1. Introduction
In the last few decades, power generation started to turn to alternative energy sources, such as wind energy and photovoltaics, as well as unconventional sources of heat, such as geothermal energy, solar heat, biomass-firing plants, or waste heat from other industrial processes. Generally, these unconventional heat sources do not have as high energy density as the conventional ones nor a high cycle efficiency, since the heat is supplied at a lower temperature level. Nevertheless, they can perform better under specific circumstances.
Conventional Rankine Cycle-based thermal power plants use water as working fluid. Because of the thermal properties of the water, the cycle cannot exploit such unconventional heat sources efficiently. Organic Rankine Cycle (ORC) provides an alternative solution, because it uses non-conventional working fluids, mainly organic ones. These unconventional working fluids are suitable for power generation from various low-temperature heat sources [
1,
2], including geothermal heat [
3,
4] or low-temperature industrial waste heat [
5]. According to the ORC World Map [
6], in the middle of 2016, the number of installed major ORC units exceeded 700.
Obviously, using different working fluids would also influence the actual layout. In some cases, it requires a superheater, a recuperator, or other auxiliary units [
7]. Also, the type of expander in use depends on the fluid properties [
8,
9,
10,
11], but this problem is not addressed in this paper.
Traditionally, the working fluid for a given ORC is selected using a trial-and-error procedure through experience from chemically similar materials, although recently there were more and more attempts to use chemical or physical properties to predict the applicability of a given fluid as an ORC working fluid. In some cases, simple optimization algorithms are used to select the ideal existing working fluid, while, in other cases, the optimization is based on molecular or equation-of-state parameters [
12,
13,
14,
15,
16,
17]. Usually, possible candidates are identified using information collected from similar systems. In this way, one might risk excluding novel, previously unused working fluids, which could be better than any traditional ones.
A novel classification method was developed by us [
18] not only to describe and categorize all the potential working fluids, but also to use some elements of the classification as a tool for finding the best working fluid for a given heat source. A short review of the novel classification is given in
