1. Introduction
Extensive use of fossil fuels causes environmental pollution, global warming, and depletion of non-renewable energy resources [
1]. Thus, exploring alternative energy sources can help in the reduction of fossil fuel consumption. Worldwide production of biodiesel increased in the last twenty years; therefore, it is one of the significant bio-based fuels [
2,
3]. Biodiesel is a mixture of fatty acid methyl esters (FAMEs) obtained by the triglycerides transesterification. Its properties, e.g., cetane number, density, flash point, oxidative stability, and viscosity, depend on the structure of its components, especially FAMEs [
4]. On the other hand, the properties of an individual fatty acid depend on the chain length, branched chains, and double bonds [
5]. For instance, the low-temperature fluidity decreases with chain length increase, but the calorific value of biodiesel increases. Moreover, the longer the chain length, the higher the fuel viscosity, but
cis double bonds lower the viscosity. All biodiesel samples contain saturated (SFAs), monounsaturated (MUFAs), and polyunsaturated (PUFAs) fatty acids. However, an ideal biodiesel composition should contain mainly MUFAs, and should have fewer PUFAs and SFAs [
6,
7].
The first-generation (conventional) biofuels mainly come from soybean, vegetable oils (e.g., palm [
8], oil [
9], and rapeseed oils [
10]), and algae [
11]. It has wildly reported that biodiesel is environmentally friendly and can help in the reduction of greenhouse gases [
12]. At low blending ratios, biodiesel can be injected into diesel engines without any modification of the engine itself. Unfortunately, bio-based fuel also has some disadvantages [
13]. It can cause corrosion of engine parts, carbon deposition, injector coking, and oxidation, due to the presence of higher unsaturated fatty acids (UNFAs). Research also indicated that some of the biodiesel types showed lower oxidative stability and therefore decreased storage period [
14]. One of the ways is a production of liquid fuel from industrial wastes, including leather industry wastes [
15], oil from fish canning industry wastes [
16], agro-industrial wastes [
17], and food processing wastes [
18]. In this study, for the first time, the herbal industry wastes as a potential feedstock for bio-based fuel production were proposed.
In Poland, many specialized farms related to the cultivation of specific herb species on the acreage above 20 ha do exist. The total area of herb plantation in the country covers over 30,000 ha [
19]. The herbal industries also produce wastes. They are generated in the herbal production process, mainly during packing. The amount of wastes is relatively large, but its utilization is problematic due to the tiny particle sizes of waste fractions [
20]. For example, approximately 30 tons of fine wastes biomass are produced in “Herbapol-Lublin” S.A. (Branch in Bialystok, Poland) annually during this process [
21]. These wastes are usually sold by the company for small sums or even given away for the price of transport [
22]. This is important because the raw materials of biodiesel have a high manufacturing cost due to the complexity of the purification process. Generally, biodiesel production with edible oils is more expensive than using non-edible oils [
23].
The postproduction herbal wastes are currently used in the biogas [
24], pellets, and briquettes production [
25], as well as bioconversion into vermicompost [
26] and food wastes composting [
27]. However, the most common method of herbal wastes utilization is using them as a fodder additive [
28]. Therefore, the research aimed to:
quantify the content of FAMEs from herbal industry wastes and determine dominative ones;
compare different herbal waste to determine the most suitable for biofuel production;
estimate cetane number (CN) for different herbal wastes based on FAMEs content;
compare fatty acid composition in herbal wastes with previously published data from other plant materials.
