Effect of Aerobic Hydrolysis on Anaerobic Fermentation Characteristics of Various Parts of Corn Stover and the Scum Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
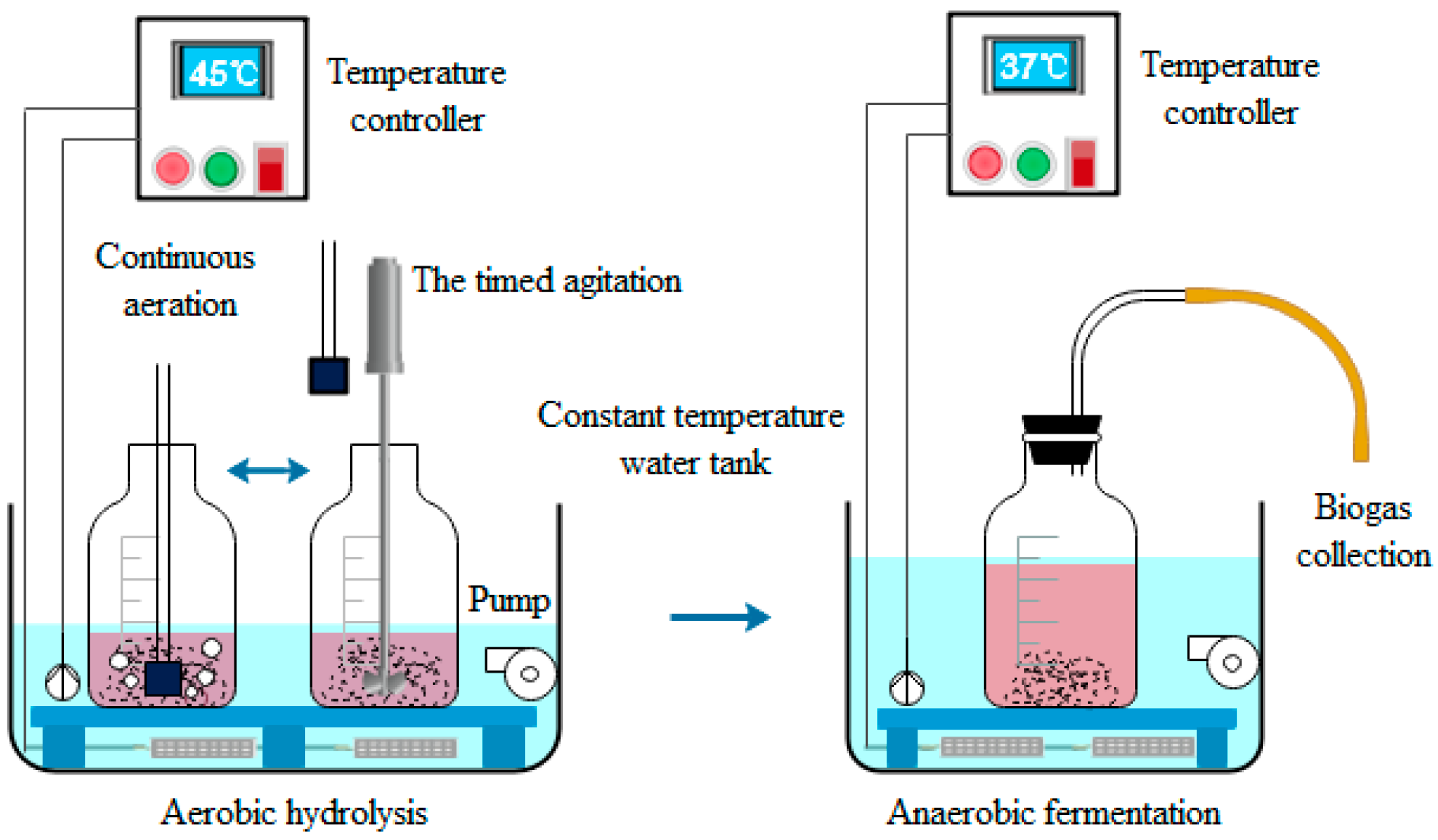
2.2.1. Aerobic Hydrolysis Stage
2.2.2. Anaerobic Fermentation Stage
2.3. Measurement Method
2.4. Methane Production Model and Data Analysis
3. Results and Discussion
3.1. Aerobic Hydrolysis Stage
3.1.1. pH and VFAs
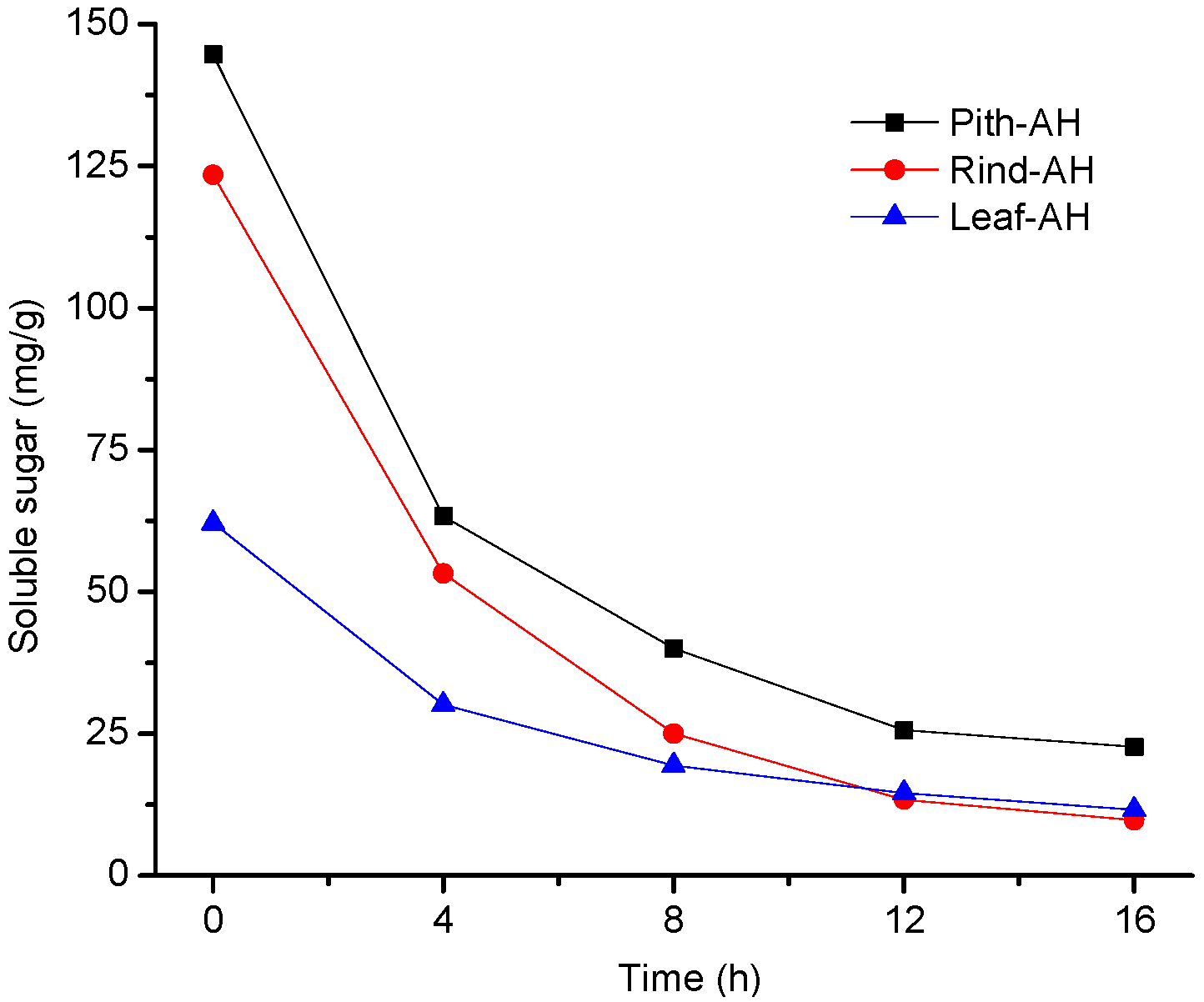
3.1.2. Soluble Sugars
3.1.3. Lignocellulose
3.1.4. FTIR Analysis
3.2. Anaerobic Fermentation Stage
3.2.1. Changes in the Scum Layer
3.2.2. Cumulative Yield of Methane
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, L.; Li, X.; Zheng, M. Influence on inoculum sources on the anaerobic digestion of corn stover. In Proceedings of the International Conference on Biomass Energy Technologies, Guangzhou, China, 3–5 December 2008; pp. 459–463. [Google Scholar]
- Guo, P.; Mochidzuki, K.; Cheng, W.; Zhou, M.; Gao, H.; Zheng, D.; Wang, X.; Cui, Z. Effects of different pretreatment strategies on corn stalk acidogenic fermentation using a microbial consortium. Bioresour. Technol. 2011, 102, 7526–7531. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.C. Plant fiber chemistry; China Light Industry Press: Beijing, China, 2012. [Google Scholar]
- Parawira, W.; Murto, M.; Zvauya, R.; Mattiasson, B. Anaerobic batch digestion of solid potato waste alone and in combination with sugar beet leaves. Renew. Energy 2004, 29, 1811–1823. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, L.X.; Dong, B.C.; WAN, X.C.; GAO, X.X. The status and trends of the development of biogas plants for crop straws in China. Renew. Energy Resour. 2010, 2010, 145–162. [Google Scholar]
- Cui, W.W.; Liang, J.F.; Du, L.Z.; Zhang, K.G. The current situation and problems of the large-scale biogas plants for straw in China. Chin. Agric. Sci. Bull. 2013, 29, 121–125. [Google Scholar]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste—Activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Yang, D.Y.; Pang, Y.A.; Yuan, H.R.; Chen, S.L.; Ma, J.W.; Yu, L.; Li, X.J. Enhancing biogas production from anaerobically digested wheat straw through ammonia pretreatment. Chin. J. Chem. Eng. 2014, 22, 576–582. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, Q.; Ma, Z.; Li, B.; Zhao, X. Physical and chemical characterizations of corn stalk resulting from hydrogen peroxide presoaking prior to ammonia fiber expansion pretreatment. Crops Prod. 2016, 83, 86–93. [Google Scholar] [CrossRef]
- Hassan, M.; Ding, W.; Bi, J.; Mehryar, E.; Talha, Z.A.A.; Huang, H. Methane enhancement through oxidative cleavage and alkali solubilization pre-treatments for corn stover with anaerobic activated sludge. Bioresour. Technol. 2016, 200, 405–412. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Liu, C.; Sun, Y.; Yang, F.; Liu, J. Effect of solar irradiance on photo biochemical transformation process of direct absorption methane digester. Energy Convers. Manag. 2018, 172, 173–181. [Google Scholar] [CrossRef]
- Fu, S.F.; He, S.; Shi, X.S.; Katukuri, N.R.; Dai, M.; Guo, R.B. The chemical properties and microbial community characterization of the thermophilic microaerobic pretreatment process. Bioresour. Technol. 2015, 198, 497–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.Q.; Xie, H.; Chen, W.; Wang, E.T.; Du, F.G.; Song, A.D. Biological pretreatment of corn stover with ligninolytic enzyme for high efficient enzymatic hydrolysis. Bioresour. Technol. 2013, 144, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.O.; Schwarzenauer, T.; Illmer, P. Improvement of methane generation capacity by aerobic pre-treatment of organic waste with a cellulolytic Trichoderma viride culture. J. Environ. Manag. 2013, 129, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Satjaritanun, P.; Khunatorn, Y.; Vorayos, N.; Shimpalee, S.; Bringley, E. Numerical analysis of the mixing characteristic for napier grass in the continuous stirring tank reactor for biogas production. Biomass Bioenergy 2016, 86, 53–64. [Google Scholar] [CrossRef]
