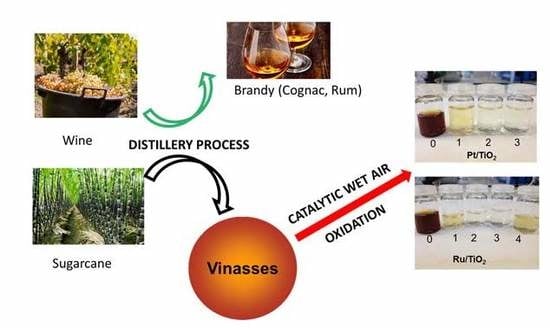
Catalytic Wet Air Oxidation Using Supported Pt and Ru Catalysts for Treatment of Distillery Wastewater (Cognac and Sugarcane Vinasses)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalysts
2.3. Apparatus and Procedure
2.3.1. Reactors
2.3.2. Analyses
3. Results and Discussion
3.1. Characterization of Catalysts
3.2. Oxidation of vVinasse “de Bonne Chauffe” (VBC)
3.2.1. Blank Experiment without Catalyst
3.2.2. Oxidation Over the Supported Ru Catalysts
3.2.3. Oxidation Over Supported Pt Catalysts
3.2.4. Summary of the VBC Treatment
3.3. Treatment of the Sugarcane Effluent
3.3.1. Oxidation in the Batch Reactor
3.3.2. Oxidation of Sugarcane Vinasse in a Trickle-Bed Reactor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| BOD | biological oxygen demand |
| COD | chemcal oxygen demand |
| CWAO | catalytic wet air oxidation |
| HPLC | High Performance Liquid Chromatography |
| ICP-OES | inductively coupled plasma optical emission spectroscopy |
| TEM | transmission electron microscopy |
| TN | Total Nitrogen |
| TOC | total organic carbon |
| XRD | X-ray diffraction |
| VBC | vinasse “bonne chauffe” |
References
- Tsakiris, A.; Kallithraka, S.; Kourkoutas, Y. Grape brandy production, composition and sensory evaluation. J. Sci. Food Agric. 2013, 94, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Lee, K.J.; Mulla, S.I.; Garg, N.; Chae, J.C. Chapter 2–Production of bioethanol from sugarcane bagasse: Current approaches and perspectives. In Applied Microbiology and Bioengineering; Academic Press: Cambridge, MA, USA, 2019; pp. 21–42. [Google Scholar] [CrossRef]
- Ravikumar, R.; Saravanan, R.; Vasanthi, N.S.; Swetha, J.; Akshaya, N.; Rajthilak, M.; Kanna, K.P. Biodegradation and decolorization of biomethanated distillery spent wash. Indian J. Sci. Technol. 2007, 1, 1–6. [Google Scholar]
- Patel, S. Jamaluddin, Treatment of distillery waste water: A review. Int. J. Theor. Appl. Sci. 2018, 10, 117–139. [Google Scholar]
- Available online: http://www.cognac.fr/cognac/_en/2_cognac/index.aspx (accessed on 9 September 2019).
