Electrochemical Removal of Ammonium Nitrogen and COD of Domestic Wastewater using Platinum Coated Titanium as an Anode Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode Preparation
2.2. Influent Source and Its Characteristics
2.3. Experimental Setup
2.4. Analytical Methods
2.5. Calculations
3. Results and Discussion
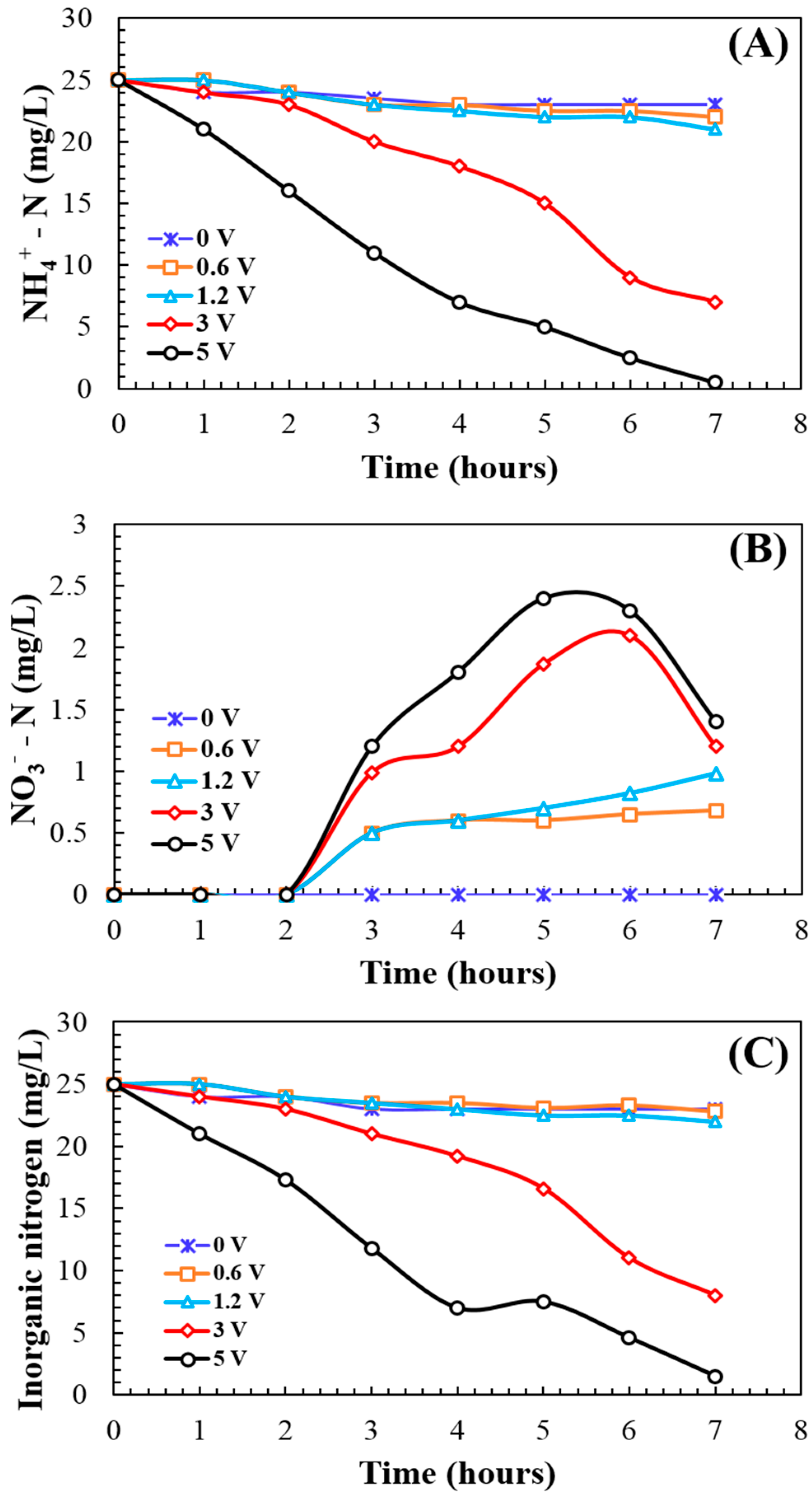
3.1. Ammonium Nitrogen
3.2. Nitrate Nitrogen
3.3. COD Removal
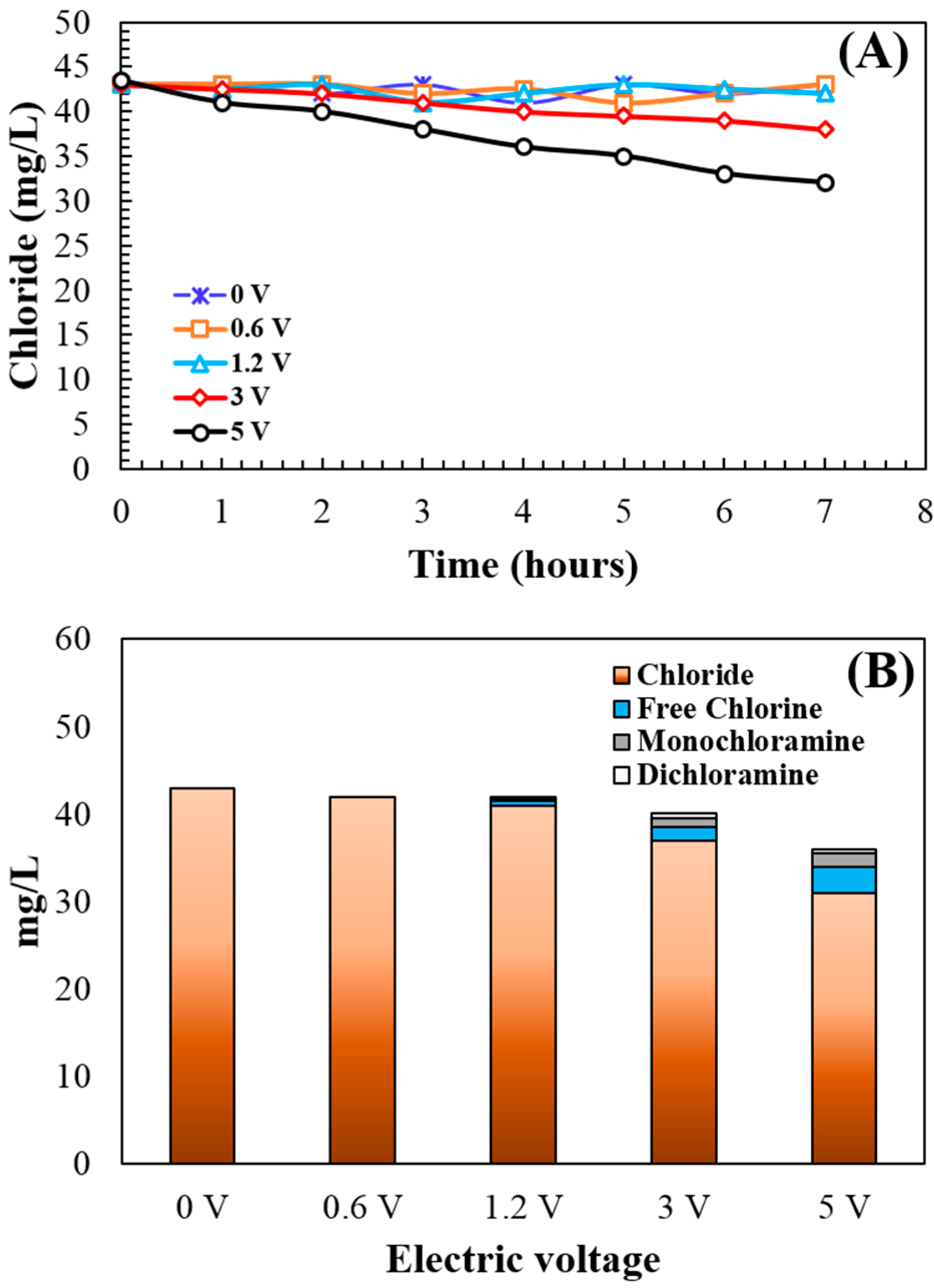
3.4. Chloride () Concentration
3.5. pH
3.6. Current Density and Energy Consumption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Della Monica, M.; Agostiano, A.; Ceglie, A. An electrochemical sewage treatment process. J. Appl. Electrochem. 1980, 10, 527–533. [Google Scholar] [CrossRef]
- Trompette, J.-L.; Vergnes, H. On the crucial influence of some supporting electrolytes during electrocoagulation in the presence of aluminum electrodes. J. Hazard. Mater. 2009, 163, 1282–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Liu, Y. Ammonia removal in electrochemical oxidation: Mechanism and pseudo-kinetics. J. Hazard. Mater. 2009, 161, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.P.; Chaudhari, S. Electrochemical denitrificaton of simulated ground water. Water Res. 2005, 39, 4065–4072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, J.; Bessarabov, D.; Sanderson, R. Review of electro-assisted methods for water purification. Desalination 1998, 115, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Sahu, O.P.; Chaudhari, P.K. Electrochemical treatment of sugar industry wastewater: COD and color removal. J. Electroanal. Chem. 2015, 739, 122–129. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.E.; Ra, C.S. Effects of electric voltage and sodium chloride level on electrolysis of swine wastewater. J. Hazard. Mater. 2010, 180, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Can, O.T.; Kobya, M.; Demirbas, E.; Bayramoglu, M. Treatment of the textile wastewater by combined electrocoagulation. Chemosphere 2006, 62, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Al-Shannag, M.; Bani-Melhem, K.; Al-Anber, Z.; Al-Qodah, Z. Enhancement of COD-nutrients removals and filterability of secondary clarifier municipal wastewater influent using electrocoagulation technique. Sep. Sci. Technol. 