- Kaparaju, P.; Angelidaki, I. Effect of temperature and active biogas process on passive separation of digested manure. Bioresour. Technol. 2008, 99, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.T.; Bolte, J.P. Methane production from low solid concentration liquid swine waste conventional anaerobic fermentation. Bioresour. Technol. 2000, 74, 241–247. [Google Scholar] [CrossRef]
- Li, S.; Mei, Z.; Liu, P.; Xi, J.; Kong, C. Harmfulness of Scum in the Corn Straw Fermentation and Influence Factors of Scum Forming. China Biogas 2015, 33, 42–44. [Google Scholar]
- Li, W.; Ding, Q.; Wei, D.; Luo, W.; Wang, Q.; Wang, J. Aerobic and Anaerobic Two-phase Fermentation Process and Biogas Production Characteristic of Rice Straw. Trans. Chin. Soc. Agric. Mach. 2016, 47, 150–157. [Google Scholar]
- Ramos, I.; Fdz-Polanco, M. The potential of oxygen to improve the stability of anaerobic reactors during unbalanced conditions: Results from a pilot-scale digester treating sewage sludge. Bioresour. Technol. 2013, 140, 80–85. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Luo, L.; Zhang, H.; Li, P.; Zhang, X.; Xu, X.; Zhang, B. Effects of Aerobic Pre-Treatment on Anaerobic Batch Digestion of Corn Straw. Biobased Mater. Bioenergy 2017, 11, 622–628. [Google Scholar] [CrossRef]
- Hu, Y.; Pang, Y.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Chuf, W.A.; Jaffar, M.; Li, X. Promoting anaerobic biogasification of corn stover through biological pretreatment by liquid fraction of digestate (LFD). Bioresour. Technol. 2015, 175, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Ximenes, E.; Ladisch, M.R.; Mosier, N.S.; Vermerris, W.; Huang, C.P.; Sherman, D.M. Tissue-specific biomass recalcitrance in corn stover pretreated with liquid hot-water:enzymatic hydrolysis (part 1). Biotechnol. Bioeng. 2012, 109, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H. Kinetic study of the anaerobic digestion of swine manure at mesophilic temperature: A lab scale batch operation. J. Biosyst. Eng. 2012, 37, 233–244. [Google Scholar] [CrossRef]
- Benbelkacem, H.; Bayard, R.; Abdelhay, A.; Zhang, Y.; Gourdon, R. Effect of leachate injection modes on municipal solid waste degradation in anaerobic bioreactor. Bioresour. Technol. 2010, 101, 5206–5212. [Google Scholar] [CrossRef] [PubMed]