- Song, L.; Wei, Y.; Bergiel, B.J. Cognac consumption: A comparative study on American and Chinese consumers. Wine Econ. Policy 2018, 7, 24–34. [Google Scholar] [CrossRef]
- Chandra, R.; Bharagava, R.N.; Rai, V. Melanoidins as major colourant in sugarcane molasses based distillery effluent and its degradation. Biores. Technol. 2008, 99, 4648–4660. [Google Scholar] [CrossRef] [PubMed]
- Wedzicha, B.L.; Kaputo, M.T. Melanoidins from glucose and glycine: Composition, characteristics, and reactivity towards sulphite ion. Food Chem. 1992, 43, 359–367. [Google Scholar] [CrossRef]
- Christofoletti, C.A.; Escher, J.P.; Correia, J.E.; Marinho, J.F.U.; Fontanetti, C.S. Sugarcane vinasse: Environmental implications of its use. Waste Manag. 2013, 33, 2752–2761. [Google Scholar] [CrossRef]
- Kumar, V.; Wati, L.; FitzGibbon, F.J.; Nigam, P.; Banat, I.M.; Singh, D.; Marchant, R. Bioremediation and decolourisation of anaerobically digested distillery spent wash. Biotechnol. Lett. 1997, 19, 311–313. [Google Scholar] [CrossRef]
- Agarwal, C.S.; Pandey, G.S. Soil pollution by spent wash discharge: Depletion of manganese (II) and impairment of its oxidation. J. Environ. Biol. 1994, 15, 49–53. [Google Scholar]
- Hoareau, J.; Caro, Y.; Grondin, I.; Petit, T. Sugarcane vinasse processing: Towards a status shift from waste to valuable resource. A review. J. Water Process. Eng. 2018, 24, 11–25. [Google Scholar] [CrossRef]
- Chowdhary, P.; Raj, A.; Bharagava, R.N. Environmental pollution and health hazards from distillery wastewater and treatment approaches to combat the environmental threats: A review. Chemosphere 2018, 194, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Gosh Ray, S.; Ghangrekar, M.M. Comprehensive review on treatment of high-strength distillery wastewater in advanced physico-chemical and biological degradation pathways. Int. J. Environm. Sci. Technol. 2019, 16, 527–546. [Google Scholar] [CrossRef]
- Ioannou, L.A.; Li Puma, G.; Fatta-Kassinos, D. Treatment of winery wastewater by physicochemical, biological and advanced processes: A review. J. Hazard. Mater. 2015, 286, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.; Adholeya, A. Biological approaches for treatment of distillery wastewater: A review. Biores. Technol. 2007, 98, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Bolzonella, D.; Papa, M.; Da Ros, C.; Muthukumar, L.A.; Rosso, D. Winery wastewater treatment: A critical overview of advanced biological processes. Critical Rev. Biotech. 2019, 39, 489–507. [Google Scholar] [CrossRef]
- Melamane, X.L.; Strong, P.J.; Burgess, J.E. Treatment of wine distillery wastewater: A review on anaerobic membrane reactors. S. Afr. J. Enol. Vitic. 2007, 28, 25–36. [Google Scholar] [CrossRef]
- Moraes, B.S.; Zaiat, M.; Bonomi, A. Anaerobic digestion of vinasse from sugarcane ethanol production. Renew. Sustain. Energy Rev. 2015, 44, 888–903. [Google Scholar] [CrossRef]
- Moletta, R. Winery and distillery wstewater treatment by anaerobic digestion. Water Sci. Technol. 2005, 51, 137–144. [Google Scholar] [CrossRef]
- Magalhaes, N.C.; Silva, A.L.D.; Amaral, M.C.S.; Lange, L.C.; Neta, L.F. Treatment of vinasse employing ultrafiltration combined with the aerobic bioreactor with membrane and post-treatment with nanofiltration allowing its reuse. Procedia Eng. 2012, 44, 1923–1924. [Google Scholar] [CrossRef]
- Singh, N.; Petrinic, I.; Helix-Nielsen, C.; Basu, S.; Balakrishnan, M. Concentrating molasses distillery wastewater using biomimetic forward osmosis (FO) membranes. Water Res. 2018, 130, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Prazeres, A.R.; Lelis, J.; Alves-Ferreira, J.; Carvalho, F. Treatment of vinasse from sugarcane ethanol industry: H2SO4, NaOH and Ca(OH)2 precipitations, FeCl3 coagulation-flocculation and atmospheric CO2 carbonation. J. Environ. Chem. Eng. 2019, 7, 103203. [Google Scholar] [CrossRef]
- Martins, R.C.; Pinto, F.L.; Castro-Silva, S.; Quinta-Ferreira, R.M. Flocculation, ozonation and Fenton’s process in the treatment of distillery effluents. J. Environ. Eng. 2013, 139, 110–116. [Google Scholar] [CrossRef]