2013, 48, 673–680. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Qian, Y.; Wang, Y.; Zhang, L. Simultaneous removal of chemical oxygen demand, turbidity and hardness from biologically treated citric acid wastewater by electrochemical oxidation for reuse. Sep. Purif. Technol. 2013, 107, 281–288. [Google Scholar] [CrossRef]
- Bukhari, A.A. Investigation of the electro-coagulation treatment process for the removal of total suspended solids and turbidity from municipal wastewater. Bioresour. Technol. 2008, 99, 914–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouhezila, F.; Hariti, M.; Lounici, H.; Mameri, N. Treatment of the OUED SMAR town landfill leachate by an electrochemical reactor. Desalination 2011, 280, 347–353. [Google Scholar] [CrossRef]
- Chou, W.-L.; Wang, C.-T.; Chang, S.-Y. Study of COD and turbidity removal from real oxide-CMP wastewater by iron electrocoagulation and the evaluation of specific energy consumption. J. Hazard. Mater. 2009, 168, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.-C.; Chang, J.-E.; Wen, T.-C. Indirect oxidation effect in electrochemical oxidation treatment of landfill leachate. Water Res. 1995, 29, 671–678. [Google Scholar] [CrossRef]
- Alfaro, M.A.Q.; Ferro, S.; Martínez-Huitle, C.A.; Vong, Y.M. Boron doped diamond electrode for the wastewater treatment. J. Braz. Chem. Soc. 2006, 17, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, L.; Goel, R. Kinetic study of electrolytic ammonia removal using Ti/IrO2 as anode under different experimental conditions. J. Hazard. Mater. 2009, 167, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, M.; Eyvaz, M.; Kobya, M. Treatment of the textile wastewater by electrocoagulation: Economical evaluation. Chem. Eng. J. 2007, 128, 155–161. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Ibanez, J.G.; Swain, G.M. Electrochemistry and the environment. J. Appl. Electrochem. 1994, 24, 1077–1091. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Comninellis, C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim. Acta 1994, 39, 1857–1862. [Google Scholar] [CrossRef]
