Assessing CO
2-rock interaction is an important part of seal integrity studies, as these potentially affect physical properties through highly coupled processes. The driving process of CO
2-rock interactions includes dissolution of CO
2 in brines, acid-induced reactions, reactions due to brine concentration, clay desiccation, pure CO
2-rock interactions and reactions induced by other gasses than CO
2 [
9]. The observed changes in the petrophysical properties of the after-exposure samples are generally attributed to the three mechanisms of mineral dissolution, mineral precipitation and physical compaction [
46]. The fine migration of susceptible minerals such as kaolinite is another important mechanism which can alter the properties of rock samples. The migration of clay fines generated and released by mineral dissolution is believed to have occurred in the exposure experiment conducted in this work. The occurrence and the extent of the effects of these mechanisms on a sample depend on the exact mineral composition, presence of core-scale heterogeneities and the original textural features of the sample and therefore, may vary from one sample to the next. Much of the discussions presented in the upcoming sections of this section are to determine how CO
2-induced dissolution and precipitation reactions affect the pore space evolution and thus the physical properties of after-exposure samples, as revealed by the complementary measurements conduced.
5.1. The Effect on Mineralogy
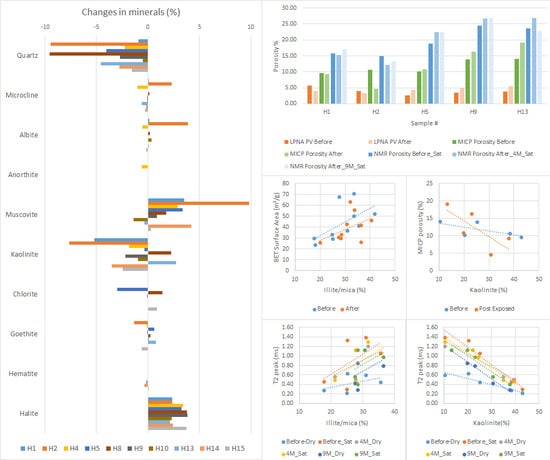
The XRD analysis of the original caprock samples showed that samples H8 and H13 are quartz-rich, samples H1, H9, H10, and H15 are clay-rich (total clay > 60%), and samples H2, H4, H5, H13, and H14 have more clay content than quartz. The siliceous samples investigated in the current study are marked by a strong component of clay minerals, with up to 75% kaolinite and Illite. Samples are rich in both kaolinite (average: 26% and maximum 43%) and Illite (average: 29% and maximum 42%). Quartz content is 33% on average, with a maximum of 59%. The dominant feldspar in the samples is microcline (average 7% and maximum 10%). Samples H14 and H15 originated from the Eneabba shale formation, and samples H1–H13 originated from the same formation (Yalgroup member of Lesueur formation), but different localities. The different mineralogical composition of the samples leads to different reaction responses to the CO2-brine and brine systems.
XRD analysis of samples was carried out to measure the exact weight percentage of each mineral phase. The results were compared to the after-exposure results to monitor any changes in the amount of mineral phases. The X-ray diffraction (XRD) results show several changes in mineralogy because of rock-brine-CO
2 reactions (
Figure 13). Quartz, kaolinite, and goethite were dissolved in most samples and muscovite and halite were precipitated in general. Feldspars showed mixed behaviour across the samples.
Previous experimental work [
11] showed some reactions occurring on a measurable time scale only at high temperatures of 200 °C. Other works [
16] noted only a limited reaction of CO
2 with pure mineral phases (anorthite and glauconite) at 50 °C and 150 °C and a low pressure. Changes in caprock fluid transport properties may happen regardless of the extent and scale of the mineralogical composition alteration. Wollenweber et al. (2010) reported carbonate dissolution and re-precipitation, leading to the alteration of shaly caprock transport parameters. In this case, no detectable mineral alterations were observed. The loss of quartz [
47], feldspars [
14], kaolinite [
18], and chlorite [
21] has been reported in other studies, resulting in an increase in porosity and opening up pore throats, leading to an increase in permeability.
The observed mineralogical alterations can be explained by the generation of carbonic acid (H
2CO
3). Changes in pH control the dissolution of minerals, release of chemical species, and formation of new compounds and minerals [
7,
8,
10,
17,
48,
49]. In the absence of calcite, reactive minerals such as feldspars, kaolinite and chlorite start to dissolve. The dissolution of these minerals releases Si, Al, Na, K, Fe, and Ca, which can form new compounds. The dissolution of feldspars and kaolinite results in the formation of muscovite/Illite.