- Hadavifar, M.; Zinatizadeh, A.A.; Younesi, H.; Galehdar, M. Fenton and photo-Fenton treatment of distillery effluent and optimization of treatment conditions with response surface methodology. Asia-Pacific J. Chem. Eng. 2009, 5, 454–464. [Google Scholar] [CrossRef]
- Sangave, P.C.; Gogate, P.R.; Pandit, A.B. Combination of ozonation with conventional aerobic oxidation for distillery wastewater treatment. Chemosphere 2007, 68, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Benitez, F.J.; Real, F.J.; Acero, J.L.; Garcia, J.; Sanchez, M. Kinetics of the ozonation and aerobic biodegradation of wine vinasses in discontinuous and continuous processes. J. Hazard. Mater. 2003, 101, 203–218. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A.; Li Puma, G. Treatment of winery wastewater by ozone-based advanced oxidaion processes (O3, O3/UV and O3/UV/H2O2) in a pilot-scale bubble column reactor and process economics. Sep. Purif. Technol. 2010, 72, 235–241. [Google Scholar] [CrossRef]
- De Souza, R.P.; Ferrari-Lima, A.M.; Pezoti, O.; Slusarski-Santana, V.; Luiz-Gimenez, M.; Fernandes-Machado, N.R.C. Photodegradation of sugarcane vinasse: Evaluation of the effect of vinasse pre-treatment and the crystalline phase of TiO2. Acta Sci. Technol. 2016, 38, 217–226. [Google Scholar] [CrossRef]
- Santana, V.S.; Machado, N.R.C.F. Photocatalytic degradation of the vinasse under solar radiation. Catal. Today 2008, 133, 606–610. [Google Scholar] [CrossRef]
- Manisankar, P.; Viswanathan, S.; Rani, S. Electrochemical treatment of distillery effluent using catalytic anodes. Green Chem. 2003, 5, 270–274. [Google Scholar] [CrossRef]
- Sangave, P.C.; Pandit, A.B. Ultrasound pretreatment for enhanced biodegradability of the distillery wastewater. Ultrason. Sonochem. 2004, 11, 197–203. [Google Scholar] [CrossRef]
- Sangave, P.C.; Gogate, P.R.; Pandit, A.B. Ultrasound and ozone assisted biological degradation of thermally pretreated and anaerobically pretreated distillery wstewater. Chemosphere 2007, 68, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Robles-Gonzales, V.; Galindez-Mayer, J.; Rinderknecht-Seijas, N.; Poggi-Varaldo, H.M. Treatment of mezscal vinasses: A review. J. Biotech. 2012, 157, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.E.R.; Bento, H.B.S.; Alves, T.M.; Carvalho, A.K.F.; De Castro, H.F. Vinasse treatment within the sugarcane-ethnaol industry using ozone combined with anaerobic and aerobic microbial processes. Environments 2019, 6, 5. [Google Scholar] [CrossRef]
- Luck, F. A review of industrial catalytic wet air oxidation processes. Catal. Today 1996, 27, 195–202. [Google Scholar] [CrossRef]
- Levec, J.; Pintar, A. Catalytic wet-air oxidation processes: A review. Catal. Today 2007, 124, 172–184. [Google Scholar] [CrossRef]
- Kim, K.H.; Ihm, S.K. Heterogeneous catalytic wet air oxidation of refractory organic pollutants in industrial wastewaters: A review. J. Hazard. Mater. 2011, 186, 16–34. [Google Scholar] [CrossRef]
- Malik, S.N.; Saratchandra, T.; Tembhekar, P.D.; Padolev, K.V.; Mudliar, S.L.; Mudliar, S.N. Wet air oxidation induced enhanced biodegradability of distillery effluent. J. Environ. Manag. 2014, 136, 132–138. [Google Scholar] [CrossRef]
- Bhoite, G.M.; Vaidhya, P.D. Improved biogas generation from biomethanated distillery wastewater by pretreatment with catalytic wet air oxidation. Ind. Eng. Chem. Res. 2018, 57, 2698–2704. [Google Scholar] [CrossRef]
- Domínguez, C.M.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Treatment of real winery wastewater by wet oxidation at mild temperature. Sep. Purif. Technol. 2014, 129, 121–128. [Google Scholar] [CrossRef]
- Belkacemi, K.; Hamoudi, S.; Larachi, F.; Sayari, A. Catalytic wet oxidation of high-strength alcohol-distillery liquors. Appl. Catal. A General 2000, 199, 199–209. [Google Scholar] [CrossRef]
- Kazemi, N.; Tavakoli, O.; Nahangi, M. High-strength distillery wastewater treatment using catalytic sub- and supercritical water. J. Supercrit. Fluids 2015, 97, 74–80. [Google Scholar] [CrossRef]
- Le, P.T.; Besson, M. Carbon and nitrogen removal from glucose-glycine melanoidins solution as a model of distillery wastewater by catalytic wet air oxidation. J. Hazard. Mat. 2016, 310, 108–116. [Google Scholar] [CrossRef]
- Available online: https://www.ercane.re/index.php?lang=en (accessed on 9 September 2019).