- Díaz, I.; Donoso-Bravo, A.; Fdz-Polanco, M. Effect of microaerobic conditions on the degradation kinetics of cellulose. Bioresour. Technol. 2011, 102, 10139–10142. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Wang, P.; Guo, S. Improving hydrolysis acidification by limited aeration in the pretreatment of petrochemical wastewater. Bioresour. Technol. 2015, 194, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Jagadabhi, P.S.; Kaparaju, P.; Rintala, J. Effect of micro-aeration and leachate replacement on COD solubilization and VFA production during mono-digestion of grass-silage in one-stage leach-bed reactors. Bioresour. Technol. 2010, 101, 2818–2824. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Li, P.; Luo, Z.; Qin, P.; Chen, C.; Wang, Y.; Wang, Z.; Tan, T. Effect of dilute alkaline pretreatment on the conversion of different parts of corn stalk to fermentable sugars and its application in acetone-butanol-ethanol fermentation. Bioresour. Technol. 2016, 211, 117–124. [Google Scholar] [CrossRef]
- Carrillo, F.; Colom, X.; Sunol, J.J.; Saurina, J. Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Luo, Z.; Li, P.; Cai, D.; Chen, Q.; Qin, P.; Tan, T.; Cao, H. Comparison of performances of corn fiber plastic composites made from different parts of corn stalk. Ind. Crops Prod. 2017, 95, 521–527. [Google Scholar] [CrossRef]
- Xiong, X.; Shi, G.Z.; Mei, Z.L.; Kong, C.X. Influence of Different Corn stalk Particle-size on Scum Crusting in Anaerobic Fermentation. China Biogas 2017, 35, 56–61. [Google Scholar]
- Zhang, J.; Wang, Y.H.; Qu, Y.S.; Wei, Q.Y.; Li, H.Q. Effect of the organizational difference of corn stalk on hemicellulose extraction and enzymatic hydrolysis. Ind. Crops Prod. 2018, 112, 698–704. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Liu, L. The inhomogeneity of corn stover and its effects on bioconversion. Biomass Bioenergy 2011, 35, 1940–1945. [Google Scholar]
- Zhang, B.; Jiang, T.; Gao, L.; Wei, X.; Liu, K.; Yang, X. Aerogenesis characteristics of swine feces mixed with corn straw under mesophilic fermentation. Chin. J. Environ. Eng. 2014, 8, 4991–4997. [Google Scholar]
