Development of Greener D-Metal Inorganic Crosslinkers for Polymeric Gels Used in Water Control in Oil and Gas Applications
Abstract
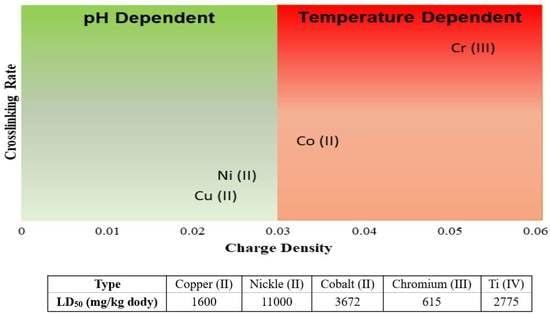
:1. Introduction
2. Gelation Mechanism of Inorganic Crosslinkers
3. Experimental Work
3.1. Materials and Preparation Steps
3.2. Characterization
3.3. Rheology Measurements
4. Results and Discussion
4.1. Rheological Behavior and Viscoelastic Properties of the Inorganic Gels
4.2. Investigation of the Effect of Temperature on Gel Strength
4.3. Effect of PH
4.4. Gel Compatibility with Additives
4.5. Environmental Impact of Inorganic Crosslinkers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elsharafi, M.O.; Bai, B. Influence of strong preformed particle gels on low permeable formations in mature reservoirs. Pet. Sci. 2016, 13, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Hamza, A.; Shamlooh, M.; Hussein, I.A.; Nasser, M.; Salehi, S. Polymeric formulations used for loss circulation materials and wellbore strengthening applications in oil and gas wells: A review. J. Pet. Sci. Eng. 2019, 180, 197–214. [Google Scholar] [CrossRef]
- Bai, Y.; Xiong, C.; Wei, F.; Li, J.; Shu, Y.; Liu, D. Gelation study on a hydrophobically associating polymer/polyethylenimine gel system for water shut-off treatment. Energy Fuels 2015, 29, 447–458. [Google Scholar] [CrossRef]
- Bai, Y.; Wei, F.; Xiong, C.; Li, J.; Jiang, R.; Xu, H.; Shu, Y. Effects of fracture and matrix on propagation behavior and water shut-off performance of a polymer gel. Energy Fuels 2015, 29, 5534–5543. [Google Scholar] [CrossRef]
- Li, T.; Fang, J.; Jiao, B.; He, L.; Dai, C.; You, Q. Study on a novel gelled foam for conformance control in high temperature and high salinity reservoirs. Energies 2018, 11, 1364. [Google Scholar] [CrossRef] [Green Version]
- Bkour, Q.; Faqir, N.; Shawabkeh, R.; Ul-Hamid, A.; Bart, H.J. Synthesis of a Ca/Na-aluminosilicate from kaolin and limestone and its use for adsorption of CO2. J. Environ. Chem. Eng. 2016, 4, 973–983. [Google Scholar] [CrossRef]
- El-Karsani, K.S.M.; Al-Muntasheri, G.A.; Sultan, A.S.; Hussein, I.A. Gelation of a water-shutoff gel at high pressure and high temperature: Rheological investigation. SPE J. 2015, 20, 1103–1112. [Google Scholar] [CrossRef]
- Godwin Uranta, K.; Rezaei-Gomari, S.; Russell, P.; Hamad, F. Studying the effectiveness of Polyacrylamide (PAM) Application in hydrocarbon reservoirs at different operational conditions. Energies 2018, 11, 2201. [Google Scholar] [CrossRef] [Green Version]
- Raghav Chaturvedi, K.; Kumar, R.; Trivedi, J.; Sheng, J.J.; Sharma, T. Stable silica nanofluids of an oilfield polymer for enhanced CO2 absorption for oilfield applications. Energy Fuels 2018, 32, 12730–12741. [Google Scholar] [CrossRef]
- Azad, M.S.; Dalsania, Y.K.; Trivedi, J.J. Capillary breakup extensional rheometry of associative and hydrolyzed polyacrylamide polymers for oil recovery applications. J. Appl. Polym. Sci. 2018, 135, 46253. [Google Scholar] [CrossRef]
- Zhu, D.; Hou, J.; Chen, Y.; Wei, Q.; Zhao, S.; Bai, B. Evaluation of Terpolymer-Gel Systems crosslinked by polyethylenimine for conformance improvement in high-temperature reservoirs. SPE J. 2019, 24, 1726–1740. [Google Scholar] [CrossRef]
- Hughes, A.E. The Jahn–Teller effect in molecules and crystals by R. Englman. Acta Crystallogr. Sect. A 1973, 29, 108–109. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Huang, R. Study on gelation of partially hydrolyzed polyacrylamide with titanium(IV). Eur. Polym. J. 2001, 37, 1553–1559. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Shiyou Kantan Yu Kaifa Pet. Explor. Dev. 2015, 42, 481–487. [Google Scholar] [CrossRef]
