Effect of Dispersion Solvents in Catalyst Inks on the Performance and Durability of Catalyst Layers in Proton Exchange Membrane Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
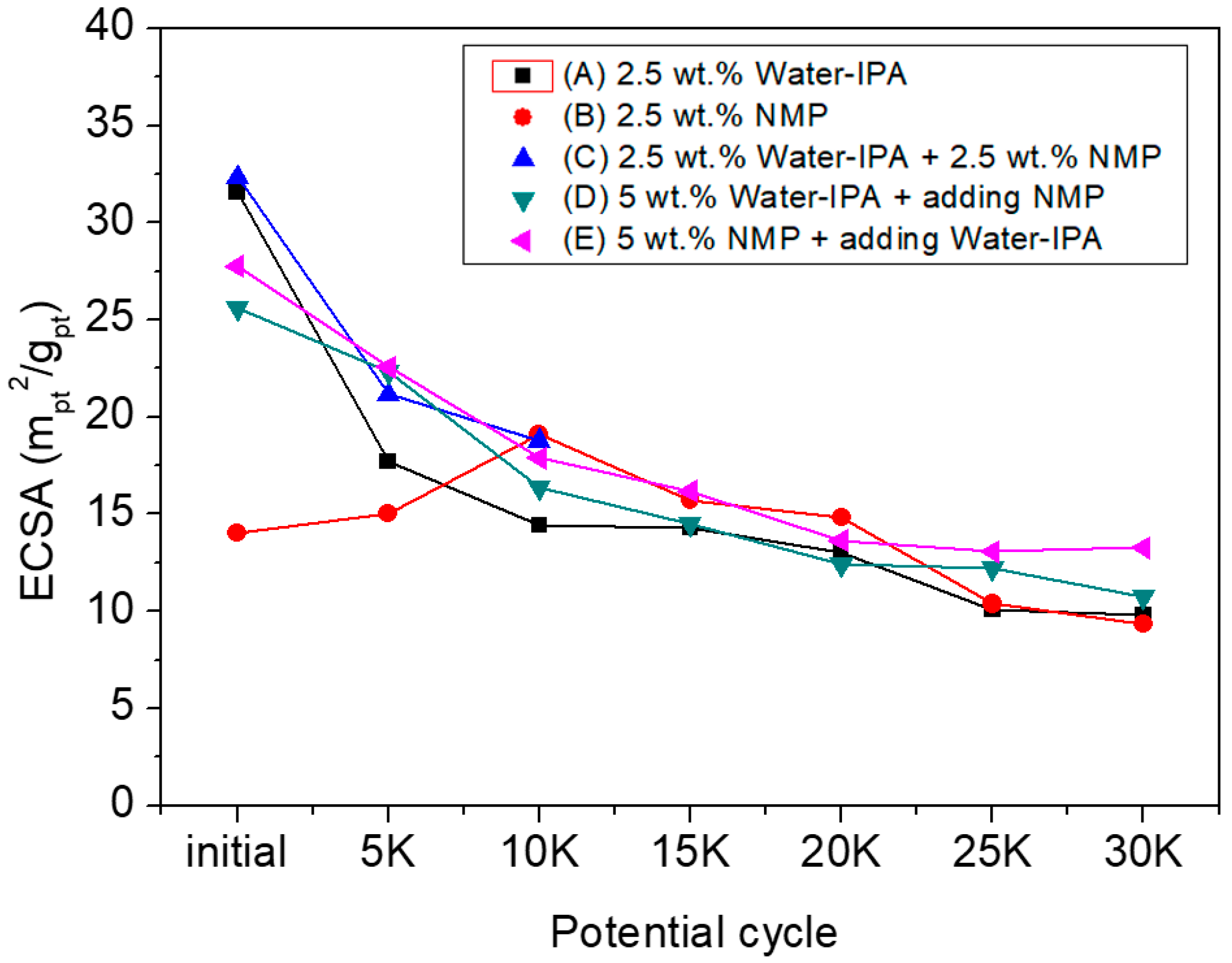
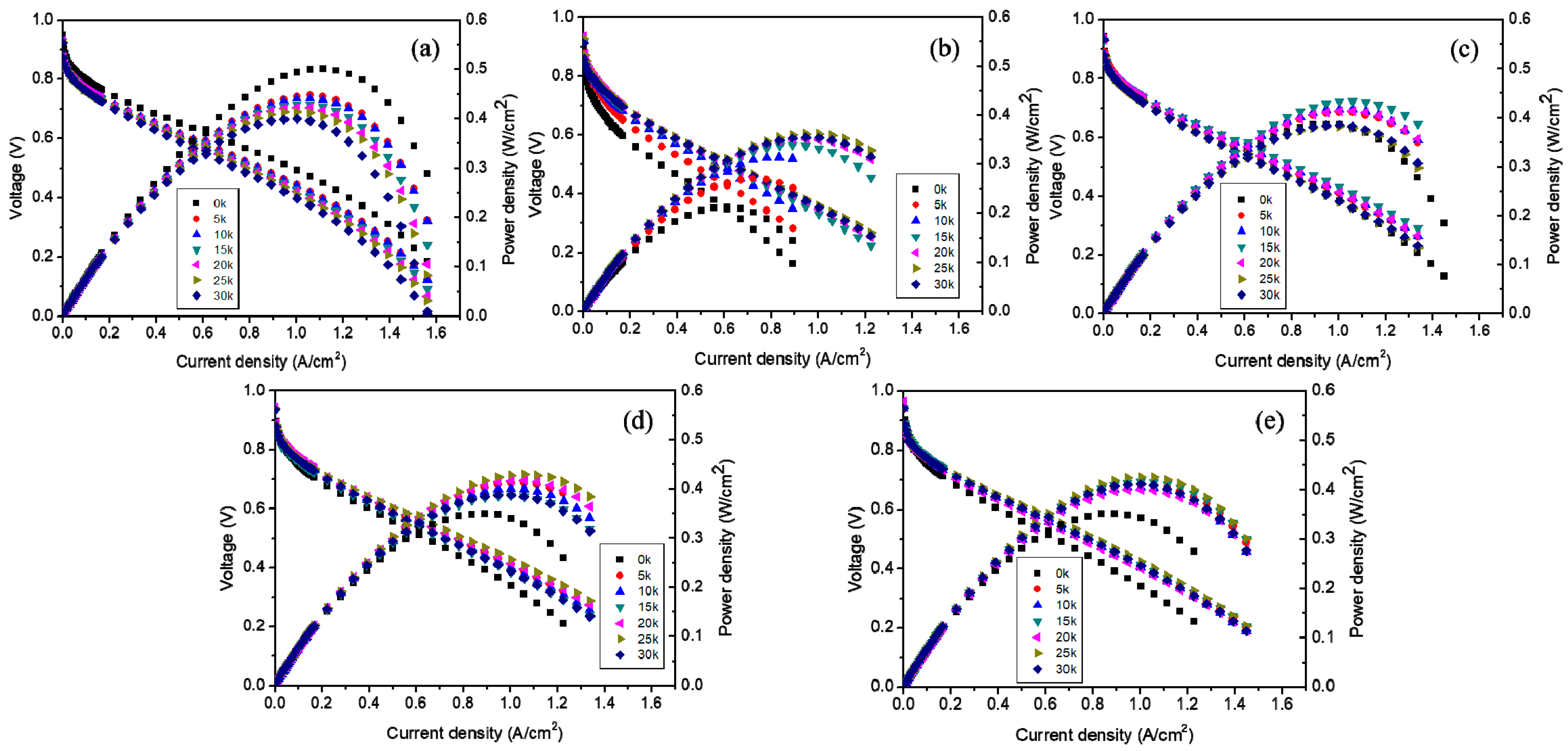
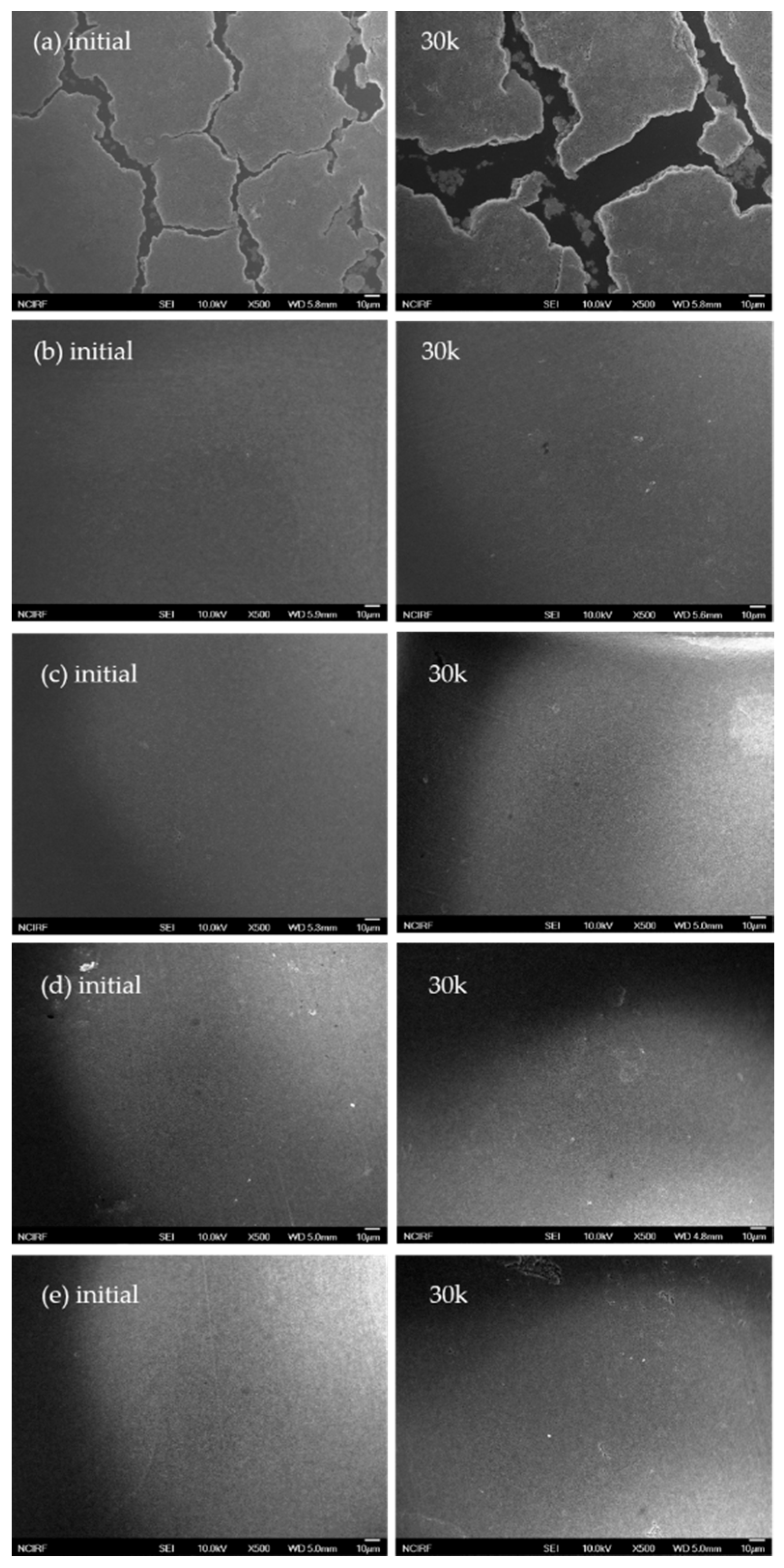
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, J.-S.; Shin, M.-S.; Kim, C.-S. Proton exchange membranes for fuel cell operation at low relative humidity and intermediate temperature: An updated review. Curr. Opin. Electrochem. 2017, 5, 43–55. [Google Scholar] [CrossRef]
- Park, J.-S.; Choi, Y.-W. High durable anion-conducting ionomer binder formed by on-site crosslinking. Chem. Lett. 2013, 42, 998–1000. [Google Scholar] [CrossRef]
- Song, C.-H.; Park, J.-S. Membrane-electrode assemblies with patterned electrodes for proton exchange membrane fuel cells. Chem. Lett. 2018, 47, 196–199. [Google Scholar] [CrossRef]
- Radev, I.; Koutzarov, K.; Pfrang, A.; Tsotridis, G. The influence of the membrane thickness on the performance and durability of PEFC during dynamic aging. Int. J. Hydrogen Energy 2012, 37, 11862–11870. [Google Scholar] [CrossRef]
- Kim, K.-H.; Lee, K.-Y.; Kim, H.-J.; Cho, E.; Lee, S.-Y.; Lim, T.-H.; Yoon, S.P.; Hwang, I.C.; Jang, J.H. The effects of Nafion® ionomer content in PEMFC MEAs prepared by a catalyst-coated membrane (CCM) spraying method. Int. J. Hydrogen Energy 2010, 35, 2119–2126. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, J.; Rios, G.M.; Kim, H.