Carbon Nanofibers Production via the Electrospinning Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Solution Preparation
2.3. Electrospinning Method
2.4. Physico-Chemical Characterization
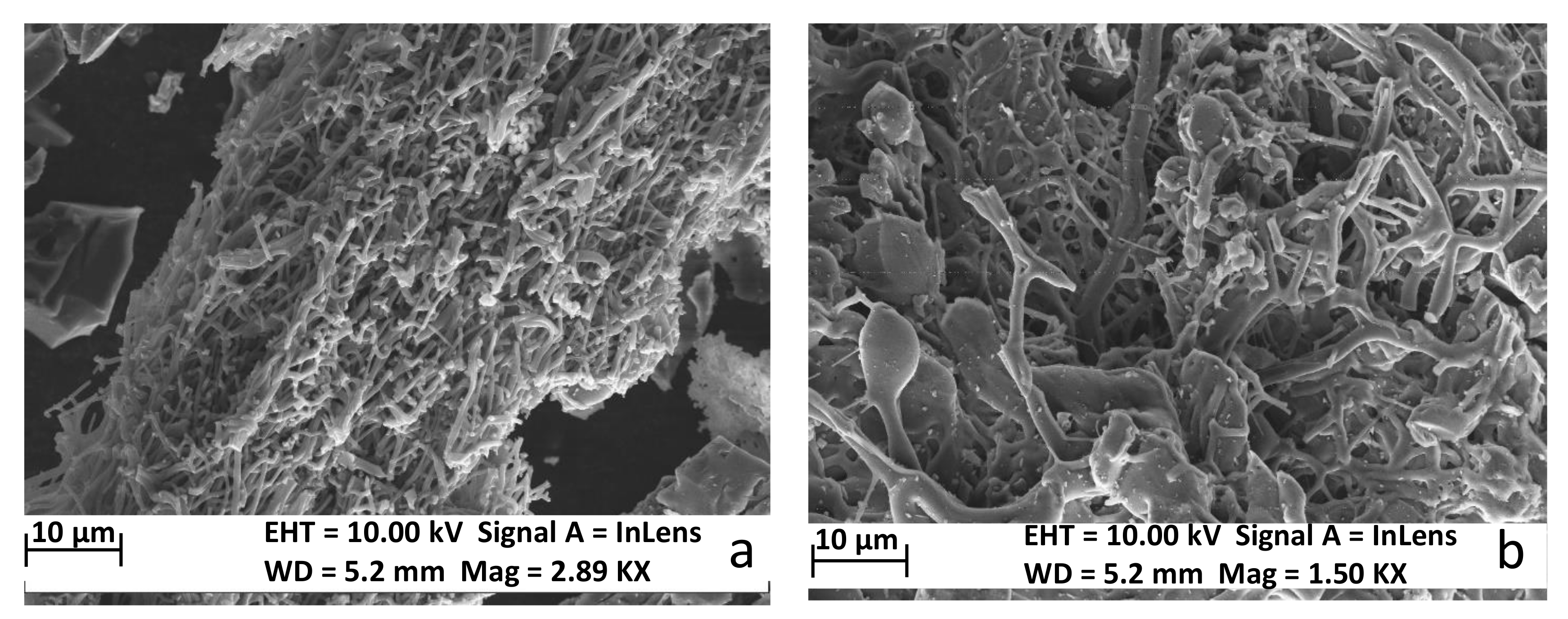
3. Results and Discussion
4. Conclusions
- An increased fiber diameter is obtained as the temperature increases, from 400–700 nm at 1200 °C to 1000–1400 nm at 1300 °C and 1400 °C. Additionally, the percentage of carbon increases from ~95% to ~97%, by increasing the heat treatment temperature. Meanwhile, the concentration of nitrogen and hydrogen decreases and the oxygen content increases as the temperature rises.