- Zhong, W.; Zhang, Z.; Luo, Y.; Sun, S.; Qiao, W.; Xiao, M. Effect of biological pretreatments in enhancing corn straw biogas production. Bioresour. Technol. 2011, 102, 11177–11182. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, R.; He, Y.; Li, Y.; Feng, L.; Chen, C.; Liu, G. Influence of particle size and alkaline pretreatment on the anaerobic digestion of corn stover. Bioresources 2013, 8, 5850–5860. [Google Scholar] [CrossRef]





| Parameters | Units | Pith | Rind | Leaf | Inoculum |
|---|---|---|---|---|---|
| Total solids (TS) | % | 94.14 ± 0.65 | 93.41 ± 0.55 | 93.08 ± 0.44 | 1.78 ± 0.22 |
| Volatile solids (VS) | % | 90.14 ± 0.59 | 90.46 ± 0.78 | 84.98 ± 0.26 | 0.98 ± 0.13 |
| Carbon (C) | % | 40.05 ± 0.05 | 42.06 ± 0.06 | 40.28 ± 0.07 | 30.04 ± 0.04 |
| Nitrogen (N) | % | 0.51 ± 0.01 | 0.61 ± 0.01 | 0.95 ± 0.02 | 2.55 ± 0.10 |
| pH | - | - | - | - | 7.29 ± 0.01 |
| Ash | % | 3.73 ± 0.15 | 3.2 ± 0.03 | 8.26 ± 0.25 | - |
| Soluble sugar | mg/g | 144.67 ± 2.05 | 123.49 ± 2.36 | 62.10 ± 1.45 | - |
| Cellulose | %TS | 23.44 ± 0.19 c | 33.20 ± 0.15 a | 25.12 ± 0.13 b | 15.55 ± 0.06 |
| Hemicellulose | %TS | 24.54 ± 0.30 b | 22.73 ± 0.29 c | 32.05 ± 0.25 a | 8.73 ± 0.22 |
| Lignin | %TS | 5.30 ± 0.09 b | 7.85 ± 0.07 a | 5.35 ± 0.06 b | 12.9 ± 0.21 |
| Hydrolysis Time | Gompertz | First-Order | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P∞ (mLg−1VS) | Rm (mLg−1VS d) | (d) | R2 | T90 (d) | B∞ (mL g−1VS) | kH (d−1) | Lp (d) | R2 | |
| Pith-AH-0h | 239.80 ± 2.19 | 29.10 ± 1.08 | 1.62 ± 0.16 | 0.9955 | 12 | 260.45 ± 7.27 | 0.15 ± 0.01 | 1.39 ± 0.17 | 0.9819 |
| Pith-AH-4h | 280.89 ± 2.88 | 30.54 ± 1.26 | 1.05 ± 0.20 | 0.9930 | 10 | 295.01 ± 2.81 | 0.17 ± 0.01 | 0.82 ± 0.08 | 0.9967 |
| Pith-AH-8h | 302.69 ± 1.47 | 51.84 ± 1.49 | 0.80 ± 0.09 | 0.9973 | 9 | 312.07 ± 4.55 | 0.26 ± 0.02 | 1.05 ± 0.12 | 0.9834 |
| Pith-AH-12h | 323.77 ± 1.56 | 56.81 ± 1.70 | 0.74 ± 0.09 | 0.9969 | 8 | 331.17 ± 2.58 | 0.28 ± 0.01 | 0.94 ± 0.07 | 0.9939 |
| Pith-AH-16h | 309.34 ± 3.24 | 49.66 ± 3.18 | 0.24 ± 0.22 | 0.9842 | 8 | 315.55 ± 2.08 | 0.28 ± 0.01 | 0.75 ± 0.06 | 0.9952 |
