Combustion Characteristics of Hydrochar and Pyrochar Derived from Digested Sewage Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Hydrochar and Pyrochar Production
2.3. Physical and Chemical Characterization of Raw SSL, Hydrochar and Pyrochar
2.4. Thermogravimetric Analysis
2.4.1. Calculation of Combustion Parameters
2.4.2. Calculation of Comprehensive Combustibility Index (S)
- (dw/dt) max = maximum rate of weight loss (wt.%/min)
- (dw/dt) mean = mean rate of weight loss (wt.%/min)
2.4.3. Calculation of Kinetic Parameters
- f (α) = general expression of the reaction mechanism
- n = order of reaction
- α = thermal conversion fraction (Equation (13))
- A = pre-exponential factor (min−1)
- EA = activation energy of the reaction (kJ/mol)
- R = gas constant (8.314 J/(mol K))
- T = temperature (K)
3. Results and Discussion
3.1. Yield of Biochar and Higher Heating Value
3.2. Carbon (daf) and Ash Recovery, Energy Yield
3.3. Proximate and Ultimate Analyses
3.4. The pH of Biochars
3.5. FTIR Analysis
3.6. Combustion Behavior of Raw SSL and Produced Biochars
3.6.1. Thermogravimetric Analysis
3.6.2. Combustion Behavior and Thermal Characteristics
3.6.3. Combustion Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Statistics|Eurostat. Available online: https://ec.europa.eu/eurostat/databrowser/view/ten00030/default/table?lang=en (accessed on 30 March 2020).
- Siebielska, I. Comparison of changes in selected polycyclic aromatic hydrocarbons concentrations during the composting and anaerobic digestion processes of municipal waste and sewage sludge mixtures. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2014, 70, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Ignatowicz, K. The impact of sewage sludge treatment on the content of selected heavy metals and their fractions. Environ. Res. 2017, 156, 19–22. [Google Scholar] [CrossRef]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- German Biogas Market Data. Available online: https://www.biogas.org/edcom/webfvb.nsf/id/EN-German-biogas-market-data (accessed on 30 March 2020).
- Parshetti, G.K.; Liu, Z.; Jain, A.; Srinivasan, M.P.; Balasubramanian, R. Hydrothermal carbonization of sewage sludge for energy production with coal. Fuel 2013, 111, 201–210. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Hydrothermal carbonization of anaerobically digested sludge for solid fuel production and energy recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