- Cordova, M.; Cheng, M.; Trejo, J.; Johnson, S.J.; Willhite, G.P.; Liang, J.T.; Berkland, C. Delayed HPAM gelation via transient sequestration of chromium in polyelectrolyte complex nanoparticles. Macromolecules 2008, 41, 4398–4404. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, B.; Hou, J. Polymer Gel Systems for water management in high-temperature petroleum reservoirs: A chemical review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Tessarolli, F.G.C.; Gomes, A.S.; Mansur, C.R.E. Hydrogels applied for conformance-improvement treatment of oil reservoirs. In Hydrogels; InTech: London, UK, 2018; Available online: https://www.intechopen.com/books/hydrogels/hydrogels-applied-for-conformance-improvement-treatment-of-oil-reservoirs (accessed on 12 July 2020).
- Li, H.; Yang, P.; Pageni, P.; Tang, C. Recent advances in metal-containing polymer hydrogels. Macromol. Rapid Commun. 2017, 38, 1700109. [Google Scholar] [CrossRef]
- Bhatt, V. Essentials of Coordination Chemistry: A Simplified Approach with 3D Visuals; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780128039373. [Google Scholar]
- Irving, H.; Williams, R.J.P. The stability of transition-metal complexes. J. Chem. Soc. 1953, 3192–3210. [Google Scholar] [CrossRef]
- Weller, M.; Overton, T.; Rourke, J.; Armstrong, F.; Atkins, P. Inorganic Chemistry, 6th ed.; Oxford University Press: Oxford, UK, 2014; ISBN 0198757174. [Google Scholar]
- Atkins, P.W. Shriver & Atkins’ Inorganic Chemistry; Oxford University Press: Oxford, UK, 2010; ISBN 0199236178. [Google Scholar]
- Shen, C.H.; Hsieh, B.Z.; Tseng, C.C.; Chen, T.L. Case study of CO2-IGR and storage in a nearly depleted gas-condensate reservoir in Taiwan. Energy Procedia 2014, 63, 7740–7749. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, T.P.; Albonico, P.; Burrafato, G. Slow-gelling Cr3/polyacrylamide solutions for reservoir profile modification: Dependence of the gelation time on pH. J. Appl. Polym. Sci. 1991, 43, 1527–1532. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.A.; Nasr-El-Din, H.A.; Peters, J.A.; Zitha, P.L.J. Investigation of a high-temperature organic water-shutoff gel: Reaction mechanisms. SPE J. 2006, 11, 497–504. [Google Scholar] [CrossRef]
- El Karsani, K.; Al-Muntasheri, G.A.; Sultan, A.S.; Hussein, I.A. Impact of salts on polyacrylamide hydrolysis and gelation: New insights. J. Appl. Polym. Sci. 2014, 131, 41185. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.A.; Sadler, P.J. Advances in Inorganic Chemistry: Inorganic Reaction Mechanisms; van Eldik, R., Hubbard, C.D., Eds.; Academic Press: Cambridge, MA, USA, 2003; ISBN 9780080915807. [Google Scholar]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Sydansk, R.D. A new Conformance-Improvement-Treatment Chromium(III) Gel technology. In Proceedings of the SPE Enhanced Oil Recovery Symposium, Society of Petroleum Engineers, Tulsa, Oklahoma, 16–21 April 1988; pp. 99–111. [Google Scholar]
- Shamlooh, M.; Hamza, A.; Hussein, I.A.; Nasser, M.S.; Magzoub, M.; Salehi, S. Investigation of the rheological properties of nanosilica-reinforced Polyacrylamide/Polyethyleneimine Gels for wellbore strengthening at high reservoir temperatures. Energy Fuels 2019, 33, 6829–6836. [Google Scholar] [CrossRef]
- Albonico, P.; Lockhart, T.P.P. Divalent ion-resistant polymer gels for high-temperature applications: Syneresis inhibiting additives. In Proceedings of the 1993 SPE International Symposium on Oilfield Chemistry, Society of Petroleum Engineers, 1993, Singapore, 8–10 February 1993; pp. 651–665. Available online: https://www.onepetro.org/conference-paper/SPE-25220-MS (accessed on 12 July 2020).