The decrease in the quartz content of the samples, observed in previous experiments, is attributed to the dissolution of quartz itself. Rathnaweera et al. (2016) reported significant quartz dissolution under the 40 °C after a 1.5-year interaction with scCO
2 and suggested that over a short period and under low temperature, quartz dissolution is too minor to be observed [
50]. Szabó et al. (2016) also reported moderate dissolution of quartz, however in durations ranging 28–57 days [
24]. Kaszuba et al. (2003) also observed dissolution of quartz along with oligoclase and biotite dissolution during their batch experiment at an elevated temperature and pressure (2000 °C and 200 bars) [
11]. Other cases when quartz content was increased have been reported in other studies [
22]. In such cases, the additional quartz is the product of the dissolution of other minerals such as feldspars and clay minerals. In an experiment with two types of shale caprocks, Alemu, et al. (2011) reported no major mineralogical alteration in the case of clay-rich shale, whereas in the case of carbonate-rich shale, plagioclase and clay minerals were dissolved, carbonates dissolved and re-precipitated, and smectite was formed [
51]. Credoz et al. (2009) used purified clay minerals to evaluate their reactivity and found kaolinite to be the most reactive clay mineral in the long-term experiments [
18].
Carbonate precipitation, such as the precipitation of dawsonite, siderite, calcite, dolomite, and magnesite, has been reported in several studies [
52,
53]. However, carbonate formation was not observed during this study. Since the shale sample used in this experimental study did not contain any carbonates and a very limited amount (2%) of Ca-feldspar in just one of the samples, a potential source for Ca was not available to form calcite and kaolinite assemblage. Also, low cation concentrations with an acidic pH were observed, which showed that dissolution prevailed over carbonate precipitation [
17].
The formation of halite was interpreted as an experimental artefact. NaCl precipitated from Na and Cl coming from the solution, since the solid was not rinsed with deionised water before analysis [
18].
5.2. The Effect on Petrophysical Properties
The low-pressure nitrogen adsorption method showed a slight increase in porosity observed in seven out of ten samples (
Figure 4), except for samples H1, H2, and H4. Samples recorded a pore volume range of 1.7–5.7 cm
3/g before exposure, which was changed to 2.1–6.4 cm
3/g after exposure to CO
2. There was an average 1.17 cm
3/g increase in the pore volume of ten samples and an average 1.4 cm
3/g decrease in the pore volume of three other samples tested.
The porosities were also estimated with the MICP and NMR methods for five samples. Samples H1 and H2 recorded a reduction in their porosity in both of these methods and samples H5, H9, and H13 show an enhancement in their porosities. The MICP porosities range is 9.6–16.3% for pre-exposure samples and 4.6–19.1% for after-exposure samples (
Table 4). There was an average 2.7% increase in MICP porosity for three samples and an average 3.2% decrease in MICP porosity for two other samples tested.
The NMR porosities were measured in both dry and saturated states. The dry samples before exposure (as received) had porosities in the range of 6.35–9.67% and the range of 9.67–19.38% after four months of treatment and 10.36–19.08% after nine months of CO
2 treatment. NMR porosities in saturated states are generally higher than dry states and their ranges for before, after four months, and after nine months of CO
2 exposure are 14.90–24.55%, 12.12–26.93%, and 13.20–26.79%, respectively (
Figure 2). While the NMR porosity of samples in the dry state increased across the sample by about 7.7%, the NMR porosity of saturated samples increased by an average 3% in three samples and decreased by an average 1.7% in two other samples tested. The increased porosity after the initial saturation stage is simply because the NMR method measures the response of hydrogen nuclei and then converts it to porosity. The received samples from storage were relatively dry and showed smaller porosities, which was significantly increased after the saturation. Each following exposure to CO
2 reduced the porosities because of the evaporation of some of the water content. The following re-saturation stages did not increase the porosity as much as the initial saturation because the samples contained much of the water from the first saturation stage.
The received samples have a systematic monomodal distribution, with a relaxation time (T2) centred around 0.3–0.7 ms. After saturation, the previous population showed a shift toward a longer T2, centred between 9–1.6 ms, except for samples H1 and H2 which stayed centred between 0.33–0.46 ms. This first population is defined as the short relaxation time. A second population defined by long relaxation time was also recorded for the samples at around 13.5 ms. This second population is more likely the effect of the macropores filled by brine during the saturation process. The saturated samples show porosity values significantly higher than partially saturated samples.