- Mock, C.; Lee, H.; Choi, S.; Manovic, V. Flame structures and ignition characteristics of torrefied and raw sewage sludge particles at rapid heating rates. Fuel 2017, 200, 467–480. [Google Scholar] [CrossRef]
- Ma, P.; Yang, J.; Xing, X.; Weihrich, S.; Fan, F.; Zhang, X. Isoconversional kinetics and characteristics of combustion on hydrothermally treated biomass. Renew. Energy 2017, 114, 1069–1076. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis, and Torrefaction. In Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Cao, Z.; Jung, D.; Olszewski, M.P.; Arauzo, P.J.; Kruse, A. Hydrothermal carbonization of biogas digestate: Effect of digestate origin and process conditions. Waste Manag. 2019, 100, 138–150. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Li, A. Hydrothermal carbonization for energy-efficient processing of sewage sludge: A review. Renew. Sustain. Energy Rev. 2019, 108, 423–440. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Liu, G.; Mian, M.M.; Zhou, C.; Sun, M.; Wu, D.; Liu, Y. Co-combustion of industrial coal slurry and sewage sludge: Thermochemical and emission behavior of heavy metals. Chemosphere 2019, 233, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, R.I. An investigation of co-combustion municipal sewage sludge with biomass in a 20kW BFB combustor under air-fired and oxygen-enriched condition. Waste Manag. 2017, 70, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Volpe, M.; Lucian, M.; Fiori, L.; Goldfarb, J.L. Does hydrothermal carbonization as a biomass pretreatment reduce fuel segregation of coal-biomass blends during oxidation? Energy Convers. Manag. 2019, 181, 93–104. [Google Scholar] [CrossRef]
- Arauzo, P.; Olszewski, M.; Kruse, A. Hydrothermal Carbonization Brewer’s Spent Grains with the Focus on Improving the Degradation of the Feedstock. Energies 2018, 11, 3226. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez Correa, C.; Hehr, T.; Voglhuber-Slavinsky, A.; Rauscher, Y.; Kruse, A. Pyrolysis vs. hydrothermal carbonization: Understanding the effect of biomass structural components and inorganic compounds on the char properties. J. Anal. Appl. Pyrolysis 2019, 140, 137–147. [Google Scholar] [CrossRef]
- Mott, R.A.; Spooner, C.E. The calorific value of carbon in coal: The Dulong relationship. Fuel 1940, 19, 242. [Google Scholar]
- Yang, W.; Wang, H.; Zhang, M.; Zhu, J.; Zhou, J.; Wu, S. Fuel properties and combustion kinetics of hydrochar prepared by hydrothermal carbonization of bamboo. Bioresour. Technol. 2016, 205, 199–204. [Google Scholar] [CrossRef]