3. Results and Discussion
Gas chromatography-mass spectrometry in the selected ion monitoring mode (GC-MS/SIM) of herbal industry wastes quantified the level of up to thirty-one FAMEs out of thirty-seven analyzed (
Table 2,
Table 3 and
Table 4). As shown in
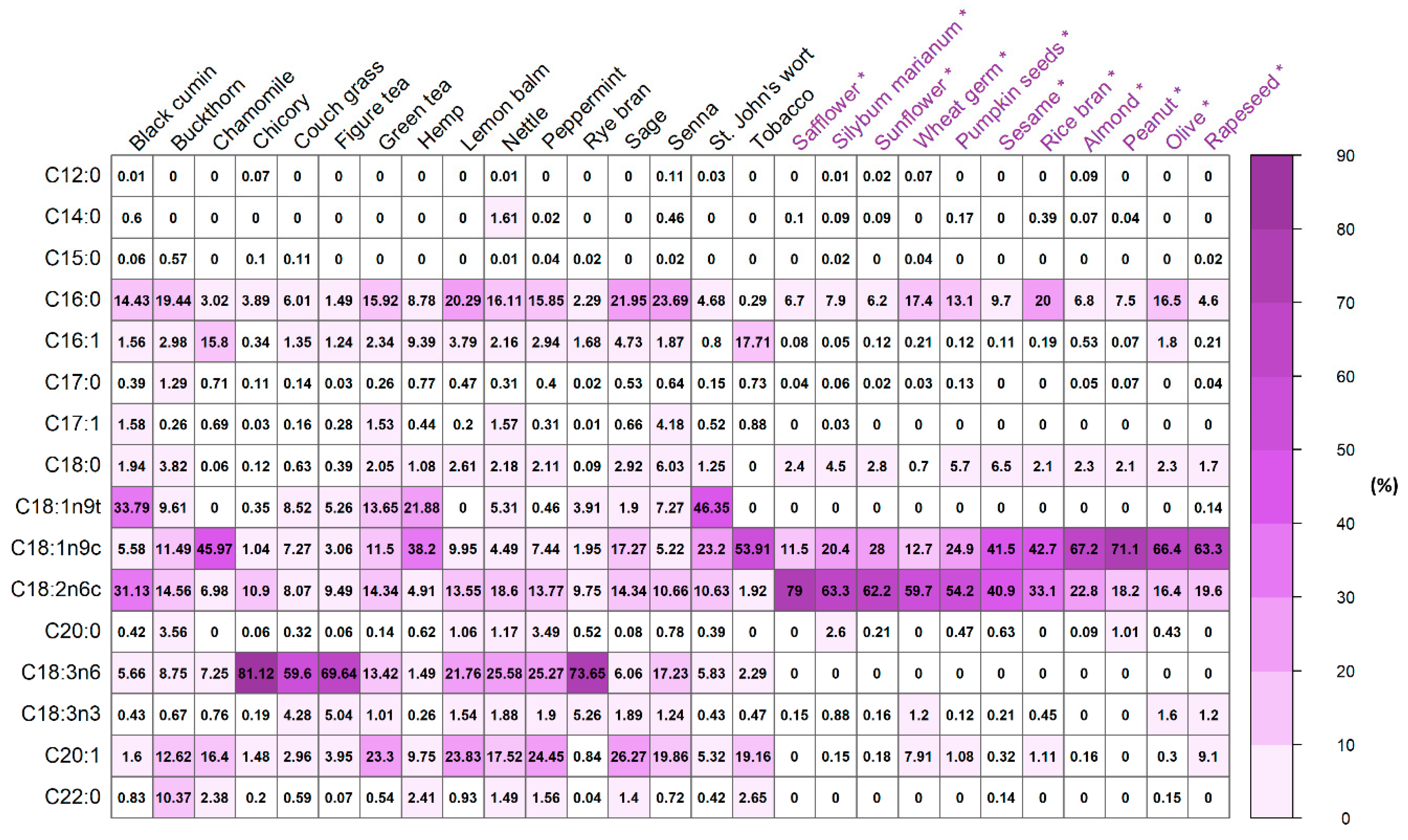
Figure 1, the qualitative differentiation in fatty acids types in plant materials was found. The total amount of different fatty acids types ranged from 20 to 31 — nettle and senna had 31, while chamomile had 20. The composition of SFAs is varied the most in comparison to MUFAs and PUFAs. Among herbal wastes, tobacco had only four SFAs (C16:0, C17:0, C22:0, and C23:0), while chicory, nettle, and senna were the richest in SFAs, having all twelve (
Table 4). Most of the herbal wastes had nine or ten PUFAs, which presence may suggest great oxidative stability of biodiesel like in algal oil biofuel [
44]. The susceptibility to oxidation of the double bonds during storage reduces the acceptability of microalgal oil for production of biodiesel [
45].