- Marinčić, L.; Leitz, F.B. Electro-oxidation of ammonia in waste water. J. Appl. Electrochem. 1978, 8, 333–345. [Google Scholar] [CrossRef]
- Vigo, F.; Uliana, C.; Novi, M. Electro-oxidation of sodium lauryl sulfate aqueous solutions. J. Appl. Electrochem. 1988, 18, 904–908. [Google Scholar] [CrossRef]
- Murphy, O.J.; Hitchens, G.D.; Kaba, L.; Verostko, C.E. Direct electrochemical oxidation of organics for wastewater treatment. Water Res. 1992, 26, 443–451. [Google Scholar] [CrossRef]
- Schultze, J.W. Sergio Trasatti (Ed.): Electrodes of Conductive Metallic Oxides, Part A. Elsevier Scientific Publishing Company: Amsterdam, The Netherlands; New York, NY, USA, 1980. Preis: 83.00 US $, 170.00 Dfl. Ber. Bunsenges. Phys. Chem. 1981, 85, 461–462. [Google Scholar] [CrossRef]
- Bryant, E.A.; Fulton, G.P.; Budd, G.C. Disinfection Alternatives for Safe Drinking Water; Van Nostrand Reinhold: New York, NY, USA, 1992; ISBN 0442318413. [Google Scholar]
- Association, A.P.H.; Association, A.W.W.; Federation, W.P.C.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1915; Volume 2, ISBN 8755-3546. [Google Scholar]
- Kim, K.-W.; Kim, Y.-J.; Kim, I.-T.; Park, G.-I.; Lee, E.-H. The electrolytic decomposition mechanism of ammonia to nitrogen at an IrO2 anode. Electrochim. Acta 2005, 50, 4356–4364. [Google Scholar] [CrossRef]
- Bard, A.J.; Ketelaar, J.A.A. Encyclopedia of Electrochemistry of the Elements. J. Electrochem. Soc. 1974, 121, 212C. [Google Scholar] [CrossRef]
- Jung, H.; Choi, Y.; Oh, J.; Lim, G. Recent trends in temperature and precipitation over South Korea. Int. J. Climatol. A J. R. Meteorol. Soc. 2002, 22, 1327–1337. [Google Scholar] [CrossRef] [Green Version]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)—Science and applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef]