- Wang, C.A.; Zhang, X.; Liu, Y.; Che, D. Pyrolysis and combustion characteristics of coals in oxyfuel combustion. Appl. Energy 2012, 97, 264–273. [Google Scholar] [CrossRef]
- Olszewski, M.P.; Arauzo, P.J.; Maziarka, P.A.; Ronsse, F.; Kruse, A. Pyrolysis Kinetics of Hydrochars Produced from Brewer’s Spent Grains. Catalysts 2019, 9, 625. [Google Scholar] [CrossRef] [Green Version]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2014, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S.; Guan, Y.; Cai, L. Influence of particle size on pyrolysis and gasification performance of municipal solid waste in a fixed bed reactor. Bioresour. Technol. 2010, 101, 6517–6520. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Correa, C.; Bernardo, M.; Ribeiro, R.P.P.L.; Esteves, I.A.A.C.; Kruse, A. Evaluation of hydrothermal carbonization as a preliminary step for the production of functional materials from biogas digestate. J. Anal. Appl. Pyrolysis 2017, 124, 461–474. [Google Scholar] [CrossRef]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Atienza-Martínez, M.; Mastral, J.F.; Ábrego, J.; Ceamanos, J.; Gea, G. Sewage Sludge Torrefaction in an Auger Reactor. Energy Fuels 2015, 29, 160–170. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Huang, H.; Zhao, D.; Kobayashi, N.; Chen, Y. Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge. J. Anal. Appl. Pyrolysis 2015, 112, 284–289. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Hu, S.; Yu, Z.; Fang, S. Effect of hydrothermal carbonization temperature on combustion behavior of hydrochar fuel from paper sludge. Appl. Therm. Eng. 2015, 91, 574–582. [Google Scholar] [CrossRef]
- Peng, X.; Ye, L.L.; Wang, C.H.; Zhou, H.; Sun, B. Temperature- and duration-dependent rice straw-derived biochar: Characteristics and its effects on soil properties of an Ultisol in southern China. Soil Tillage Res. 2011, 112, 159–166. [Google Scholar] [CrossRef]
- Yan, W.; Acharjee, T.C.; Coronella, C.J.; Vásquez, V.R. Thermal pretreatment of lignocellulosic biomass. Environ. Prog. Sustain. Energy 2009, 28, 435–440. [Google Scholar] [CrossRef]
- Volpe, R.; Volpe, M.; Fiori, L.; Messineo, A. Upgrading of Olive Tree Trimmings Residue as Biofuel by Hydrothermal Carbonization and Torrefaction: A Comparative Study. Chem. Eng. Trans. 2016, 50, 13–18. [Google Scholar] [CrossRef]
