge. Biomass Bioenergy 2015, 80, 30–37. [Google Scholar] [CrossRef]
- Vassalle, L.; Díez-Montero, R.; Machado, A.T.R.; Moreira, C.; Ferrer, I.; Mota, C.R.; Passos, F. Upflow anaerobic sludge blanket in microalgae-based sewage treatment: Co-digestion for improving biogas production. Bioresour. Technol. 2020, 300, 9. [Google Scholar] [CrossRef] [PubMed]
- Díez-Montero, R.; Solimeno, A.; Uggetti, E.; García-Galán, M.J.; García, J. Feasibility assessment of energy-neutral microalgae-based wastewater treatment plants under Spanish climatic conditions. Process. Saf. Environ. Prot. 2018, 119, 242–252. [Google Scholar] [CrossRef]
- Acien, F.G.; Sevilla, J.M.F.; Grima, E.M. Microalgae: The Basis of Mankind Sustainability. In Case Study of Innovative Projects–Successful Real Cases; InTech: London, UK, 2017; Volume 1, p. 17. [Google Scholar]
- García-Galán, M.J.; Gutiérrez, R.; Uggetti, E.; Matamoros, V.; García, J.; Ferrer, I. Use of full-scale hybrid horizontal tubular photobioreactors to process agricultural runoff. Biosyst. Eng. 2018, 166, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Passos, F.; Uggetti, E.; Carrère, H.; Ferrer, I. Pretreatment of microalgae to improve biogas production: A review. Bioresour. Technol. 2014, 172, 403–412. [Google Scholar] [CrossRef]
- Ehimen, E.A.; Holm-Nielsen, J.B.; Poulsen, M.; Boelsmand, J.E. Influence of different pre-treatment routes on the anaerobic digestion of a filamentous algae. Renew. Energy 2013, 50, 476–480. [Google Scholar] [CrossRef]
- Passos, F.; Ferrer, I. Microalgae conversion to biogas: Thermal pretreatment contribution on net energy production. Environ. Sci. Technol. 2014, 48, 7171–7178. [Google Scholar] [CrossRef] [PubMed]
- Solé-Bundó, M.; Salvadó, H.; Passos, F.; Garfí, M.; Ferrer, I. Strategies to optimize microalgae conversion to biogas: Co-digestion, pretreatment and hydraulic retention time. Molecules 2018, 23, 2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavinato, C.; Ugurlu, A.; de Godos, I.; Kendir, E.; Gonzalez-Fernandez, C. Biogas Production from Microalgae; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081010273. [Google Scholar]
- García, J.; Ortiz, A.; Álvarez, E.; Belohlav, V.; García-Galán, M.J.; Díez-Montero, R.; Álvarez, J.A.; Uggetti, E. Nutrient removal from agricultural run-off in demonstrative full scale tubular photobioreactors for microalgae growth. Ecol. Eng. 2018, 120, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Rueda, E.; García-Galán, M.J.; Díez-Montero, R.; Vila, J.; Grifoll, M.; García, J. Polyhydroxybutyrate and glycogen production in photobioreactors inoculated with wastewater borne cyanobacteria monocultures. Bioresour. Technol. 2020, 295, 122233. [Google Scholar] [CrossRef] [PubMed]
- Uggetti, E.; García, J.; Álvarez, J.A.; García-Galán, M.J. Start-up of a microalgae-based treatment system within the biorefinery concept: From wastewater to bioproducts. Water Sci. Technol. 2018, 78, 114–124. [Google Scholar] [CrossRef]
- Passos, F.; García, J.; Ferrer, I. Impact of low temperature pretreatment on the anaerobic digestion of microalgal biomass. Bioresour. Technol. 2013, 138, 79–86. [Google Scholar] [CrossRef]
- Ras, M.; Lardon, L.; Bruno, S.; Bernet, N.; Steyer, J.P. Experimental study on a coupled process of production and anaerobic digestion of Chlorella vulgaris. Bioresour. Technol. 2011, 102, 200–206. [Google Scholar] [CrossRef]
- APHA-AWWA-WEF. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Clesceri, L.S., Eds.; The American Water Works Association; The American Public Health Association; The Water Environment Federation: Washington, DC, USA, 2012; ISBN 9780875530130. [Google Scholar]
- Solórzano, L. Determination of ammonia in natural seawater by the phenol-hypochlorite method. Limnol. Oceanogr. 1969, 14, 799–801. [Google Scholar] [CrossRef]
- Streble, H.; Krauter, D. Atlas de los Microorganismos de Agua Dulce, La Vida en Una Gota de Agua; Omega: Barcelona, Spain, 1987; ISBN 842820800X 9788428208000. [Google Scholar]
- Palmer, C.M. Algas en Abastecimientos de Agua: Manual Ilustrado Acerca de la Identificación, Importancia y Control de las Algas en los Abastecimientos de Agua; Interamericana: Ciudad de México, Mexico, 1962. [Google Scholar]