The average pore width results of the LPNA method show an increase in most samples (H5, H8, H9, H10, H13, H14, and H15 and a decrease in three samples, H1, H2, and H4 (
Figure 6). The average pore width range according to this method is 2.9–6.9 nm for pre-exposure samples, which is changed to 3.3–5.8 nm after exposure. However, the MICP average pore width is increased across all samples. The average pore diameter range of before-exposure samples is 11.3–22 nm, and for after-exposure samples the range is 12.7–28.1 nm (
Table 4).
The leaching of the mineral constituents of the shale is the main reason for porosity increase. Mineral dissolution and precipitation can induce changes in porosity and permeability. In this study, an exposure period of nine months was selected, based on the reaction time required to complete the kinetically slow reactions of existing minerals with CO
2 and brine to identify the fate of these major rock minerals. However, the timescales required to complete the reactions are certainly more than one year, up to hundreds or even thousands of years [
54,
55]. Therefore, considering the time frame available for this study and in order to record the maximum impact possible, nine months was selected as being a reasonable period for the CO
2-brine-rock interactions. However, this period is not nearly enough to capture all the possible mineral reactions that occur in CO
2 and brine environment, such as precipitation of feldspars and secondary precipitation of calcite and quartz. Therefore, it would be possible to see the dominant reactions such as the initial dissolution of quartz and kaolinite and the salt drying-out effect [
50]. The significant increase in porosity observed in most experimental samples would potentially deteriorate the sealing capacity of the caprock.
The bulk porosities from LPNA, MICP, and NMR present some differences (
Figure 14). The porosities from NMR, averaging about 20% for saturated samples, are much higher than the LPNA and MICP porosities, which are about 12% and 4 cm
3/g, respectively. This scale of differences has also been investigated by [
56] for mudstone samples. Possible explanations include: 1—the ability of NMR to measure both connected and isolated pores (pores located within the grains and clay-bound water spaces) in contrast with MICP that only measures the connected pores; 2—NMR samples are saturated with brine which make them prone to clay swelling and cracking, while MICP samples were dried which induced potential clay shrinkage [
57].
Two groups of samples were identified from the capillary pressure profiles (
Figure 11). The capillary pressure curves in samples H1 and H2 start to plateau earlier than the other three samples and remain mostly flat for a considerably larger portion of the curves. This suggests a unimodal type of pores in the first two samples compared with more complex pore sizes in the last three samples covering a large range of pore sizes. The general peak pore throat radius shows, in the H1 and H2 samples, a value of 12.5 nm, smaller than H5, H9, and H13 samples which have values around 21.1 nm (
Table 5 and
Figure 11). More specifically, pore throat distribution reveals a second minor population in the second group of samples that have a pore throat size >250 nm that is easily invaded by mercury injection at low pressure. Samples H1 and H2 (group one) have the smallest average pore diameter at 11.3–12.7nm with low permeability at around 160–430 nD, while other samples are 16.2–22 nm.
Similarly, threshold pressure shows distinctive values in these two groups. The group one threshold pressures are in the range of 6700–8350 psi and the group two threshold pressure range is 412–1900 psi, which is significantly lower than group one (
Table 4). The threshold pressure is reduced in all five samples. However, the decrease is significant in the case of samples H2, where it is decreased from 6310 psi to 1736 psi.
When the buoyancy pressure, due to an accumulated CO
2 plume, dominates the capillary pressure of caprock, the plume intrudes into the pore throats, and the occurrence of capillary leakage is inevitable in the caprock. The capillary pressure of the CO
2-brine can be equated to the buoyancy pressure of the injected CO
2 column [
38]. Here, the experimental investigation of capillary pressure was conducted, and the result was used to calculate the height of the CO
2 column. Mercury injection capillary pressure (MICP) indicated a reduction in the capillary pressure and the calculated maximum column heights of CO
2.
There is a slight positive relationship between MICP porosity and the average pore diameter. However, a strong relationship exists between MICP porosity and peak pore throat diameter and threshold pressure (
Figure 15). The general trend follows the expected relationship between these three parameters. Smaller porosities and peak pore diameter (group one) correspond to higher threshold pressure and vice versa (group two).
These two groups are distinctive in their content of kaolinite clays. Group one, compromising samples H1 and H2, with high kaolinite content, records a high threshold pressure due to low pore throat diameter. The second group of samples, H5, H9, and H13, with lower kaolinite content, has a lower entry pressure and more diverse range of pore throat diameters.