- Wüst, D.; Rodriguez Correa, C.; Suwelack, K.U.; Köhler, H.; Kruse, A. Hydrothermal carbonization of dry toilet residues as an added-value strategy – Investigation of process parameters. J. Environ. Manag. 2019, 234, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Wüst, D.; Merzari, F.; Lucian, M.; Andreottola, G.; Kruse, A.; Fiori, L. One stage olive mill waste streams valorisation via hydrothermal carbonisation. Waste Manag. 2018, 80, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lin, Q.; He, R.; Zhao, X.; Li, G. Hydrochar production from watermelon peel by hydrothermal carbonization. Bioresour. Technol. 2017, 241, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Bach, Q.-V.; Tran, K.-Q.; Skreiberg, Ø. Accelerating wet torrefaction rate and ash removal by carbon dioxide addition. Fuel Proc. Technol. 2015, 140, 297–303. [Google Scholar] [CrossRef]
- Ovsyannikova, E.; Arauzo, P.J.; Becker, G.C.; Kruse, A. Experimental and thermodynamic studies of phosphate behavior during the hydrothermal carbonization of sewage sludge. Sci. Total Environ. 2019, 692, 147–156. [Google Scholar] [CrossRef]
- Lang, Q.; Zhang, B.; Li, Y.; Liu, Z.; Jiao, W. Formation and toxicity of polycyclic aromatic hydrocarbons during CaO assisted hydrothermal carbonization of swine manure. Waste Manag. 2019, 100, 84–90. [Google Scholar] [CrossRef]
- Hu, Q.; Yang, H.; Yao, D.; Zhu, D.; Wang, X.; Shao, J.; Chen, H. The densification of bio-char: Effect of pyrolysis temperature on the qualities of pellets. Bioresour. Technol. 2016, 200, 521–527. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.-Y. Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Atienza-Martínez, M.; Fonts, I.; Ábrego, J.; Ceamanos, J.; Gea, G. Sewage sludge torrefaction in a fluidized bed reactor. Chem. Eng. J. 2013, 222, 534–545. [Google Scholar] [CrossRef]
- Sharma, H.B.; Panigrahi, S.; Dubey, B.K. Hydrothermal carbonization of yard waste for solid bio-fuel production: Study on combustion kinetic, energy properties, grindability and flowability of hydrochar. Waste Manag. 2019, 91, 108–119. [Google Scholar] [CrossRef]
- Atienza-Martínez, M.; Ábrego, J.; Gea, G.; Marías, F. Pyrolysis of dairy cattle manure: Evolution of char characteristics. J. Anal. Appl. Pyrolysis 2020, 145, 104724. [Google Scholar] [CrossRef]
- Hammes, K.; Smernik, R.J.; Skjemstad, J.O.; Herzog, A.; Vogt, U.F.; Schmidt, M.W.I. Synthesis and characterisation of laboratory-charred grass straw (Oryza sativa) and chestnut wood (Castanea sativa) as reference materials for black carbon quantification. Org. Geochem. 2006, 37, 1629–1633. [Google Scholar] [CrossRef]