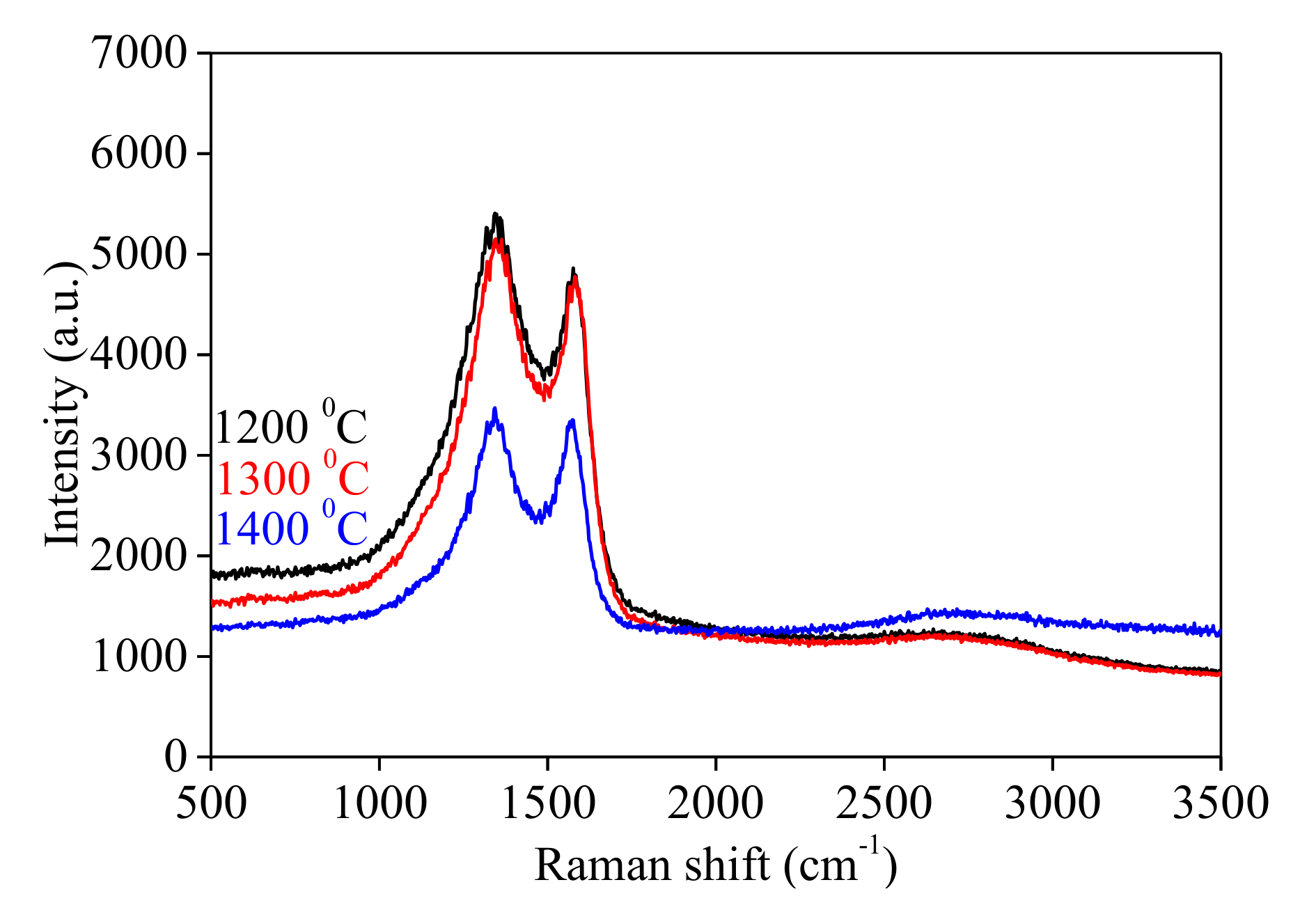
- A relaxation of the molecular orbitals at high temperatures is noticed, the G band from Raman measurements being shifted to a lower value (i.e., 1568 cm−1 at 1400 °C compared to 1576 cm−1 at 1200 °C).
- From XRD measurements, it was revealed that all diffractograms are fitted on the same hexagonal graphite crystal structure, but a higher degree of crystallinity for the sample treated at 1400 °C was obtained, as compared to the samples prepared at lower temperatures.