The total area of pores is estimated from both the LPNA and MICP methods. Ten samples were tested with LPNA before and after exposure. However, only five of them were measured with the MICP method (H1, H2, H5, H9, and H13). The LPNA surface area shows slight changes in most samples (
Figure 16). A slight positive change is observed in samples H1, H2, H5, H8, H9 and a slight negative change in sample H10. However, in samples H5 and H15, the total pore area is decreased significantly, and, in the case of sample H13, it is increased significantly. The MICP total pore area shows more dramatic changes. The total pore area is decreased in samples H1, H2, and H5 and is increased in samples H9 and H13. In the case of sample H2, the reduction is very significant (−77%). MICP confirms the LPNA total pore area results in terms of the direction of change in samples H9 and H13 where both methods show an enhancement in total surface area. However, the slight LPNA pore area increase in samples H1, H2, and H5 contradicts the MICP significant decrease after exposure to scCO
2.
The BET surface areas were found to be 23.9–70.6 m
2/g (
Table 2). There was a direct relationship found between the BET surface area and the occurrence of Illite/mica (
Figure 17). Group one of samples has a lower percentage of Illite/mica clays but higher kaolinite percentages compared with group two of the samples. The second group of samples with a higher kaolinite content has higher threshold pressure due to its low pore throat diameter and lower porosities (
Figure 15 and
Figure 17). Furthermore, the presence of kaolinite influences the NMR response (
Figure 17). As the kaolinite content increases, the
T2 relaxation time peak tends to decrease, with corresponding smaller pore sizes or a restricted environment.
The high presence of total clay (mostly kaolinite) could cause the blocking of the pore throats and could give access to the neighbouring larger pores during the saturation process, leading to lower T2 amplitude values. The long T2 in group two of the samples is an indication of macropores, and potentially new cracks induced by artificial saturation under pressure and brine reactivity with shales, during the sample recovery and preparation steps.
There is no strong relationship between the LPNA average pore width and either total pore area or total pore volume (
Figure 18). Average pore width shows a strong relationship with fractions of micro-, meso-, and macropores. Before and after exposure samples show a decrease in micropore percentage and an increase in mesopore and macropore percentage with increasing pore diameter (
Figure 19 and
Figure 20). Based on IUPAC pore classification, LPNA pore volume showed a pore range from 65.3% to 86.2% mesopores, 6.1% to 33.7% micropores, and a small portion of 0.5% to 8% macropores.
PSD Comparisons
The LPNA results show different changes in the sample PSD. After nine months of exposure, an increase was observed in the pore volume of micropores and mesopores smaller than about 8 nm in samples H1, H2, and H4. Sample H4 is different in this matter to the other two samples in that the pore volume of micropore range was decreased (
Figure 7). However, the pore volume of pores larger than about 8 nm is decreased in samples H1, H2, and H4. In samples H5, H8, H9, and H13, the pore volume is increased across all pore size range. Samples H10, H14, and H15 follow a deferent pattern. Their pore volume is increased in pore sizes smaller than about 20 nm and is decreased bellow this turning point. Their after-exposure isotherms are lower than the before-exposure isotherms.
The MICP results are two-fold too. MICP shows a decrease in the porosity of samples H1 and H2 and an increase in samples H5, H9, and H13. Although H5 porosity increased, which puts it in the second group, its total pore volume is decreased from 10.26 m
2/g to 6.35 m
2/g (
Table 4). The MICP PSD did not include the micropore range as the LPNA did. MICP advocates substantial pore volume in the meso- and macropores range. Samples H1 and H2 obtained from MICP analysis have the largest mesopore volume and the lowest macropore volume. The slight inconsistency between MICP and LPNA is because MICP only quantifies pore throat sizes and not the pore bodies, whereas LPNA quantifies both of them [
57].
The grouping mentioned above is also obvious in the MICP pore size distribution curves (
Figure 10). Samples H1 and H2 with the most uniform pore size distributions have the largest proportion of their pores in narrow pore sizes (peak at about 10 nm) which is reduced after exposure. A dual behaviour is observed in the pore volume distribution of samples H5, H9, and H13. The pore sizes are reduced below around 200 nm and enhanced above this point. PSD analysis using the NMR and MICP methods gives similar results, with pore distribution made of meso- and macropores. The pressure injection of mercury in the MICP method is not enough to override the strong capillary pressure of pores <2 nm and some of the nanopore signal associated with micro-porosity is not detectable by low-field NMR [
57]. The same trend is also observed in the NMR results (
Figure 3). The NMR pore size distribution of samples H1 and H2 are in the smaller pore size range and show a lower porosity than other samples (H5, H8, H9, H13).