- Lockhart, T.P.; Albonico, P. New chemistry for the placement of chromium(III)/polymer gels in high-temperature reservoirs. SPE Prod. Facil. 1994, 9, 273–279. [Google Scholar] [CrossRef]
- Kangwansupamonkon, W.; Klaikaew, N.; Kiatkamjornwong, S. Green synthesis of titanium dioxide/acrylamide-based hydrogel composite, self degradation and environmental applications. Eur. Polym. J. 2018, 107, 118–131. [Google Scholar] [CrossRef]
- Murugan, R.; Mohan, S.; Bigotto, A. FTIR and polarised raman spectra of acrylamide and polyacrylamide. J. Korean Phys. Soc. 1998, 32, 505. [Google Scholar]
- Jain, R.; Mahto, V. Evaluation of polyacrylamide/clay composite as a potential drilling fluid additive in inhibitive water based drilling fluid system. J. Pet. Sci. Eng. 2015, 133, 612–621. [Google Scholar] [CrossRef]
- Adesina, A.A.; Hussein, I.A. Impact of organoclay and maleated polyethylene on the rheology and instabilities in the extrusion of high density polyethylene. J. Appl. Polym. Sci. 2012, 123, 866–878. [Google Scholar] [CrossRef]
- Mohamed, A.I.A.; Hussein, I.A.; Sultan, A.S.; Al-Muntasheri, G.A. Gelation of Emulsified Polyacrylamide/Polyethylenimine under high-temperature, high-salinity conditions: Rheological investigation. Ind. Eng. Chem. Res. 2018, 57, 12278–12287. [Google Scholar] [CrossRef]
- Najafiazar, B.; Wessel-Berg, D.; Bergmo, P.E.; Simon, C.R.; Yang, J.; Torsæter, O.; Holt, T. Polymer Gels Made with functionalized organo-silica nanomaterials for conformance control. Energies 2019, 12, 3758. [Google Scholar] [CrossRef] [Green Version]
- Lai, N.; Guo, X.; Zhou, N.; Xu, Q. Shear resistance properties of modified Nano-SiO2/AA/AM copolymer oil displacement agent. Energies 2016, 9, 1037. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Wei, L.; Wang, B.; Feng, Y. Aqueous hybrids of silica nanoparticles and hydrophobically associating hydrolyzed polyacrylamide used for eor in high-temperature and high-salinity reservoirs. Energies 2014, 7, 3858–3871. [Google Scholar] [CrossRef] [Green Version]
- Masten, S. Cobalt Dust [7440-48-4] Review of Toxicological Literature. 2002. Available online: https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/cobaltdust_508.pdf (accessed on 12 July 2020).