Section 3. The basis of the new classification is the relative location of some characteristic points on the saturation curve of a given material in specific entropy–temperature diagram. Using one or two of these points and correlating them with the isochoric molar specific heat capacities of the equilibrium vapor phase, we are able to give a “rule of thumb” to select working fluids, where droplet formation during expansion and, hence, the potentially harmful droplet erosion of turbine blades—common for traditional and some organic Rankine processes using wet working fluids [
19,
20]—can be avoided. In this way, several new potential working fluids can be found and used for further evaluation, using general criteria for ORC working fluid selection including thermodynamics [
18,
21], safety and environmental impact [
22,
23], availability, compatibility, and cost-effectiveness [
24], or most often, a combination of these criteria.
2. Traditional Classification
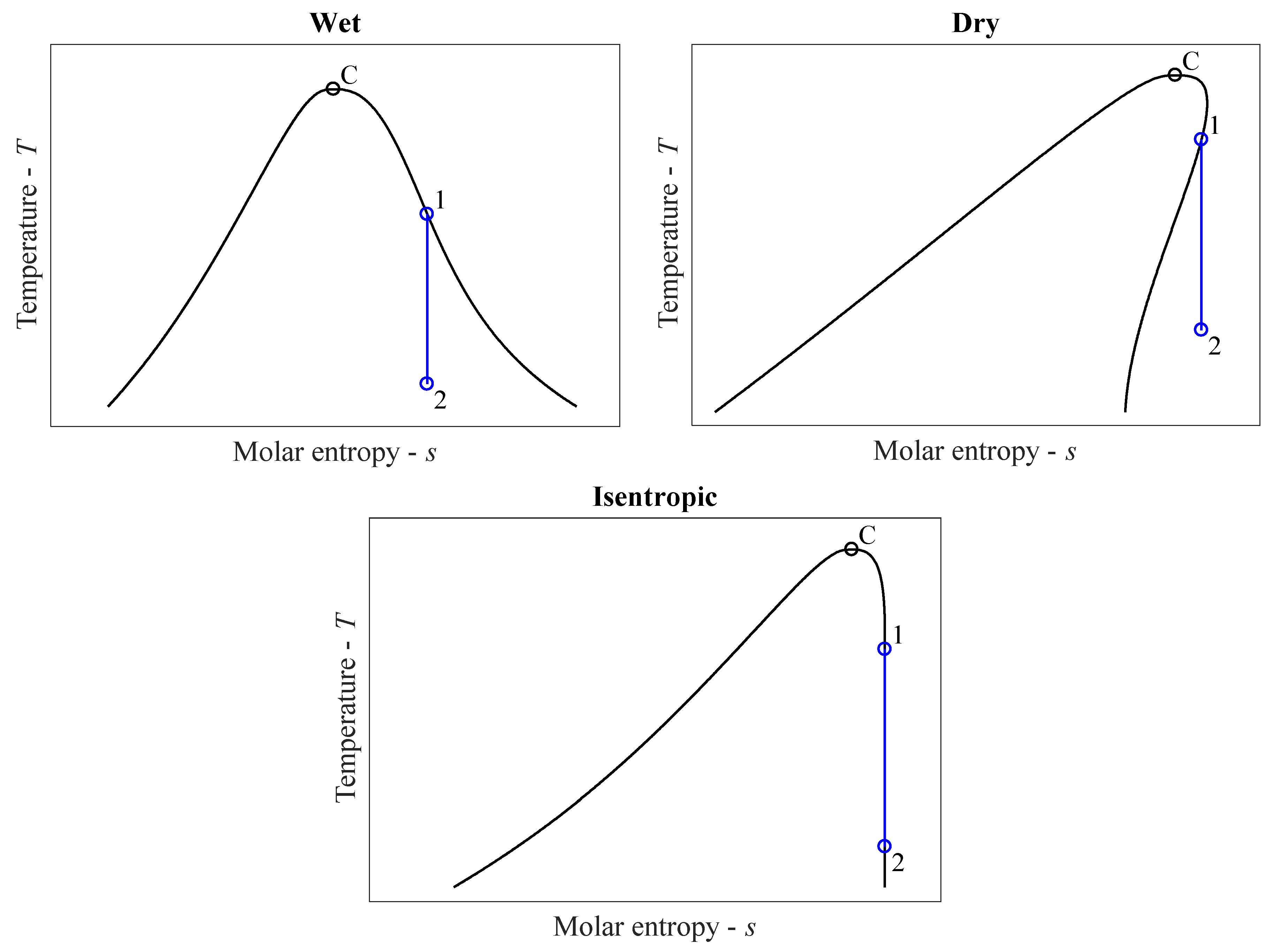
The widespread traditional classification of working fluids is based on the shape of the vapor saturation curve of the fluids and, hence, the quality of the expanded vapor at the outlet of the turbine [
21,
25]. Expander is a more common term in ORC technology, which refers to the differences in size and construction compared to steam turbines. The traditional classification of ORC working fluids differentiates three categories, namely wet, dry, and isentropic, usually with a smooth transition between them [
12,
24,
26,
27,
28].
The working fluid is called wet or wet-type if an isentropic (adiabatic and reversible) expansion starting from a saturated vapor state will end in the two-phase region, which is also called “saturated liquid vapor region” or “wet region”. The vapor curve of a wet fluid in a temperature–entropy diagram has a negative slope at each point (dT/ds < 0). The working fluid is called dry if an isentropic expansion starting from a saturated vapor state will end in the single-phase vapor region, which is also called “superheated vapor region” or sometimes simply “dry region”. The vapor curve of dry fluids has mainly a positive slope (dT/ds > 0). At high temperatures, the vapor curve has necessarily a negative slope, where the entropy increases until reaching a maximum value (ds/dT = 0), and then the curve bends back to smaller entropy values. Finally, the working fluid is called isentropic if an isentropic expansion starts and ends in a saturated vapor state. The saturated vapor curve of an ideal isentropic fluid has an infinite slope (dT/ds → ∞) at each point after reaching the point where ds/dT = 0.