- Lin, S.H.; Wu, C.L. Electrochemical removal of nitrite and ammonia for aquaculture. Water Res. 1996, 30, 715–721. [Google Scholar] [CrossRef]
- De Vooys, A.C.A.; Van Santen, R.A.; Van Veen, J.A.R. Electrocatalytic reduction of NO3− on palladium/copper electrodes. J. Mol. Catal. A Chem. 2000, 154, 203–215. [Google Scholar] [CrossRef]
- Kapałka, A.; Cally, A.; Neodo, S.; Comninellis, C.; Wächter, M.; Udert, K.M. Electrochemical behavior of ammonia at Ni/Ni (OH)2 electrode. Electrochem. Commun. 2010, 12, 18–21. [Google Scholar] [CrossRef]
- Bockris, J.; Kim, J. Electrochemical treatment of low-level nuclear wastes. J. Appl. Electrochem. 1997, 27, 623–634. [Google Scholar] [CrossRef]
- Rashid, M.M.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen production by water electrolysis: A review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int. J. Eng. Adv. Technol. 2015, 4, 2249–8958. [Google Scholar]
- Díaz, V.; Ibáñez, R.; Gómez, P.; Urtiaga, A.M.; Ortiz, I. Kinetics of electro-oxidation of ammonia-N, nitrites and COD from a recirculating aquaculture saline water system using BDD anodes. Water Res. 2011, 45, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Ramanujam, T.K.; Balasubramanian, N. In Situ Hypochlorous Acid Generation for the Treatment of Distillery Spentwash. Ind. Eng. Chem. Res. 1999, 38, 2264–2267. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Fóti, G.; Gandini, D.; Comninellis, C.; Perret, A.; Haenni, W. Oxidation of organics by intermediates of water discharge on IrO2 and synthetic diamond anodes. Electrochem. Solid State Lett. 1999, 2, 228–230. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Shim, Y.-B.; Lee, B.; Choi, S.-Y.; Won, M.-S. Electrochemical degradation of phenol and 2-chlorophenol using Pt/Ti and boron-doped diamond electrodes. Bull. Korean Chem. Soc. 2012, 33, 2274–2278. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Yue, P.L. Separation of pollutants from restaurant wastewater by electrocoagulation. Sep. Purif. Technol. 2000, 19, 65–76. [Google Scholar] [CrossRef]