The analysis of the composition of biodiesel produced from these samples revealed that the USFA methyl esters amount (97.06% in rye bran) was higher than that of SFAs (
Figure 2). The overall share of SFAs was the lowest among all herbal wastes except for buckthorn (51.36%). MUFAs and PUFAs were the dominant fatty acids types; however, various herbal wastes had different MUFAs to PUFAs ratio. The highest MUFAs share was in hemp (66.77%,) the lowest in chicory (5.14%); the highest PUFAs level was in chicory (90.33), the lowest in buckthorn (19.99%). The dominant FAMEs were C18 fatty acids, i.e., C18:3n6, C18:1n9t/c, and C18:2n6, as well as C16:0 (
Figure 3), which are suitable for fuel production. The richest in C18:3n6 (> 50%) herbal wastes were couch grass, Figure tea, chicory, and rye bran. C15:1, C16:0, C20:1, C20:5n3, C22:2n6, C24:0 were also reported to occur in a significant amount, having the percentage share > 5% among many different herbal wastes. The only exception was the presence of C20:3n6, C22:1n9, and C20:4n6 in sage (13.84–35.88%). The other FAMEs, mostly SFA type, had the lowest impact on the composition of FAMEs, indicated by both the low percentage shares (
Figure 4) (i.e., approximately less than 5%) and low content (
Table 2,
Table 3 and
Table 4) (i.e., less than about 100 µg g
−1dry weight, dw).
According to the literature data, among the vegetable oils and fats, the higher contents of SFAs were found in palm kernel oil (76.0%) and coconut oil (90.5%) with a dominant presence of C12:0 and C14:0 methyl esters, while lower in linseed oil (9.65%), sunflower oil (8.8%), and safflower oil (7.2%) [
46]. The total content of C16:0 in analyzed herbal samples is higher than in castor [
47], rapeseed, and sunflower oils [
48]. Presented findings are coherent with previous research on other plant species, where MUFAs were also at higher levels than SFAs (
Figure 2,
Figure 3 and
Figure 4). Interestingly, MUFA was the main part of FAME compositions in oils, such as sesame (42.0%), rice brain (44.0%), almond (67.9%), olive(68.2%), peanut (71.1%), and rapeseed (72.8%). C18:1 was found as the most abundant MUFA in oil samples, except for hemp oil, where C20:1 was predominant [
49]. Among the vegetable oils and fats, the highest percentage of long-chain MUFA and PUFA, such as C18:1, C18:2n6c, and C18:3n6 methyl esters contain the sunflower oil, safflower oil, and linseed oil [
46].
Some of the FAMEs, viz one MUFA (C22:1n9), one PUFA (C20:3n6), and five SFAs (C11:0, C12:0, C14:0, C15:0, and C24:0), were only present in about half of herbal wastes, e.g., lemon balm and tobacco. The highest concentration of total FAMEs was determined in rye bran (35.79 mg g
−1dw), Figure tea (11.69 mg g
−1dw), and chicory (8.78 mg g
−1dw) (
Table 2,
Table 3 and
Table 4). C18:3n3, C18:1n9t, and C22:1n9 were found in large amounts (> 1 mg g
−1dw) in rye bran, St. John’s wort, and sage (
Table 5). It is essential because, among all FAMEs, C16:0, and C18:1 are the ideal components of biodiesel [
50,
51]. Moreover, the highest content of C16:0 was found in senna (0.92 mg g
−1dw), and also in rye bran and nettle the C16:0 content was high (0.79 and 0.57 mg g
−1dw, respectively). The lowest content of total FAMEs was reported in tobacco (0.26 mg g
−1dw), chamomile (0.27 mg g
−1dw), and hemp (0.36 mg g
−1dw). However, most herbal wastes (ten out of sixteen – 62.5%) had the total FAMEs content between 1.42 and 5.02 mg g
−1dw (
Table 5). In comparison, the total FAMEs amounts in some halophytic plants ranged from 1.00 to 7.27 mg g
−1dw [
52]. Also, the SFA and USFA methyl esters’ contents among
Paeonia species varied from 10.8 to 29.8 mg g
−1dw and from 98.6 to 210.8 mg g
−1dw, respectively. Thus, the total content of FAMEs among
Paeonia species varied from 109.4 to 240.6 mg g
−1dw [
50].