-J.; Lee, S.-Y.; Cho, E.; Lim, T.-H.; Jang, J.H. Effect of ionomer content and relative humidity on polymer electrolyte membrane fuel cell (PEMFC) performance of membrane-electrode assemblies (MEAs) prepared by decal transfer method. Int. J. Hydrogen Energy 2010, 35, 9678–9686. [Google Scholar] [CrossRef]
- Suzuki, A.; Sen, U.; Hattori, T.; Nagumo, R.; Tsuboi, H.; Hatakeyama, N.; Takaba, H.; Williams, M.C.; Miyamoto, A. Ionomer content in the catalyst layer of polymer electrolyte membrane fuel cell (PEMFC): Effects on diffusion and performance. Int. J. Hydrogen Energy 2011, 36, 2221–2229. [Google Scholar] [CrossRef]
- Uchida, M.; Aoyama, Y.; Eda, N.; Ohta, A. Investigation of the microstructure in the catalyst layer and effects of both perfluorosulfonate ionomer and PTFE-loaded carbon on the catalyst layer of polymer electrolyte fuel cells. J. Electrochem. Soc. 1995, 142, 4143–4149. [Google Scholar] [CrossRef]
- Paganin, V.A.; Ticianelli, E.A.; Gonzalez, E.R. Development and electrochemical studies of gas diffusion electrodes for polymer electrolyte fuel cells. J. Appl. Electrochem. 1996, 26, 297–304. [Google Scholar] [CrossRef]
- Antolini, E.; Giorgi, L.; Pozio, A.; Passalacqua, E. Influence of Nafion loading in the catalyst layer of gas-diffusion electrodes for PEFC. J. Power Sources 1999, 77, 136–142. [Google Scholar] [CrossRef]