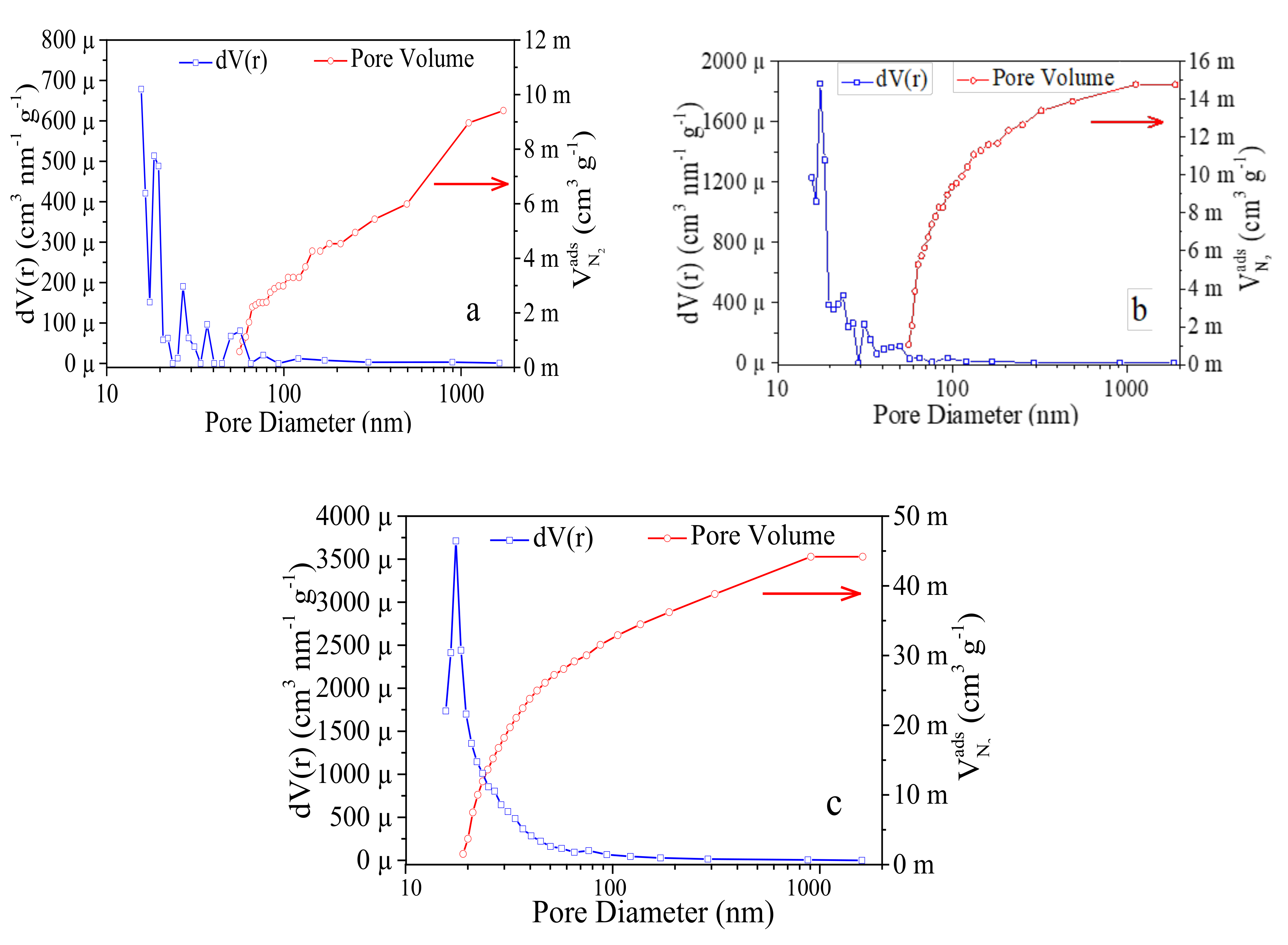
- The pore size distribution investigation done confirms the presence of both micro and mesopores, ensuring a gradual porous arrangement in developed samples.