The visualization of the three traditional categories can be seen in
Figure 1, marking isentropic expansion processes with initial (1) and final (2) states.
Deficiencies in Traditional Classification
Owing to the spread of ORC technology and, hence, the application of new working fluids, the traditional classification is deemed narrow and does not reflect significant differences between fluids from the same categories. These differences have theoretical and even practical significance. The development and the wide variety of applied working fluids raise the need for a more sophisticated classification, which contains more precise information about the applicability of the fluid.
Concerning dry fluids, the “dryness” has practical significance and an effect on the layout of the ORC power plant. After the isentropic expansion of the fluid in the expander (turbine), the vapor is in a superheated state. The extent of superheating also has a great effect on the thermal efficiency of the cycle. Also, there is a mainly theoretical issue relating to the dryness of the fluid. If the fluid is “very dry”, it bends back greatly to lower entropies; however, at least underneath the critical point, it becomes possible to fully liquefy vapor using an isentropic compression and vice versa (to fully evaporate liquid using an isentropic expansion) [
18].
Another very significant issue relates to the isentropic category. It is not proven, but neither real fluid properties available in the NIST Chemistry Webbook database (NIST) Chemistry Webbook database [
29] nor theoretical analyses of model fluids [
7,
30] confirm the existence of any fluids with a perfectly isentropic saturation vapor curve. It means that, for real or even model fluids, entropy cannot be constant in a finite temperature range. These theoretical analyses also show that probably all isentropic fluids have an S-shaped vapor saturation curve (negative–positive–negative slopes in sequence). In practice, we can find some fluids that approach the “ideal” isentropic behavior at least in a smaller part of the vapor curve, for instance, a common refrigerant, trichlorofluoromethane (CCl
3F, R-11). These facts inspired the authors to redefine the meaning of isentropic-type fluids. According to the new definition, the fluid is isentropic if it is possible to find an isentropic expansion line starting from and ending in a saturated vapor state, whereby the expansion line does not enter the two-phase (wet) region. A “real” isentropic-type working fluid can be seen in
Figure 2 (biggest diagram), which shows the importance of the reverse S-shaped vapor curve by displaying three isentropic expansion lines (blue, red, and green). In the first case (blue), the working fluid behaves like a wet type, because the expansion ends in the wet region. In the second case (red), isentropic behavior can be observed (initial and final points of the expansion located on the saturation curve). In the third case (green), the fluid shows dry behavior,
Since the expansion ends in the dry (superheated vapor) region. The quality of the vapor during the expansion is maybe the most crucial issue when designing a power plant, since it has strict limits regarding the extent of liquid droplet formation in the expander for safety and lifetime reasons.
4. Application and Correlations
Novel classification can help in finding thermodynamically optimal working fluids for a given heat source. Since other thermodynamic cycles use various types of working fluids such as refrigeration cycles or heat pumps, creation of a database that contains the main thermodynamic properties and the sequence of the working fluids or refrigerants makes the search even easier and faster. Our database includes 74 working fluids (data taken from the NIST [
29]), and continuous updates and expansions can make it valuable not only for researchers, but also for the industry. Since the triple-point temperature of some working fluids is far below the normal environmental circumstances, the database also includes the sequence of all 74 working fluids at 15 °C ambient temperature (
TA = 15 °C), as well for a more realistic limit for most applications (except cryogenic cycles [
31] where temperature can approach the triple point of most relevant working fluids). The present form of the database is included in
Table S1 (
Supplementary Materials).
After introducing the novel classification, our research turned to finding a simple, measurable physical property with the help of which it is possible to easily differentiate the eight sequences or at least the traditional categories (dry, wet, and isentropic). Based on the results of studies on model fluids [
7], isochoric molar specific heat capacity (
cV) seemed to be a proper candidate to establish some correlation with the novel classes. Therefore, our research focused on examining the
cV of the saturated vapor curve of real working fluids [
32] from the NIST database.