- Méndez, A.; Gascó, G.; Ruiz, B.; Fuente, E. Hydrochars from industrial macroalgae “Gelidium Sesquipedale” biomass wastes. Bioresour. Technol. 2019, 275, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Dieguez-Alonso, A.; Funke, A.; Anca-Couce, A.; Rombolà, A.; Ojeda, G.; Bachmann, J.; Behrendt, F. Towards Biochar and Hydrochar Engineering—Influence of Process Conditions on Surface Physical and Chemical Properties, Thermal Stability, Nutrient Availability, Toxicity and Wettability. Energies 2018, 11, 496. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Zhou, Y.; Meng, F.; Zhang, Y.; Liu, Z.; Zhang, W.; Xue, G. Preparation and characterization of hydrochar from waste eucalyptus bark by hydrothermal carbonization. Energy 2016, 97, 238–245. [Google Scholar] [CrossRef]
- Román, S.; Nabais, J.M.V.; Laginhas, C.; Ledesma, B.; González, J.F. Hydrothermal carbonization as an effective way of densifying the energy content of biomass. Fuel Proc. Technol. 2012, 103, 78–83. [Google Scholar] [CrossRef]
- Olszewski, M.P.; Nicolae, S.A.; Arauzo, P.J.; Titirici, M.-M.; Kruse, A. Wet and dry? Influence of hydrothermal carbonization on the pyrolysis of spent grains. J. Clean. Prod. 2020, 121101. [Google Scholar] [CrossRef]
- Reza, M.T.; Coronella, C.; Holtman, K.M.; Franqui-Villanueva, D.; Poulson, S.R. Hydrothermal Carbonization of Autoclaved Municipal Solid Waste Pulp and Anaerobically Treated Pulp Digestate. ACS Sustain. Chem. Eng. 2016, 4, 3649–3658. [Google Scholar] [CrossRef]
- Gai, C.; Chen, M.; Liu, T.; Peng, N.; Liu, Z. Gasification characteristics of hydrochar and pyrochar derived from sewage sludge. Energy 2016, 113, 957–965. [Google Scholar] [CrossRef]
- He, C.; Wang, K.; Yang, Y.; Amaniampong, P.N.; Wang, J.-Y. Effective nitrogen removal and recovery from dewatered sewage sludge using a novel integrated system of accelerated hydrothermal deamination and air stripping. Environ. Sci. Technol. 2015, 49, 6872–6880. [Google Scholar] [CrossRef]
- Peng, C.; Zhai, Y.; Zhu, Y.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Production of char from sewage sludge employing hydrothermal carbonization: Char properties, combustion behavior and thermal characteristics. Fuel 2016, 176, 110–118. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Boussarsar, H.; Rogé, B.; Mathlouthi, M. Optimization of sugarcane bagasse conversion by hydrothermal treatment for the recovery of xylose. Bioresour. Technol. 2009, 100, 6537–6542. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Díaz, R.M.; Xiberta, J. Pyrolysis of blends of different types of sewage sludge with one bituminous coal. Energy 2005, 30, 1079–1091. [Google Scholar] [CrossRef]