- Park, Y.-C.; Tokiwa, H.; Kakinuma, K.; Watanabe, M.; Uchida, M. Effects of carbon supports on Pt distribution, ionomer coverage and cathode performance for polymer electrolyte fuel cells. J. Power Sources 2016, 315, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Haro, M.; Guétaz, L.; Printemps, T.; Morin, A.; Escribano, S.; Jouneau, P.-H.; Bayle-Guillemaud, P.; Chandezon, F.; Gebel, G. Three-dimensional analysis of Nafion layers in fuel cell electrodes. Nat. Commun. 2014, 5, 5529. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Uchida, M.; Yano, H.; Tryk, D.A.; Uchida, H.; Watanabe, M. New evaluation method for the effectiveness of platinum/carbon electrocatalysts under operating conditions. Electrochim. Acta 2010, 55, 8504–8512. [Google Scholar] [CrossRef]
- Kim, T.-H.; Yi, J.-Y.; Jung, C.-Y.; Jeong, E.; Yi, S.-C. Solvent effect on the Nafion agglomerate morphology in the catalyst layer of the proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2017, 42, 478–485. [Google Scholar] [CrossRef]
- Orfanidi, A.; Rheinländer, P.J.; Schulte, N.; Gasteiger, H.A. Ink solvent dependence of the ionomer distribution in the catalyst layer of a PEMFC. J. Electrochem. Soc. 2018, 165, F1254–F1263. [Google Scholar] [CrossRef]
- Jung, C.-Y.; Yi, S.-C. Improved polarization of mesoporous electrodes of a proton exchange membrane fuel cell using N-methyl-2-pyrrolidinone. Electrochim. Acta 2013, 113, 37–41. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, S.; Jiang, L.; Xia, Z.; Sun, H.; Sun, G. High Pt utilization catalyst prepared by ion exchange method for direct methanol fuel cells. Int. J. Hydrogen Energy 2012, 37, 14543–14548. [Google Scholar] [CrossRef] [Green Version]