- Díez-Montero, R.; Belohlav, V.; Ortiz, A.; Uggetti, E.; García-Galán, M.J.; García, J. Evaluation of daily and seasonal variations in a semi-closed photobioreactor for microalgae-based bioremediation of agricultural runoff at full-scale. Algal Res. 2020, 47, 101859. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse, 4th ed.; Metcalf & Eddy, McGraw-Hill Education: New York, NY, USA, 2003; ISBN 9780070418783. [Google Scholar]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Thermal pretreatment to improve methane production of Scenedesmus biomass. Biomass Bioenergy 2012, 40, 105–111. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Passos, F.; Romero-Güiza, M.S.; Ferrer, I.; Astals, S. Co-digestion strategies to enhance microalgae anaerobic digestion: A review. Renew. Sust. Energy Rev. 2019, 112, 471–482. [Google Scholar] [CrossRef]
- McCarty, P.L. Anaerobic Waste Treatment Fundamentals. Public Work 1964, 95, 91–94. [Google Scholar]
- Sutherland, D.L.; Park, J.; Heubeck, S.; Ralph, P.J.; Craggs, R.J. Size matters—Microalgae production and nutrient removal in wastewater treatment high rate algal ponds of three different sizes. Algal Res. 2020, 45, 101734. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from microalgae: Technologies, challenges and opportunities. Renew. Sust. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Chow, S.; Ketter, B.; Fung Shek, C.; Yacar, D.; Tang, Y.; Zivojnovich, M.; Betenbaugh, M.J.; Bouwer, E.J. Phytoremediation of agriculture runoff by filamentous algae poly-culture for biomethane production, and nutrient recovery for secondary cultivation of lipid generating microalgae. Bioresour. Technol. 2016, 222, 294–308. [Google Scholar] [CrossRef] [PubMed]


| Average | SD | |
|---|---|---|
| HRT (h) | 20.0 | 4.5 |
| Influent | ||
| Soluble COD before (mg/L) | 456 | 265 |
| VS/TS | 0.47 | 0.08 |
| Effluent | ||
| Soluble COD after (mg/L) | 2625 | 1262 |
| VS/TS | 0.45 | 0.08 |
| Parameter | Laboratory Scale * | Demonstration-Scale (Period 1) | Laboratory Scale ** | Demonstration-Scale (Period 2) |
|---|---|---|---|---|
| Operational conditions | ||||
| Thermal pre-treatment HRT (h) | 10 | 21.3 (0.0) | 10 | 21.7 (5.6) |
| Anaerobic digester HRT (days) | 20 | 20 (0) | 30 | 32 (10) |
| OLR (g VS/L·day) | 0.68 (0.10) | 0.28 (0.11) | 0.81 (0.02) | 0.50 (0.28) |
| Influent composition | ||||
| VS (g/L) | 11.2 (1.40) | 6.4 (0.7) | 23.7 (1.00) | 18.1 (7.2) |
| TS (g/L) | 21.1 (3.10) | 16.6 (1.7) | 34.2 (2.80) | 36.1 (15.4) |
| COD (g/L) | 11.84 (0.71) | 9.04 (0.98) | 25.2 (1.8) | 20.92 (11.98) |
| N-NH4 (mg/L) | 218 (9.54) | 156 (120) | 260 (6.00) | 312 (300) |
| Effluent composition | ||||
| VS (g/L) | 9.50 (1.0) | 1.8 (1.2) | 14.5 (1.10) | 9.9 (5.5) |
| TS (g/L) | 19.80 (2.70) | 4.6 (3.1) | 26.7 (2.70) | 23.4 (13.2) |
| COD (g/L) | 10.6 (0.5) | 11.3 (8.9) | 25.2 (2.1) | 14.7 (10.1) |
| N-NH4 (mg/L) | 323 (17.15) | 458 (250) | 8.0 (1.0) | 456 (310) |
| Anaerobic digester pH | 7.6 (0.4) | 7.0 (0.2) | 7.55 (0.08) | 7.4 (0.1) |
| VFA (mg COD/L) | 150 (58.6) | - | 130 (<596 1) | - |
| Anaerobic digestion performance | ||||
| VS removal (%) | 52.3 (3.8) | 70.0 (23.6) | 39.5 (3.7) | 45.7 (18.0) |
| Methane production rate (L CH4/L·day) | 0.20 (0.10) | 0.072 (0.035) | 0.19 (0.07) | 0.064 (0.053) |
| Methane yield (L CH4/g VS) | 0.30 (0.09) | 0.24 (0.08) | 0.24 (0.07) | 0.16 (0.05) |
| Methane content in biogas (%) | 68.1 (0.6) | 76.7 (0.0) | 69.5 (1.7) | 76.8 (2.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez-Montero, R.; Vassalle, L.; Passos, F.; Ortiz, A.; García-Galán, M.J.; García, J.; Ferrer, I. Scaling-Up the Anaerobic Digestion of Pretreated Microalgal Biomass within a Water Resource Recovery Facility. Energies 2020, 13, 5484. https://doi.org/10.3390/en13205484
Díez-Montero R, Vassalle L, Passos F, Ortiz A, García-Galán MJ, García J, Ferrer I. Scaling-Up the Anaerobic Digestion of Pretreated Microalgal Biomass within a Water Resource Recovery Facility. Energies. 2020; 13(20):5484. https://doi.org/10.3390/en13205484
Chicago/Turabian StyleDíez-Montero, Rubén, Lucas Vassalle, Fabiana Passos, Antonio Ortiz, María Jesús García-Galán, Joan García, and Ivet Ferrer. 2020. "Scaling-Up the Anaerobic Digestion of Pretreated Microalgal Biomass within a Water Resource Recovery Facility" Energies 13, no. 20: 5484. https://doi.org/10.3390/en13205484