- Inoue, S.; Sawayama, S.; Ogi, T.; Yokoyama, S.-Y. Organic composition of liquidized sewage sludge. Biomass Bioenerg. 1996, 10, 37–40. [Google Scholar] [CrossRef]
- Biagini, E.; Tognotti, L. Comparison of Devolatilization/Char Oxidation and Direct Oxidation of Solid Fuels at Low Heating Rate. Energy Fuels 2006, 20, 986–992. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Ma, Q.; Han, L.; Huang, G. Evaluation of different water-washing treatments effects on wheat straw combustion properties. Bioresour. Technol. 2017, 245, 1075–1083. [Google Scholar] [CrossRef]

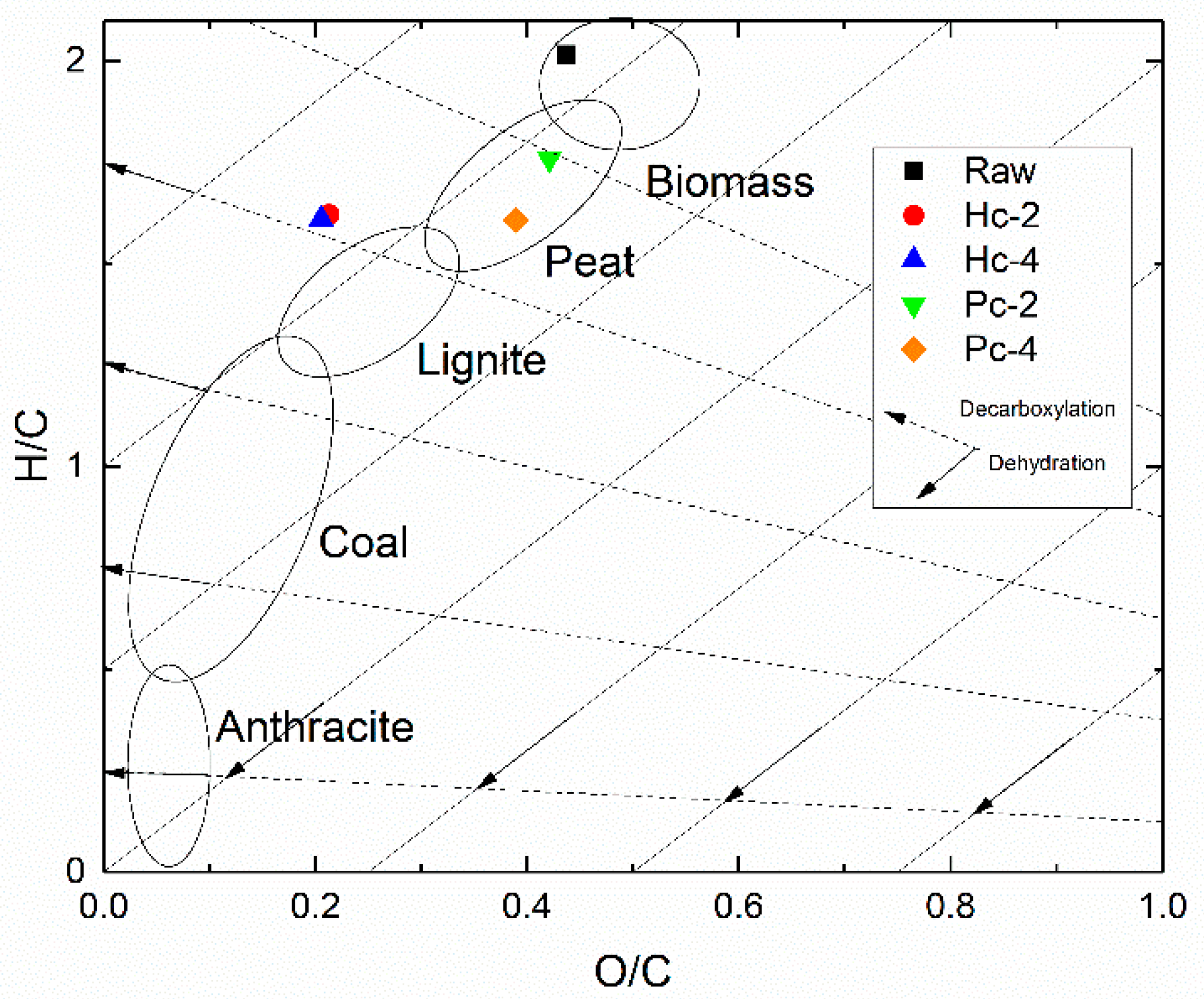
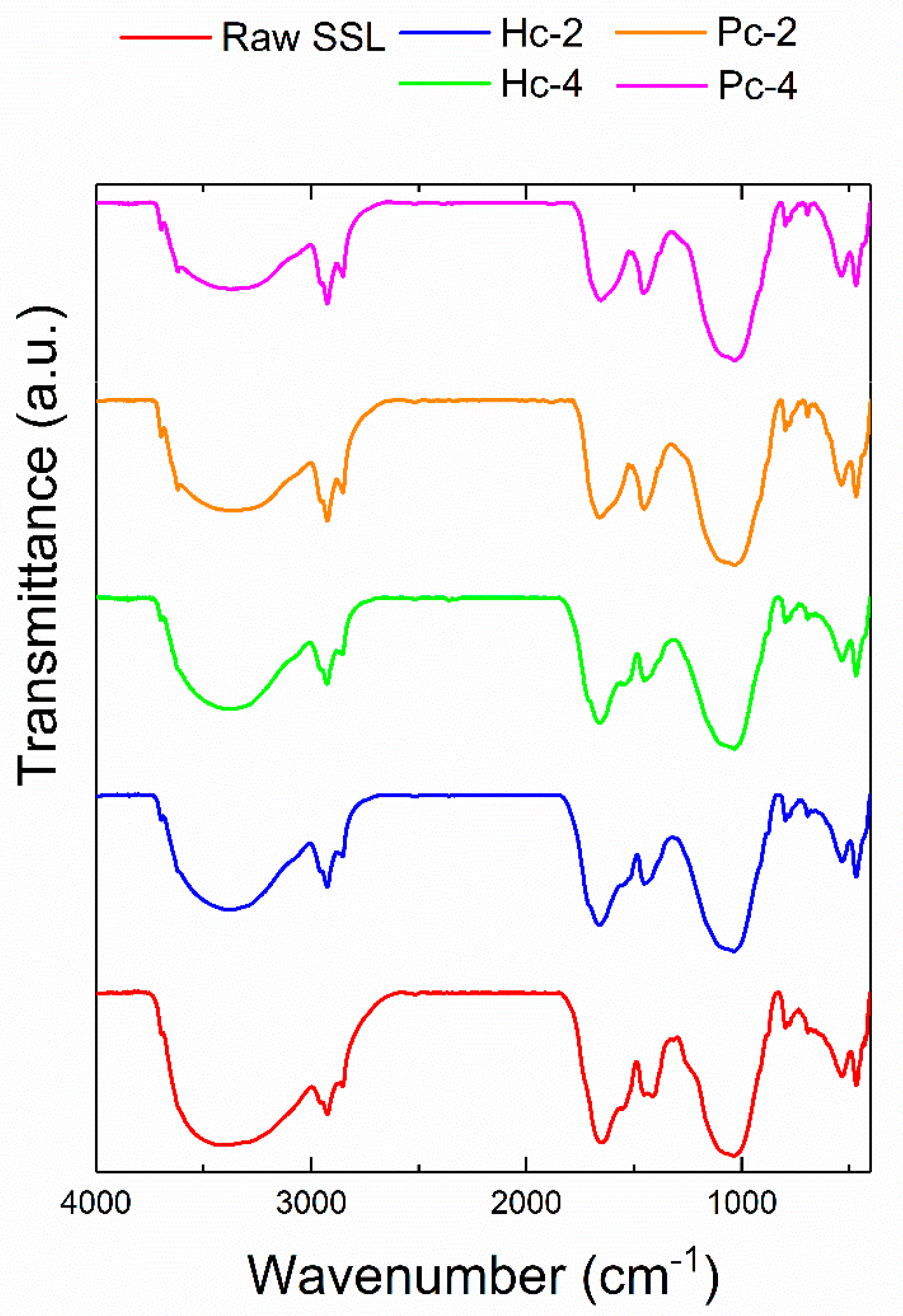

| Material | η Biochar (wt.%) | Ed | FR | HHV (MJ/kg) | HHV (MJ/kg, daf 1) |
|---|---|---|---|---|---|
| Raw SSL | - | - | 0.07 (0.01) | 13.7 (0.2) | 25.9 (0.2) |
| Hc-2 | 71.2 (0.5) | 0.9 (0.1) | 0.34 (0.02) | 12.4 (0.2) | 30.8 (0.2) |
| Hc-4 | 71.8 (0.3) | 0.9 (0.1) | 0.36 (0.03) | 12.6 (0.3) | 31.1 (0.3) |
| Pc-2 | 88.1 (0.3) | 0.9 (0.1) | 0.21 (0.02) | 12.9 (0.2) | 24.1 (0.2) |
| Pc-4 | 88.0 (0.7) | 0.9 (0.1) | 0.25 (0.03) | 12.9 (0.2) | 25.0 (0.24) |
| Material | Proximate Analysis (wt.% db) | Ultimate Analysis (wt.% db) | pH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| VM | Ash | FC | N% | C% | H % | S% | O% | ||
| Raw SSL | 50.0 (2.1) | 46.5 (2.0) | 3.5 (1.9) | 3.9 (0.1) | 27.9 (0.1) | 4.7 (0.1) | 0.8 (0.1) | 16.3 (0.1) | 7.5 (0.1) |
| Hc-2 | 29.7 (1.3) | 60.4 (1.4) | 10.0 (1.7) | 2.6 (0.2) | 25.6 (0.4) | 3.5 (0.3) | 0.7 (0.1) | 7.27 (0.1) | 6.7 (0.1) |
| Hc-4 | 29.3 (0.6) | 60.1 (0.8) | 10.6 (0.9) | 2.6 (0.1) | 25.6 (0.2) | 3.5 (0.2) | 0.7 (0.1) | 7.1 (0.1) | 6.5 (0.1) |
| Pc-2 | 42.3 (0.1) | 49.0 (0.1) | 8.7 (0.1) | 3.7 (0.1) | 27.7 (0.2) | 4.1 (0.1) | 0.7 (0.1) | 15.5 (0.1) | 6.9 (0.1) |
| Pc-4 | 40.8 (1.7) | 49.1 (1.4) | 10.1 (1.4) | 3.8 (0.2) | 28.5 (0.1) | 3.8 (0.1) | 0.7 (0.2) | 14.8 (0.1) | 6.8 (0.1) |
| Air Flow Rate | 20 mL/min | 90 mL/min | ||||||
|---|---|---|---|---|---|---|---|---|
| Material | Ti (°C) | Tm (°C) | Tb (°C) | S (wt. %2/(min2 × °C3)) | Ti (°C) | Tm (°C) | Tb (°C) | S (wt. %2/(min2 × °C3)) |