- Krstajić, N.; Nakić, V.; Spasojević, M. Hypochlorite production. I. A model of the cathodic reactions. J. Appl. Electrochem. 1987, 17, 77–81. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Szpyrkowicz, L.; Naumczyk, J.; Zilio-Grandi, F. Electrochemical treatment of tannery wastewater using TiPt and Ti/Pt/Ir electrodes. Water Res. 1995, 29, 517–524. [Google Scholar] [CrossRef]
- Wang, B.; Kong, W.; Ma, H. Electrochemical treatment of paper mill wastewater using three-dimensional electrodes with Ti/Co/SnO2-Sb2O5 anode. J. Hazard. Mater. 2007, 146, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Can, W.; Yao-Kun, H.; Qing, Z.; Min, J. Treatment of secondary effluent using a three-dimensional electrode system: COD removal, biotoxicity assessment, and disinfection effects. Chem. Eng. J. 2014, 243, 1–6. [Google Scholar] [CrossRef]
- Fockedey, E.; Van Lierde, A. Coupling of anodic and cathodic reactions for phenol electro-oxidation using three-dimensional electrodes. Water Res. 2002, 36, 4169–4175. [Google Scholar] [CrossRef]



| Characteristics | Concentration |
|---|---|
| pH | 7.12 |
| Electrical conductivity | 0.65 mS/cm |
| Ammonium nitrogen | 25.4 mg/L |
| Nitrate | <20 µg/L |
| Nitrite | <20 µg/L |
| COD | 283 mg/L |
| Chloride | 43.1 mg/L |
| Turbidity | 27 NTU |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, U.; Jang, M.; Jung, S.P.; Park, D.; Park, S.J.; Yu, H.; Oh, S.-E. Electrochemical Removal of Ammonium Nitrogen and COD of Domestic Wastewater using Platinum Coated Titanium as an Anode Electrode. Energies 2019, 12, 883. https://doi.org/10.3390/en12050883
Ghimire U, Jang M, Jung SP, Park D, Park SJ, Yu H, Oh S-E. Electrochemical Removal of Ammonium Nitrogen and COD of Domestic Wastewater using Platinum Coated Titanium as an Anode Electrode. Energies. 2019; 12(5):883. https://doi.org/10.3390/en12050883
Chicago/Turabian StyleGhimire, Umesh, Min Jang, Sokhee P. Jung, Daeryong Park, Se Jin Park, Hanchao Yu, and Sang-Eun Oh. 2019. "Electrochemical Removal of Ammonium Nitrogen and COD of Domestic Wastewater using Platinum Coated Titanium as an Anode Electrode" Energies 12, no. 5: 883. https://doi.org/10.3390/en12050883