| Rind-AH-0h | 193.36 ± 2.82 | 20.21 ± 1.05 | 1.06 ± 0.25 | 0.9897 | 13 | 208.35 ± 4.95 | 0.15 ± 0.01 | 1.16 ± 0.15 | 0.9875 |
| Rind-AH-4h | 201.24 ± 2.33 | 27.98 ± 1.74 | 0.80 ± 0.24 | 0.9849 | 9 | 206.30 ± 1.62 | 0.24 ± 0.01 | 0.77 ± 0.07 | 0.9951 |
| Rind-AH-8h | 248.70 ± 2.74 | 39.27 ± 2.62 | 0.72 ± 0.23 | 0.9829 | 8 | 253.84 ± 1.71 | 0.28 ± 0.01 | 0.74 ± 0.06 | 0.9951 |
| Rind-AH-12h | 251.00 ± 1.31 | 43.81 ± 1.41 | 0.73 ± 0.10 | 0.9963 | 8 | 256.87 ± 1.99 | 0.28 ± 0.01 | 0.94 ± 0.07 | 0.9941 |
| Rind-AH-16h | 233.82 ± 1.07 | 40.34 ± 1.10 | 0.76 ± 0.08 | 0.9975 | 8 | 240.91 ± 3.54 | 0.26 ± 0.02 | 1.05 ± 0.12 | 0.9829 |
| Leaf-AH-0h | 242.78 ± 1.55 | 30.56 ± 0.82 | 1.75 ± 0.11 | 0.9977 | 13 | 263.58 ± 8.80 | 0.15 ± 0.02 | 1.35 ± 0.21 | 0.9736 |
| Leaf-AH-4h | 241.26 ± 0.76 | 39.74 ± 0.75 | 0.57 ± 0.06 | 0.9987 | 9 | 246.64 ± 2.18 | 0.27 ± 0.01 | 0.84 ± 0.08 | 0.9924 |
| Leaf-AH-8h | 264.39 ± 1.32 | 51.68 ± 1.79 | 0.57 ± 0.10 | 0.9957 | 8 | 268.94 ± 1.61 | 0.33 ± 0.01 | 0.83 ± 0.05 | 0.9951 |
| Leaf-AH-12h | 245.28 ± 1.42 | 56.66 ± 2.58 | 0.52 ± 0.11 | 0.9926 | 8 | 248.20 ± 1.00 | 0.40 ± 0.01 | 0.80 ± 0.04 | 0.9970 |
| Leaf-AH-16h | 231.23 ± 1.51 | 59.65 ± 3.33 | 0.48 ± 0.12 | 0.9903 | 7 | 233.47 ± 0.84 | 0.46 ± 0.01 | 0.76 ± 0.03 | 0.9971 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Li, W.; Xu, X.; Li, P.; Li, N.; Zhang, H.; Sun, Y. Effect of Aerobic Hydrolysis on Anaerobic Fermentation Characteristics of Various Parts of Corn Stover and the Scum Layer. Energies 2019, 12, 381. https://doi.org/10.3390/en12030381
Zhang B, Li W, Xu X, Li P, Li N, Zhang H, Sun Y. Effect of Aerobic Hydrolysis on Anaerobic Fermentation Characteristics of Various Parts of Corn Stover and the Scum Layer. Energies. 2019; 12(3):381. https://doi.org/10.3390/en12030381
Chicago/Turabian StyleZhang, Bo, Wenzhe Li, Xiang Xu, Pengfei Li, Nan Li, Hongqiong Zhang, and Yong Sun. 2019. "Effect of Aerobic Hydrolysis on Anaerobic Fermentation Characteristics of Various Parts of Corn Stover and the Scum Layer" Energies 12, no. 3: 381. https://doi.org/10.3390/en12030381