- Omata, T.; Tanaka, M.; Miyatake, K.; Uchida, M.; Uchida, H.; Watanabe, M. Preparation and fuel cell performance of catalyst layers using sulfonated polyimide ionomers. Appl. Mater. Interfaces 2012, 4, 730–737. [Google Scholar] [CrossRef]
- Rolfi, A.; Oldani, C.; Merlo, L.; Facchi, D.; Ruffo, R. New perfluorinated ionomer with improved oxygen permeability for application in cathode polymeric electrolyte membrane fuel cell. J. Power Sources 2018, 396, 95–101. [Google Scholar] [CrossRef]
- Stassi, A.; Gatto, I.; Passalacqua, E.; Antonucci, V.; Aricò, A.S.; Merlo, L.; Oldani, C.; Pagano, E. Performance comparison of long and short-side chain perfluorosulfonic membranes for high temperature polymer electrolyte membrane fuel cell opersion. J. Power Sources 2011, 196, 8925–8930. [Google Scholar] [CrossRef]
- Welch, C.; Labouriau, A.; Hjelm, R.; Orler, B.; Johnston, C.; Kim, Y.S. Nafion in dilute solvent systems: Dispersion or solution? ACS Macro Lett. 2012, 1, 1403–1407. [Google Scholar] [CrossRef]
- Johnston, C.M.; Lee, K.-S.; Rockward, T.; Labouriau, A.; Mack, N.; Kim, Y.S. Impact of solvent on ionomer structure and fuel cell durability. ECS Trans. 2009, 25, 1617–1622. [Google Scholar]