Comparison of the FAMEs percentage composition in studied herbal wastes to other plants, e.g., sunflower and rapeseed [
49], revealed that some similarities in the distribution of FAMEs do exist; however, the overall profile is different (
Figure 5), e.g., a similar share of C12:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0, and C18:3n3, but different share of C16:1, C18:1n9c, and C18:2n6c. Interestingly, in herbal wastes, C18:3n6 is present having its share at the level of even 81.12%, while in other plants, like safflower or rapeseed, it is not present at all.
The United States Standard ASTM D975-20a [
53] for conventional petro-diesel fuel requires a minimum CN of 40, while the standard for biodiesel ASTM D6751-20 [
54] that relates to biodiesel specifies a minimum of 47. British and European Standard BS EN 590:2013+A1:2017 [
55] and BS EN 14214:2012+A2:2019 [
56] specifies a minimum CN to be greater than 51 for diesel and biodiesel fuels, which is necessary for optimum operation of engines (reduce emissions, smooth running, and better cold flow) [
57]. The CN depends on the feedstock source. Biodiesel consists of FAMEs with each ester component contributing to fuel properties. Hence, those properties depend not only on the structure of the fatty acidsbut also on that of the ester moiety derived from the alcohol, e.g., methanol, propanol, and ethanol [
4].
A high value of the CN can be observed in SFAs (e.g., C16:0 and C18:0), while in the medium range in MUFAs. The longer the chain of an individual FAME and the more saturated the molecules, the higher the CN. High CN shortens the ignition delay, while lower CN (consequently longer ignition delay) results in increased nitric oxide emission [
5,
58]. Biodiesel with high level of SFAs and a low level of PUFAs has a good oxidative stability. Large amounts of SFAs have a great influence on cetane number and oxidative stability but cause poor low-temperature properties. To achieve better low-temperature performance, biodiesel should have low amounts of long-chain SFAs. On the other hand, the biodiesel with a higher amount of UNFAs exhibits better cold-flow and viscosity with a penalty in both the ignition quality and oxidative stability. Summarizing, the highest possible percentage of MUFAs is desirable for biodiesel in combination with a low percentage of SFAs, PUFAs (especially with three C=C bonds), and very-long-chain FA (more then 20 carbon atoms) [
4,
57,
59].