- The carbon fibers developed in this study are adequate to produce gas diffusion layers (GDLs) for PEMFC applications and will be further used in our experimental work.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strelko, V.V.; Stavitskaya, S.S.; Gorlov, Y.I. Proton catalysis with active carbons and partially pyrolyzed carbonaceous materials. Chin. J. Catal. 2014, 35, 815–823. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Liu, Z.; Wang, F.; Gao, B.; Chen, F.; Zhang, Q.; Xiong, R.; Han, J.; Samal, S.K.; De Smedt, S.C.; et al. pH responsive polyurethane (core) and cellulose acetate phthalate (shell) electrospun fibers for intravaginal drug delivery. Carbohydr. Polym. 2016, 151, 1240–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, D.; Wang, R.; Tang, G.; Mou, Z.; Lei, J.; Han, J.; De Smedt, S.; Xiong, R.; Huang, C. Ecofriendly Electrospun Membranes Loaded with Visible-Light-Responding Nanoparticles for Multifunctional Usages: Highly Efficient Air Filtration, Dye Scavenging, and Bactericidal Activity. ACS Appl. Mater. Interfaces 2019, 11, 12880–12889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun Nanofibers Membranes for Effective Air Filtration. Macromol. Mater. Eng. 2017, 302, 1600353–1600380. [Google Scholar] [CrossRef]
- Joshi, V.S.; Gokhale, S.P.; Patil, K.R.; Haram, S. Fabrication, characterization and electrochemical performance of single strand carbon fiber prepared by catalytic chemical vapor decomposition method. Electrochem. Acta 2012, 55, 2022–2028. [Google Scholar] [CrossRef]
- Wang, Q.; Han, X.H.; Sommers, A.; Park, Y.; Joen, C.T.; Jacobi, A. A review on application of carbonaceous materials and carbon matrix composites for heat exchangers and heat sinks. Int. J. Refrig. 2012, 35, 7–26. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Tseng, P.T. Field emission characteristics of diamond nano-tip array fabricated by anodic aluminium oxide template with nano-conical holes. Appl. Surf. Sci. 2015, 351, 1004–1011. [Google Scholar] [CrossRef]
- Gergin, I.; Ismar, E.; Sarac, A.S. Oxidative stabilization of polyacrylonitrile nanofibers and carbon nanofibers containing graphene oxide (GO): A spectroscopic and electrochemical study. Beilstein J. Nanotechnol. 1616, 8, 1616–1628. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.K.; Feng, Y.; He, H.J.; Zhang, J.; Sun, R.J.; Chen, M.Y. Effect of carbonization temperature on properties of aligned electrospun polyacrylonitrile carbon nanofibers. Mater. Des. 2015, 85, 483–486. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; He, H.; Huang, M.; Zhang, X.; Wang, S. Application of nanomaterials in the bioanalytical detection of disease-relayed genes. Biosens. Bioelectron. 2015, 74, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Su, W.; Wei, Z.; Jing, M.; Fan, X.; Liu, J.; Yan, C. Effect of the graphitization degree for electrospun carbon nanofibers on their electrochemical activity towards VO2+/VO2+ redox couple. Electrochim. Acta 2016, 199, 147–153. [Google Scholar] [CrossRef]
- Zhang, X. An electrochemical non-enzymatic immunosensor for ultrasensitive detection of microcystin-Ir using carbon nanofibers as the matrix. Sens. Actuators B 2016, 233, 624–632. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.Y.; Ren, J.; Wu, Q.L. Preparation and structures of electrospun PAN nanofibers as a precursor of carbon nanofibers. Synth. Metals 2005, 155, 157–161. [Google Scholar] [CrossRef]
- Sutasinpromprae, J.; Jitjaicham, S.; Nithitanakul, M.; Meechaisue, C.; Supaphol, P. Preparation and characterization of ultrafine electrospun polyacrylonitrile fibers and their subsequent pyrolysis to carbon fibers. Polym. Int. 2006, 55, 825–833. [Google Scholar] [CrossRef]