- Huang, D.-C.; Yu, P.-J.; Liu, F.-J.; Huang, S.-L.; Hsueh, K.-L.; Chen, Y.-C.; Wu, C.-H.; Chang, W.-C.; Tsau, F.-H. Effect of dispersion solvent in catalyst ink on proton exchange membrane fuel cell performance. Int. J. Electrochem. Sci. 2011, 6, 2551–2565. [Google Scholar]
- Choi, B.; Langlois, D.A.; Mack, N.; Johnston, C.M.; Kim, Y.S. The effect of cathode structures on Nafion membrane durability. J. Electrochem. Soc. 2014, 161, F1154–F1162. [Google Scholar] [CrossRef]
- Fuel Cell Tech Team Accelerated Stress Test and Polarization Curve Protocols for PEM Fuel Cells. Available online: https://www.energy.gov/sites/prod/files/2015/08/f25/fcto_dwg_usdrive_fctt_accelerated_stress_tests_jan2013.pdf (accessed on 28 November 2018).
- Paul, D.K.; Karan, K.; Docoslis, A.; Giorgi, J.B.; Pearce, J. Characteristics of self-assembled ultrathin Nafion films. Macromolecules 2013, 46, 3461–3475. [Google Scholar] [CrossRef]
- Karan, K. PEFC catalyst layer: Recent advances in materials, microstructural characterization, and modeling. Curr. Opin. Electrochem. 2017, 5, 27–35. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Zhang, J.; Wang, J.; Hou, Z.; Mao, Z. Diagnosis of membrane electrode assembly degradation with drive cycle test technique. Int. J. Hydrogen Energy 2014, 39, 14370–14375. [Google Scholar] [CrossRef]
- Kim, S.M.; Ahn, C.-Y.; Cho, Y.-H.; Kim, S.; Hwang, W.; Jang, S.; Shin, S.; Lee, G.; Sung, Y.-E.; Choi, M. High-performance fuel cell with stretched catalyst-coated membrane: One-step formation of cracked electrode. Sci. Rep. 2016, 6, 26503–26509. [Google Scholar] [CrossRef]