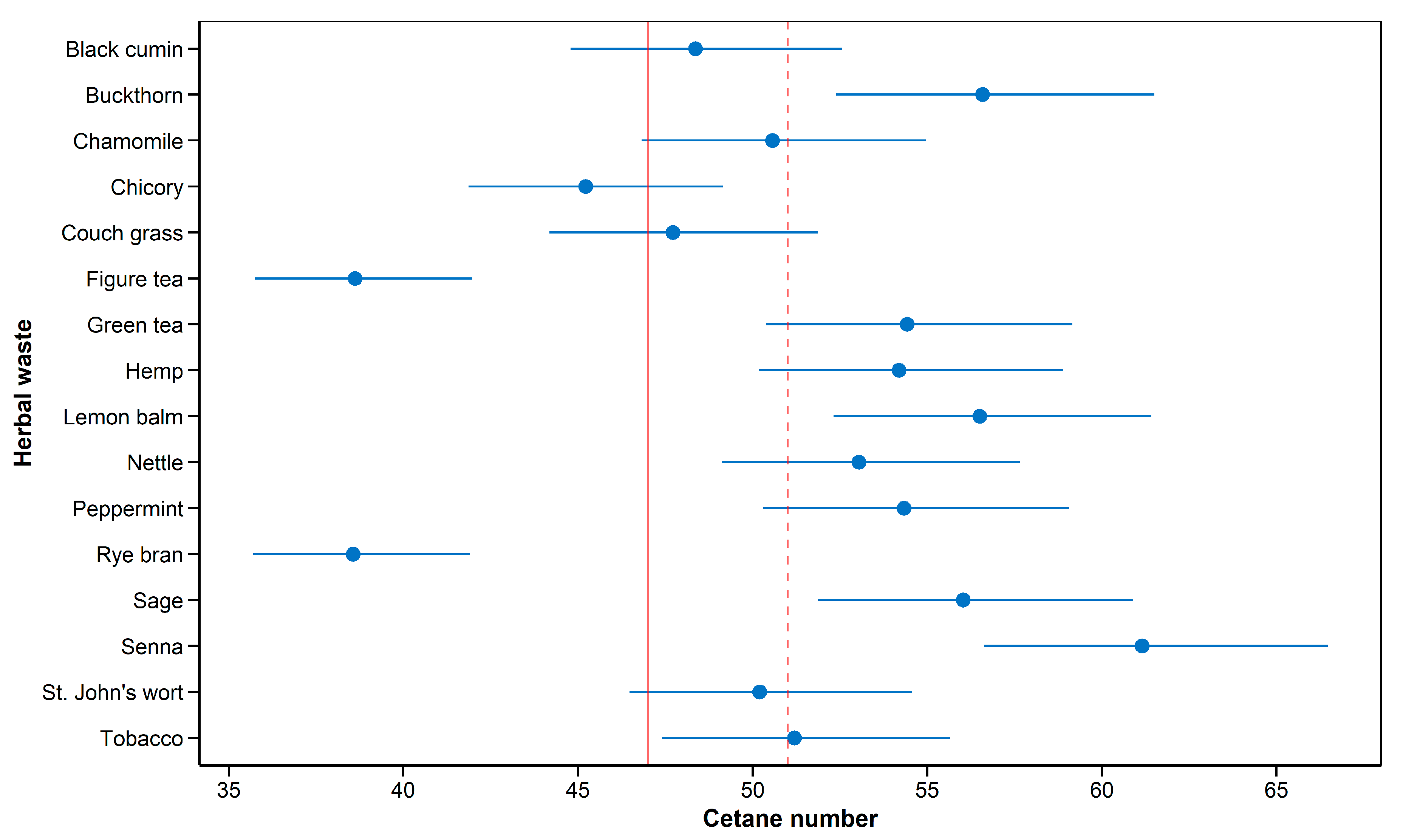
Prediction of CN allowed to classify potential herbal industry wastes for their future usage in the fuel industry. According to BS EN 14214:2012+A2:2019 [
56] values presented in
Figure 6 and
Table S1 show that buckhorn, green tea, hemp, lemon balm, peppermint, sage, and senna are the most likely to be used in biofuel. However, further assessment of other properties of the fuel, e.g., viscosity, the heat of combustion, density, oxidative stability, low-temperature properties, and lubricity, either by estimations or measurement of the actual biofuel from herbal wastes is needed [
58].