- Ko, T.H. Influence of continuous stabilization on the physical properties and microstructure of pan-based carbon-fibers. J. Appl. Polym. Sci. 1991, 42, 1949–1957. [Google Scholar] [CrossRef]
- Shokuhfar, A.; Sedghi, A.; Farsani, R.E. Effect of thermal characteristics of commercial and special polyacrylonitrile fibres on the fabrication of carbon fibres. Mater. Sci. Technol. 2006, 22, 1235–1239. [Google Scholar] [CrossRef]
- Yusof, N.; Ismail, A.F. Post spinning and pyrolysis processes of polycrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. Anal. Appl. Pyrolysis 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Faraji, S.; Yardim, M.F.; Can, D.S.; Sarac, A.S. Characterization of polyacrylonitrile, poly(acrylonitrile-co-vinyl acetate),and poly(acrylonitrile-co-itaconic acid) based activated carbonnanofibers. J. Appl. Polym. Sci. 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Arshad, S.N.; Naraghi, M.; Chasiotis, I. Strong carbon nanofibers from electrospun polyacrylonitrile. Carbon 2011, 49, 1710–1719. [Google Scholar] [CrossRef]
- De Almeida Coelho, N.M.; Furtado, J.L.B.; Pham-Huu, C.; Vieira, R. Carbon nanofibers: A versatile catalytic support. Mater. Res. 2008, 11, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M. Encyclopedia of Materials, Parts, and Finishes, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Wiles, K.B. Determination of Reactivity Ratios for Acrylonitrile/Methyl Acrylate Radical Copolymerization via Nonlinear Methodologies Using Real Time FTIR. Master’s Thesis, Faculty of the Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2002. [Google Scholar]
- Zhou, X.; Qiu, Y.; Yu, J.; Yin, J.; Gao, S. Tungsten carbide nanofibers prepared by electrospinning with high electrocatalytic activity for oxygen reduction. Int. J. Hydrogen Energy 2011, 36, 7398–7404. [Google Scholar] [CrossRef]
- Wang, S.; Dai, C.; Li, J.; Zhao, L.; Ren, Z.; Ren, Y.; Qiu, Y.; Yu, J. The effect of different nitrogen sources on the electrocatalytic properties of nitrogen-doped electrospun carbon nanofibers for the oxygen reduction reactions. Int. J. Hydrogen Energy 2015, 40, 4673–4682. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Dong, L.; Yan, B.; Shan, H.; Li, D.; Sun, X. Electrospun SnO2-ZnO nanofibers with improved electrochemical performance as anode materials for lithium-ion batteries. Int. J. Hydrogen Energy 2015, 40, 14338–14344. [Google Scholar] [CrossRef]
- Srinivasan, S.S.; Ratnadurai, R.; Niemann, M.U.; Phani, A.R.; Goswami, D.Y.; Stefanakos, E.K. Reversible hydrogen storage in electrospun polyaniline fibers. Int. J. Hydrogen Energy 2010, 35, 225–230. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Annamalai, P.; Langmi, H.W.; North, B.C.; Mathe, M. Electrospun MOF nanofibers as hydrogen storage media. Int. J. Hydrogen Energy 2015, 40, 9382–9387. [Google Scholar] [CrossRef]
- Andrei, R.D.; Marinoiu, A.; Bucura, F.; Zaharoiu, A.; Niculescu, V.; Sisu, C.; Carcadea, E. Synthesis via electrospinning of electrospun polyacrylonitrile fibers—Preliminary Results. Prog. Cryog. Isot. Sep. 2018, 21, 81–90. [Google Scholar]
- McCutcheon, J.R.; Elimelech, M. Influence of membrane support layer hydrophobicity on water flux in osmotically driven membrane processes. J. Membr. Sci. 2008, 318, 458–466. [Google Scholar] [CrossRef]
- Arena, J.T.; McCloskey, B.; Freeman, B.D.; McCutcheon, J.R. Surface modification of thin film composite membrane support layers with dopamine: Enabling use of reverse osmosis membrane in pressure retarded osmosis. J. Membr. Sci. 2011, 375, 55–62. [Google Scholar] [CrossRef]
- Huang, L.; Bui, N.N.; Meyering, M.T.; Hamlin, T.J.; McCutcheon, J.R. Novel hydrophilic nylon 6, 6 microfiltration membrane supported thin film composite membranes for engineered osmosis. J. Membr. Sci. 2013, 437, 141–149. [Google Scholar] [CrossRef]