| Raw SSL | 222.3 | 305.3 | 467.3 | 9.22 × 10−8 | 223.3 | 301.3 | 465.3 | 9.24 × 10−8 |
| Hc-2 | 268.0 | 294.0 | 437.0 | 3.21 × 10−8 | 268.0 | 295.0 | 430.0 | 3.38 × 10−8 |
| Hc-4 | 269.5 | 293.5 | 435.5 | 3.14 × 10−8 | 267.5 | 286.5 | 430.5 | 3.53 × 10−8 |
| Pc-2 | 254.1 | 304.1 | 445.1 | 8.41 × 10−8 | 246.1 | 300.1 | 440.1 | 9.77 × 10−8 |
| Pc-4 | 246.6 | 303.6 | 445.6 | 9.12 × 10−8 | 244.6 | 297.6 | 437.6 | 1.06 × 10−7 |
| Sample | Air Flow Rate (mL/min) | Temperature Range (K) | EA (kJ/mol) | R2 | A (s−1) | TA (K·10−3) |
|---|---|---|---|---|---|---|
| Raw SSL | 20 | 581–624 | 266.28 | 0.96 | 8.37 × 1047 | 32.03 |
| 624–731 | 82.15 | 0.84 | 2.92 × 1014 | 9.88 | ||
| Raw SSL | 90 | 581–622 | 167.92 | 0.85 | 1.84 × 1034 | 20.20 |
| 622–727 | 114.02 | 0.99 | 9.99 × 1024 | 13.71 | ||
| Hc-2 | 20 | 617–660 | 69.78 | 0.97 | 9.68 × 1012 | 8.39 |
| 660–733 | 73.87 | 0.99 | 1.61 × 1015 | 8.89 | ||
| Hc-2 | 90 | 600–643 | 112.31 | 0.95 | 1.27 × 1025 | 13.51 |
| 643–718 | 130.65 | 0.89 | 1.24 × 1032 | 15.71 | ||
| Hc-4 | 20 | 619–661 | 61.99 | 0.93 | 1.27 × 1011 | 7.46 |
| 661–733 | 65.07 | 0.88 | 2.95 × 1012 | 7.83 | ||
| Hc-4 | 90 | 611–651 | 129.68 | 0.89 | 4.42 × 1028 | 15.60 |
| 651–722 | 148.64 | 0.85 | 1.7 × 1037 | 17.88 | ||
| Pc-2 | 20 | 600–638 | 67.89 | 0.95 | 4.48 × 1014 | 8.17 |
| 638–722 | 49.52 | 0.88 | 5.37 × 107 | 5.96 | ||
| Pc-2 | 90 | 593–628 | 83.18 | 0.91 | 1.23 × 1017 | 9.98 |
| 628–709 | 94.17 | 0.96 | 6.66 × 1022 | 11.30 | ||
| Pc-4 | 20 | 597–635 | 83.22 | 0.90 | 1.14 × 1018 | 10.01 |
| 635–720 | 61.05 | 0.96 | 6.17 × 1010 | 7.34 | ||
| Pc-4 | 90 | 589–623 | 117.31 | 0.86 | 8.76 × 1026 | 14.11 |
| 632–707 | 99.96 | 0.86 | 1.32 × 1024 | 12.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arauzo, P.J.; Atienza-Martínez, M.; Ábrego, J.; Olszewski, M.P.; Cao, Z.; Kruse, A. Combustion Characteristics of Hydrochar and Pyrochar Derived from Digested Sewage Sludge. Energies 2020, 13, 4164. https://doi.org/10.3390/en13164164
Arauzo PJ, Atienza-Martínez M, Ábrego J, Olszewski MP, Cao Z, Kruse A. Combustion Characteristics of Hydrochar and Pyrochar Derived from Digested Sewage Sludge. Energies. 2020; 13(16):4164. https://doi.org/10.3390/en13164164
Chicago/Turabian StyleArauzo, Pablo J., María Atienza-Martínez, Javier Ábrego, Maciej P. Olszewski, Zebin Cao, and Andrea Kruse. 2020. "Combustion Characteristics of Hydrochar and Pyrochar Derived from Digested Sewage Sludge" Energies 13, no. 16: 4164. https://doi.org/10.3390/en13164164