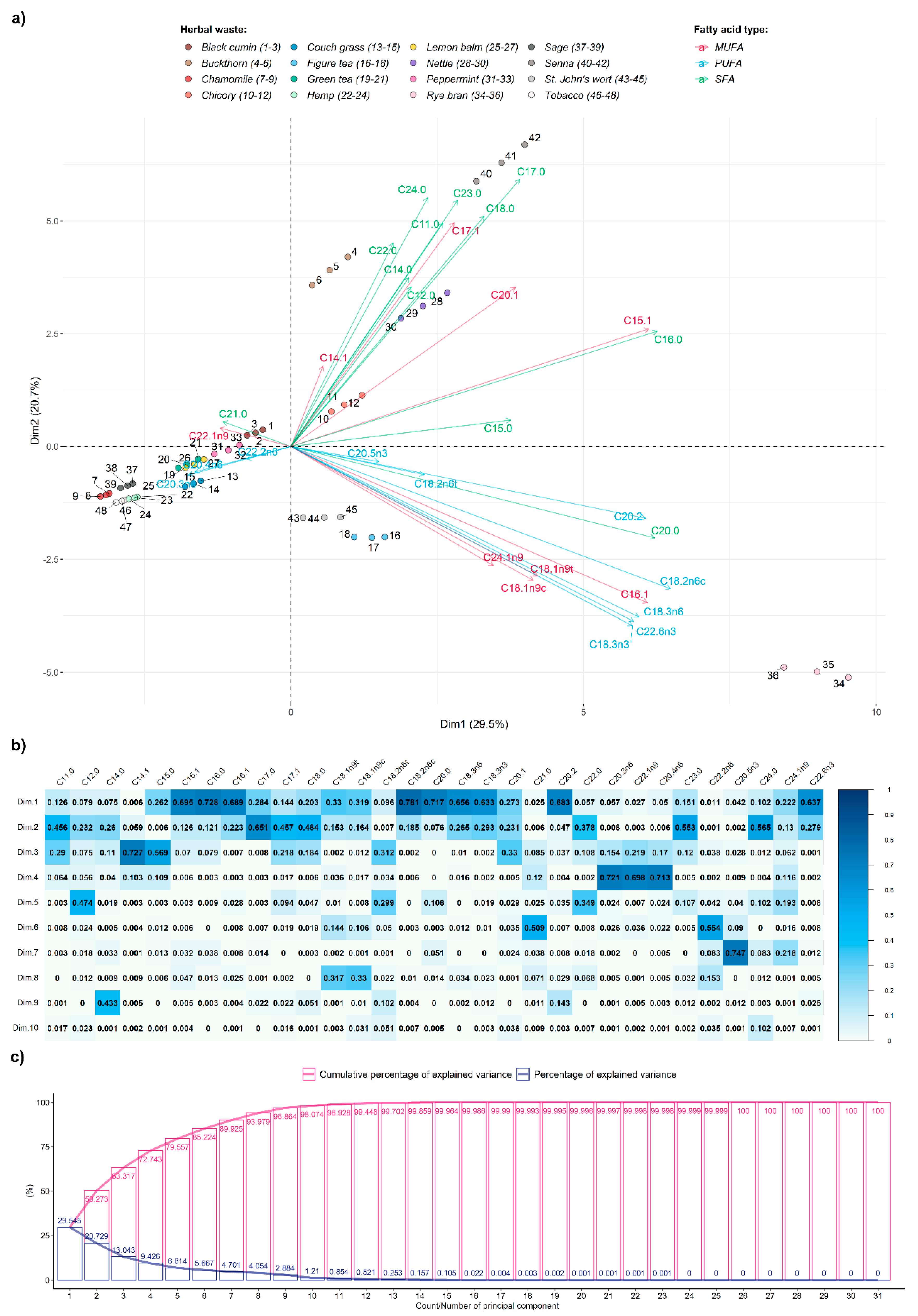
The first and second PCA components (Dim1 and Dim2, respectively) separated buckthorn, chicory, Figure tea, nettle, and St. John’s wort from other herbal wastes. Those five herbal wastes, depending on the position on the factor map, had significantly higher contents of FAMEs, e.g., C15:1, C14:1, C18:1n9t, C18:1n9c, C24:1n9, C18.2n6c, C18:216c, C18:3n6, C18:0, C20:0, and C23:0. Positive values of Dim2 are related mostly to MUFAs (e.g., C15:1) and SFAs, while negative to MUFAs (e.g., C18:1n9t) and PUFAs (
Figure 7a).