Figure 3 displays the isochoric specific heat capacity values (given in mass-related kJ/kg·K units) of the saturated vapor curve of working fluids available in the NIST database with respect to their reduced temperature (defined as actual temperature in Kelvin divided by the critical temperature of the given fluid, also in Kelvin). Blue lines represent wet-type, red lines represent isentropic-type, and green lines represents dry-type sequences. Circles and squares mark points M and N, previously defined on the temperature–entropy curve of the given material. No correlation between the fluid categories (wet, dry, and isentropic) and the isochoric specific heat capacity values can be seen here. On the other hand, a strong correlation between categories and the isochoric “molar” specific heat capacity was reported for model fluids [
7,
30].
Using molar heat capacity values might be unusual for engineering applications, but one should be aware that, in several cases, when comparing materials with the same mass—instead of the same mole number—it might be misleading. For example, the isochoric heat capacity of helium—expressed in J/g∙K or kJ/kg∙K—is one order of magnitude higher than that of argon; however, while using J/mol∙K or kJ/kmol∙K, these quantities are equal. The reason for this difference is that the molar mass of argon is ten times higher than the molar mass of helium and, accordingly, in the same mass, one can find ten times more helium atoms than argon ones. Therefore, in certain analyses, the use of properties related to the same amount (instead of the same mass) of substances is advised (i.e., using quantities with mol, instead of kg).
Figure 4 displays the molar isochoric specific heat capacity values of the saturated vapor curve of working fluids with respect to their reduced temperature (circles and squares mark points M and N; blue lines represent wet-type, red lines represent isentropic-type, and green lines represent dry-type sequences). Here, one can see a strong correlation between the fluid category and the molar heat capacity. It can easily be seen that wet-type fluids, i.e., ACZ sequences, are located exclusively at the bottom part of the diagram. Above this ACZ region, there is a narrow transition zone where isentropic-type fluids, i.e., ACNMZ sequences, appear. ACNMZ may be said to be the closest in behavior to wet-type fluids (see
Figure 2). Right until the appearance of the first dry-type fluid (AZCM), only sequence ACNMZ of isentropic-type fluids can be found. Above this region, only sequences starting with AN, where the characteristic point N has a lower entropy value than point C, exist. The disappearance of sequences starting with AC can be explained by the fact that, if we consider a wet–dry global transition, then isentropic fluids have to reflect this in some intermediate steps; thus, with the increase of
cV, they become closer and closer to dry-type behavior, with point N shifting below point C in the entropy scale. As we go higher with
cV, only the dry-type sequence AZCM and isentropic-type sequence ANZCM exist. The NIST database seems to have a shortage in dry-type fluids, but it is probable that, above a certain
cV value, only dry-type fluids with sequence AZCM can exist. Nevertheless, there are some sequences (AZCM) mixed with isentropic-type fluids; it can also be observed that these fluids have a high triple-point temperature compared to their critical temperature.
In
Figure 4, square markers represent characteristic points N, while circle markers represent points M of each fluid. Although a kind of regularity (U-shape) can be discovered in the relative location of the characteristic points, it is still not clear how to find the right way to describe them with a trend line, since these measured values should be supported with theoretical explanation as well. Nevertheless, an outer envelope (dotted curve) can be drawn, and a rule of thumb can be established by appointing the minimum of this envelope as a border point between wet and non-wet fluids. This point is plotted in
Figure 3 and is indicated by a triangle marker at the lowest molar isochoric specific heat capacity value of the envelope; here, points M and N are identical. According to this rule, if the isochoric specific heat capacity of a fluid in a saturated vapor state is smaller than 80 J/(mol∙K) at its reduced temperature of 0.74 (0.74∙
TC), corresponding to the minimum on the dotted envelope, marked with a triangle, then the fluid is very possibly a wet-type one (ACZ). However, when it is higher than 80 J/(mol∙K), then the fluid is most probably isentropic or dry.
This value almost coincides with characteristic point N of trichlorofluoromethane (CCl
3F) that was already said to be practically an “ideal” isentropic fluid; thus, it is one of the best examples for describing the start of the wet isentropic transition region. The difference between the molar entropy of the characteristic points M and N of CCl
3F is approximately 0.12 J/(mol∙K); hence, they are almost identical. A similar rule of thumb can be established for the relative position of points C and N; however, so far, it has been made only for model fluids [
33].