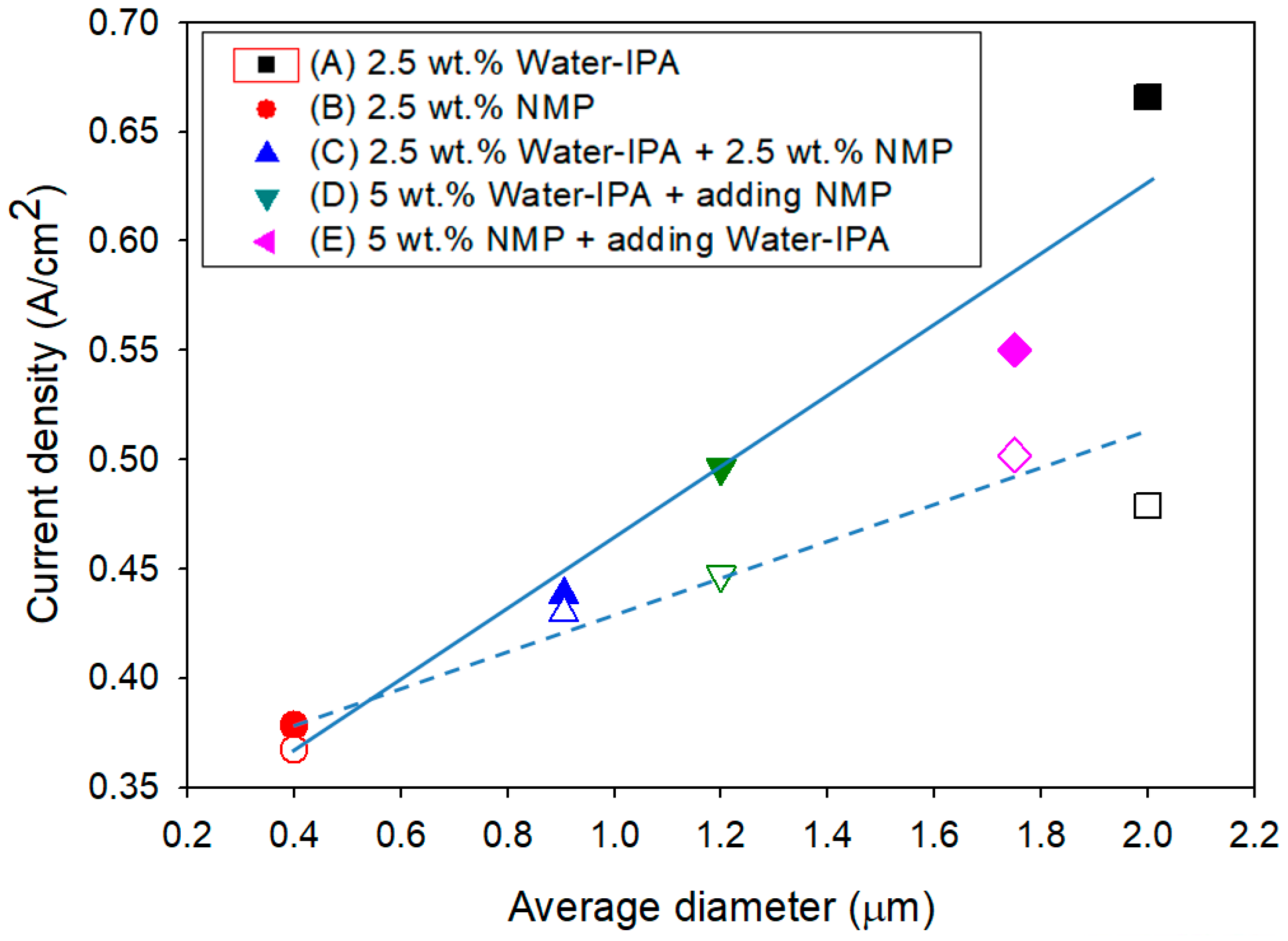
| 2.5 wt.% Dispersions | Average Diameter (μm) | D10 1 | D50 1 | D90 1 | Span 2 | |
|---|---|---|---|---|---|---|
| Main Dispersion | Additional Solvent | |||||
| 2.5 wt.% water–IPA (1:1) | - | 2.00 ± 0.03 | 0.440 ± 0.106 | 34.7 ± 7.7 | 62.4 ± 3.5 | 1.78 |
| 2.5 wt.% NMP | - | 0.400 ± 0.023 | 0.028 ± 0.023 | 0.297 ± 0.072 | 0.849 ± 0.167 | 2.76 |
| 2.5 wt.% water–IPA (1:1)+2.5 wt.% NMP | - | 0.905 ± 0.017 | 0.094 ± 0.075 | 1.32 ± 0.073 | 6.60 ± 0.77 | 4.95 |
| 5 wt.% water–IPA (1:1) | NMP | 1.20 ± 0.04 | 0.046 ± 0.017 | 2.11 ± 0.62 | 7.32 ± 1.80 | 3.45 |
| 5 wt.% NMP | Water–IPA (1:1) | 1.75 ± 0.07 | 0.20 ± 0.04 | 3.61 ± 0.21 | 12.8 ± 2.81 | 3.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.-H.; Park, J.-S. Effect of Dispersion Solvents in Catalyst Inks on the Performance and Durability of Catalyst Layers in Proton Exchange Membrane Fuel Cells. Energies 2019, 12, 549. https://doi.org/10.3390/en12030549
Song C-H, Park J-S. Effect of Dispersion Solvents in Catalyst Inks on the Performance and Durability of Catalyst Layers in Proton Exchange Membrane Fuel Cells. Energies. 2019; 12(3):549. https://doi.org/10.3390/en12030549
Chicago/Turabian StyleSong, Chan-Ho, and Jin-Soo Park. 2019. "Effect of Dispersion Solvents in Catalyst Inks on the Performance and Durability of Catalyst Layers in Proton Exchange Membrane Fuel Cells" Energies 12, no. 3: 549. https://doi.org/10.3390/en12030549