In all possible combinations of the first four dimensions, the following herbal wastes: black cumin, chamomile, couch grass, green tea, hemp, peppermint, lemon balm, and tobacco were close together (
Figure 7a,
Figures S1–S5), suggesting similarities in their fatty acid profiles. It is also worth reporting that higher PCA dimensions separated the dataset based on other FAMEs levels. The model could have been further reduced to 7 or 8 dimensions that still explain about 90% of the variance, which further suggests that the raw dataset of 31 FAMEs [C8:0 was detected only in chicory, and thus it could disturb the model and was not taken to the analysis (
Figure 4)] is highly correlated and reducible. This property might be useful for future studies involving other machine learning techniques build-up on top of PCA to classify biodiesel produced from specific plants (
Figure 7b,c). Similar conclusions were also stated by Škrbić, et al. [
51].
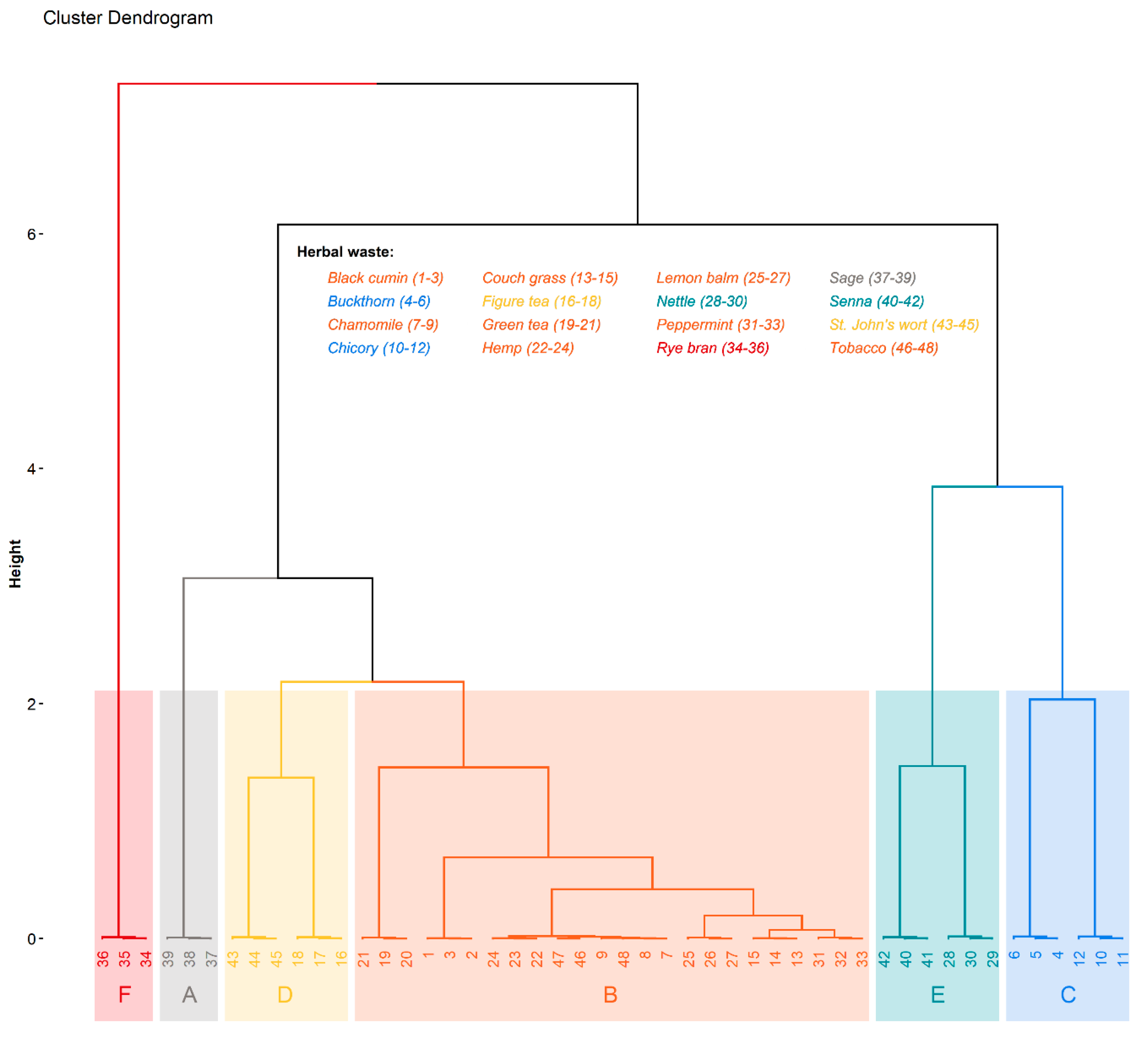
Nonetheless, HCPC analysis on the multidimensional PCA model allowed to cluster herbal wastes, preserving a high level of explained variance equal to approximately 98.07%. HCPC clustered herbal wastes based on FAMEs content into six clusters (A-F) (
Table 6,
Figure 8). The A-cluster is represented only by sage, which has the highest content of C20:3n6, C20:4n6, C22:1n9, and C21:0, making them a good biofuel candidate according to the EN standard [
55]. The next and largest cluster contains herbal wastes with a lot of different FAMEs, which content is lower than average, suggesting that plants in this cluster (e.g., black cumin, chamomile, and peppermint) are not suitable for biofuel. Herbal wastes assigned to cluster C, i.e., buckthorn and chicory, show higher than average contents of MUFAs and SFAs; however, C18:2n6t (PUFA) is also very high in this cluster. Thus, these herbal wastes are also not good for biofuel. The D-cluster, where Figure tea and St. John’s wort were taken together, shows higher average content of C18:1n9c and C18:1n9t compared to other clusters; however, other FAMEs are not present in high amounts. The E-cluster, in which nettle and senna were grouped, has 13 FAMEs (three MUFAs, a PUFA, and nine SFAs). In this cluster, MUFAs and SFAs dominate, e.g., C15:1, C16:0, C18:0, C20:1, and C24:0 are higher than average. Taking together, the high CN that could reach its value above 51 (