- Kim, J.H.; Gibb, H.J.; Howe, P.D. Cobalt and inorganic cobalt compounds. IPCS Concise Int. Chem. Assess. Doc. 2006, 1–82. [Google Scholar]
- Henderson, R.G.; Durando, J.; Oller, A.R.; Merkel, D.J.; Marone, P.A.; Bates, H.K. Acute oral toxicity of nickel compounds. Regul. Toxicol. Pharmacol. 2012, 62, 425–432. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M. Handbook on the Toxicology of Metals, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 1, ISBN 9780123973399. [Google Scholar]
- Shaker, A.M.; El-Shahawy, A.; Zaki, A.H.; Abdel-Rahim, E.F.; Khedr, M.H. Estimation The median lethal dose and inhibitory concentration of Tio2, Sio2, Zno and Cuo nanoparticles on human hepatoma HEPG2 Cells. Int. J. Pharm. Phytopharm. Res. 2017, 7, 18–23. [Google Scholar]














| Metal Ion | Electron Configuration | Ionic Radius * (Oh Geometry) in pm | Charge/Radius Ratio (pm−1) |
|---|---|---|---|
| Al (III) | [Ne] | 39.00 (4), 53.00(6) | 3/53.00 = 0.0566 |
| Cr (III) | [Ar]3d3 | 62.00 (6) | 3/62.00 = 0.0484 |
| Co (II) | [Ar]3d7 | 65.00 (6) | 2/65.00 = 0.0308 |
| Ni (II) | [Ar]3d8 | 55.00 (4), 69.00 (6) | 2/69.00 = 0.0289 |
| Cu (II) | [Ar]3d9 | 73.00 (6) | 2/73.00 = 0.0274 |
| Cobalt (II) Concentration, ppm | Sydansk Code | Observation |
|---|---|---|
| 330 | A | No gel was detected at low or high temperatures (25–150 °C) |
| 3300 | A | No gel was detected at low or high temperatures (25–130 °C) |
| 10,000 | E-D | The gel started to move when the temperature decreased from 130 °C to 25 °C. |
| 12,000 | F-E | Part of the gel started to move when the temperature decreased from 130 °C to 25 °C. |
| 13,300 | F-E | Part of the gel started to move when the temperature decreased from 130 °C to 25 °C. |
| 16,700 | A | No gel was detected at low or high temperatures (25–130 °C) |
| Wave Number, cm−1 | Assigned Bond | |
|---|---|---|
| 9 wt.% PAM | Mature Gel 9 wt.% PAM/12,000 ppm Co (II) | |
| 3317 | 3262 | Secondary amide N-H stretching |
| 2871 | 2871 | C-H bond stretching |
| 1636 | 1550 | Primary amide C=O stretching |
| 491 | 490 | C-C |
| 475 | 475 | |
| 460 | 460 | O-C-N Bending |
| 444 | 440 | |
| 430 | 428 | |
| 413 | - | |
| Concentration of Cu (II), ppm | pH | Sydansk Code | Observation |
|---|---|---|---|
| 300–14,300 | 5.23 | A | No gel was detected at low and high temperatures. |
| 11,145 | 7 | B-C | Increasing the initial pH of the solution improved the gel strength at room temperature up to class G. |
| 11,145 | 11 | E-G |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nimir, H.I.; Hamza, A.; Hussein, I.A. Development of Greener D-Metal Inorganic Crosslinkers for Polymeric Gels Used in Water Control in Oil and Gas Applications. Energies 2020, 13, 4262. https://doi.org/10.3390/en13164262
Nimir HI, Hamza A, Hussein IA. Development of Greener D-Metal Inorganic Crosslinkers for Polymeric Gels Used in Water Control in Oil and Gas Applications. Energies. 2020; 13(16):4262. https://doi.org/10.3390/en13164262
Chicago/Turabian StyleNimir, Hassan I., Ahmed Hamza, and Ibnelwaleed A. Hussein. 2020. "Development of Greener D-Metal Inorganic Crosslinkers for Polymeric Gels Used in Water Control in Oil and Gas Applications" Energies 13, no. 16: 4262. https://doi.org/10.3390/en13164262