In addition to this rule of thumb, two other results can be deduced from the presentation used in
Figure 4. All
cV lines are plotted from the triple-point temperature to the critical one. It can be seen that, for dry working fluids (green lines), the absence of point N is simply caused by the fact that freezing happens at temperatures higher than the expected crossing of the
cV line and the outer envelope (represented by the dotted line) of points N and M. It means that, by using dry working fluids, which have the tendency to supercool (i.e., remain liquid for a while below the freezing point, e.g., glycerol), one might obtain wet vapor during expansion instead of the expected dry one. For model fluids (i.e., for fluids described by van der Waals of by Redlich–Kwong equations of state) the presence of this low-temperature local entropy minimum (point N) can be found for all substances [
7,
30,
33], but this is the first indirect experimental proof for its general existence in real fluids.
Finally, one can see that sharp borders between various novel working fluid classes do not exist. However, taking a fixed reduced temperature value (for example,
T = 0.74·
Tc, given above), one can assume that all fluids with
cV > 165 J/(mol∙K) should be either dry or ANZCM-type, while those with 80 J/(mol∙K) <
cV < 105 J/(mol∙K) are probably ACNZM-type. These differences are crucial not only for ORC applications, but also for other thermodynamic cycles such as the Trilateral Flash Cycle (TFC) [
33].
5. Conclusions
The traditional classification of working fluids with its three categories (wet, dry, isentropic), is not sufficient to reliably predict or exclude the formation of liquid droplets in the low-pressure stage of the expander of an ORC power plant. In this paper, a brief overview is given for a novel and more sophisticated classification [
17], which eliminates these deficiencies and makes it easier to select working fluids for ORC applications. The novel classification contains eight categories, also called sequences. These categories have different thermodynamic characteristics and they require different layouts for ORC machinery. A database was created to make the choice of optimal working fluid even easier and faster. The database contains 74 fluids (so far) with their significant thermodynamic properties and their traditional and novel classifications.
Using the location of two of these characteristic points in isochoric (molar) heat capacity–reduced temperature space, a rule of thumb can be defined to distinguish between wet and non-wet working fluids. If the molar isochoric heat capacity of the saturated vapor phase of the given working fluid is smaller than 80 J/(mol∙K) at a temperature 0.74∙
Tc, then it is most likely a wet fluid (or ACZ according to the novel classification), while fluids with heat capacity values above this limit (at this temperature) are very likely dry or isentropic. Real fluids show the theoretically proven trend that, with the increase of molar isochoric heat capacity, the fluid is more and more probably isentropic or dry. In a similar way, one can assume that all fluids with
cV > 165 J/(mol∙K) at
T = 0.74·
Tc temperature are either dry or ANZCM-type, while the ones with 80 J/(mol∙K) <
cV < 105 J/(mol∙K) are probably ACNZM-type. These correlations can be seen only when isochoric heat capacities are plotted in molar units (in J/(mol∙K)), which shows the importance of molar quantities in comparative analysis. There are other methods—developed by Garrido et al. [
12,
13] and White et al. [
27]—to separate wet fluids from dry (and isentropic) ones, using various quantities. Combining the criterion of isochoric molar heat capacity given in this paper with the other two criteria (given for critical molar volume [
27] and for a material-dependent generalized psi-function [
12,
13]) should give an even more accurate selection method for classifying a fluid as wet or dry/isentropic. Therefore, the usage of the three methods in combination is strongly advised.
Finally, it was shown that, even for the so-called dry fluids, a local entropy maximum (point N) might exist, i.e., they might have a low-temperature part on their saturated vapor line (in the T–s diagram) with a negative slope. Therefore, having a properly deep expansion in these dry fluids, the final state might be wet. Since this part of the saturation curves usually “hides” below the freezing point, it can be relevant only for fluids where supercooling of the liquid phase is possible.