Table 6), these herbal wastes are promising for their usage in biodiesel. The last cluster contains rye bran, which has a lot of PUFAs, which explain the low estimated CN.
Literature data indicate that cluster and PCA applied to FAME profiles distinguished microalgae groups according to their potential for fuel production [
60]. A similar approach to that used in this research was applied by Škrbić, et al. [
51], where fatty acids profiles of 119 vegetable oils were analyzed and clustered using PCA and hierarchical clustering. They reported differences between clusters explained by the level of C16:0, C18:0, C18:2, and C18:3. According to Katre, et al. [
61], the feedstock used for biodiesel fuel production should have a high MUFA to PUFA ratio. Biodiesel produced through the transesterification reaction of these types of fatty acids shows numerous advantages over petro-diesel, such as low emissions of CO, CO
2, and hydrocarbons. However, such fuel also had several disadvantages, e.g., low CN, poor cold-flow, high viscosity, and low oxidative stability [
5]. In contrast, biodiesel derived from material with a high amount of SFA demonstrates better cold-flow properties and reduced NO
x emissions [
62,
63].
Fuel produced from fats has disadvantages, such as the higher cold filter plugging point. Biofuel from lard and tallow fat is less stable for oxidation than rapeseed and linseed oil [
64,
65,
66,
67]. On the other hand, blends of soybean and animal (e.g., beef and chicken) fat-based biodiesel presented higher oxidative stability than soybean-based fuel [
68,
69]. In the process of transesterification, glycerol (propane-1,2,3-triol) is generated as a by-product. Typically, the production of 100 kg of biodiesel yields approximately 10 kg of glycerol with low purity (50–55%). The amount of glycerol depends on methods of conversion, as well as used alcohol and catalyst [
70,
71]. In the biodiesel production process, nearly 70–95% of the total cost is caused by raw material [
72]. This issue can be overcome by the use of wastes, which can effectively reduce the feedstock cost even to 60–70%. For that reason, the herbal industry wastes are useful materials for biofuel production.