- Rezende, M.C.; Costa, M.L.; Botelho, E.C. Compósitos Estruturais: Tecnologia e Práctica; Artliber: São Paulo, Brazil, 2011; p. 34. [Google Scholar]
- Atuart, B. Infrared Spectroscopy: Fundamentals and Applications; John Wiley&Sons Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach, Encyclopedia of Analytical Chemistry; John Wiley&Sons Ltd.: Chichester, UK, 2000; p. 10815. [Google Scholar]
- Smith, B. Infrared Spectral Interpretation; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Lee, S.; Kim, J.; Ku, B.-C.; Kim, J.; Joh, H.-I. Structural Evolution of Polyacrylonitrile Fibers in Stabilization and Carbonization. Adv. Chem. Eng. Sci. 2012, 2, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Kakida, H.; Tashiro, K.; Kobayashi, M. Mechanism and Kinetics of Stabilization Reaction of Polyacrylonitrile and Related Copolymers I. Relationship between Isothermal DSC Thermogram and FT/IR Spectral Change of an Acrylonitrile/Methacrylic Acid Copolymer. Polym. J. 1996, 28, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. Mechanism and kinetics of the stabilization reactions of itaconic acid-modified polyacrylonitrile. Polym. Degrad. Stab. 2008, 93, 1415–1421. [Google Scholar] [CrossRef]
- Green, K.J.; Dean, D.R.; Vaidya, U.K.; Nyairo, E. Multiscale Fiber Reinforced Composites Based on a Carbon Nanofiber/Epoxy Nanophased Polymer Matrix: Synthesis, Mechanical, and Thermomechanical Behavior. Compos. Part A: Appl. Sci. Manuf. 2009, 40, 1470–1475. [Google Scholar] [CrossRef]


| Sample | % PAN | Thermal treatment, °C | % C | % N | % H | % O |
|---|---|---|---|---|---|---|
| C-1 | 4 | 1200 | 96.08 | 3.1 | 0.35 | 0.47 |
| C-2 | 6 | 1200 | 94.80 | 4.19 | 0.6 | 0.41 |
| C-3 | 8 | 1200 | 96.21 | 3.08 | 0.3 | 0.41 |
| C-4 | 10 | 1200 | 95.85 | 3.53 | 0.20 | 0.42 |
| C-5 | 12 | 1200 | 95.56 | 3.36 | 0.7 | 0.38 |
| C-6 | 4 | 1300 | 96.44 | 2.94 | 0.36 | 0.26 |
| C-7 | 6 | 1300 | 96.71 | 2.67 | 0.36 | 0.26 |
| C-8 | 8 | 1300 | 97.12 | 2.24 | 0.39 | 0.25 |
| C-9 | 10 | 1300 | 97.55 | 1.94 | 0.24 | 0.27 |
| C-10 | 12 | 1300 | 97.12 | 2.24 | 0.39 | 0.25 |
| C-11 | 4 | 1400 | 97.64 | 2.19 | 0.15 | 0.02 |
| C-12 | 6 | 1400 | 97.95 | 2.03 | – | 0.02 |
| C-13 | 8 | 1400 | 97.96 | 2.02 | – | 0.02 |
| C-14 | 10 | 1400 | 97.92 | 2.06 | – | 0.02 |
| C-15 | 12 | 1400 | 97.89 | 2.04 | 0.06 | 0.01 |
| PAN | 12 | - | 65.54 | 26.34 | 5.88 | 2.24 |
| Sample | Thermal Treatment (°C) | D band (cm−1) | G band (cm−1) | ID/IG |
|---|---|---|---|---|
| C-5 | 1200 | 1348 ± 0.001 | 1576 ± 0.001 | 1.076 ± 0.001 |
| C-10 | 1300 | 1348 ± 0.002 | 1576 ± 0.002 | 1.072 ± 0.002 |
| C-15 | 1400 | 1348 ± 0.002 | 1568 ± 0.001 | 1.008 ± 0.002 |
| Sample | Thermal Treatment, (°C) | SBET (m2g−1) | Vtot (mLg−1) | Pore Radius (Å) |
|---|---|---|---|---|
| C-5 | 1200 | 40.69 ± 0.02 | 0.009 ± 0.001 | 15.652 ± 0.004 |
| C-10 | 1300 | 89.92 ± 0.02 | 0.015 ± 0.001 | 17.512 ± 0.004 |
| C-15 | 1400 | 66.89 ± 0.02 | 0.044 ± 0.001 | 17.504 ± 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrei, R.D.; Marinoiu, A.; Marin, E.; Enache, S.; Carcadea, E. Carbon Nanofibers Production via the Electrospinning Process. Energies 2020, 13, 3029. https://doi.org/10.3390/en13113029
Andrei RD, Marinoiu A, Marin E, Enache S, Carcadea E. Carbon Nanofibers Production via the Electrospinning Process. Energies. 2020; 13(11):3029. https://doi.org/10.3390/en13113029
Chicago/Turabian StyleAndrei, Radu Dorin, Adriana Marinoiu, Elena Marin, Stanica Enache, and Elena Carcadea. 2020. "Carbon Nanofibers Production via the Electrospinning Process" Energies 13, no. 11: 3029. https://doi.org/10.3390/en13113029