- Minh, D.P.; Gallezot, P.; Besson, M. Treatment of olive oil mill wastewater by catalytic wet air oxidation. 3. Stability of supported ruthenium catalysts during oxidation of model pollutant p-hydroxybenzoic acid in batch and continuous reactors. Appl. Catal. A Environ. 2007, 75, 71–77. [Google Scholar] [CrossRef]
- Lousteau, C.; Besson, M.; Descorme, C. Catalytic wet air oxidation of ammonia over supported noble metals. Catal. Today 2015, 241, 80–85. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P. Deactivation of metal catalysts in liquid phase organic reactions. Catal. Today 2003, 81, 547–559. [Google Scholar] [CrossRef]
- Bernardi, M.; Le Du, M.; Dodouche, I.; Descorme, C.; Deleris, S.; Blanchet, E.; Besson, M. Selective removal of the ammonium-nitrogen in ammonium acetate aqueous solutions by catalytic wet air oxidation over supported Pt catalysts. Appl. Catal. B Environ. 2012, 128, 64–71. [Google Scholar] [CrossRef]
- Barbier, J., Jr.; Delanoe, F.; Jabouille, F.; Duprez, D.; Blanchard, G.; Isnard, P. Total oxidation of acetic acid in aqueous solutions over noble metal catalysts. J. Catal. 1998, 177, 378–385. [Google Scholar] [CrossRef]
- Perkas, N.; Minh, D.P.; Gallezot, P.; Gedanken, A.; Besson, M. Platinum and ruthenium catalysts on mesoporous titanium and zirconium oxides for the catalytic wet air oxidation of model compounds. Appl. Catal. B Environ. 2005, 59, 121–130. [Google Scholar] [CrossRef]
- Mikulova, J.; Barbier, J., Jr.; Rossignol, S.; Mesnard, D.; Duprez, D.; Kappenstein, C. Wet air oxidation of acetic acid over platinum catalysts supported on cerium-based materials: Influence of metal and oxide crystallite size. J. Catal. 2007, 251, 172–181. [Google Scholar] [CrossRef]
- Grosjean, N.; Descorme, C.; Besson, M. Catalytic wet air oxidation of N,N-dimethylformamide aqueous solutions: Deactivation of TiO2 and ZrO2-supported noble metal catalysts. Appl. Catal. B Environ. 2010, 97, 276–283. [Google Scholar] [CrossRef]
- Migo, V.P.; Del Rosario, E.J.; Matsumura, M. Floculation of melanoidins induced by inorganic ions. J. Ferment. Bioeng. 1997, 83, 287–291. [Google Scholar] [CrossRef]
- Plavsic, M.; Cosovic, B.; Lee, C. Copper complexing propeties of melanoidins and marine humic material. Sci. Total Environ. 2006, 366, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: Oxford, UK, 1966. [Google Scholar]

 ), and NO2− (▲).
), and NO2− (▲).
 ), and NO2− (▲).
), and NO2− (▲).
 (4)).
(4)).
 (4)).
(4)).
 ), and NO2− (▲).
), and NO2− (▲).
 ), and NO2− (▲).
), and NO2− (▲).
 ) and NO2− (▲); and (b) concentrations of carboxylic (di)acids.
) and NO2− (▲); and (b) concentrations of carboxylic (di)acids.
 ) and NO2− (▲); and (b) concentrations of carboxylic (di)acids.
) and NO2− (▲); and (b) concentrations of carboxylic (di)acids.

 ).
).
 ).
).
 ), and Pt/ZrO2 (
), and Pt/ZrO2 (  ).
).
 ), and Pt/ZrO2 (
), and Pt/ZrO2 (  ).
).
 ) NO2− (▲). Empty symbols show the results of duplicate experiments.
) NO2− (▲). Empty symbols show the results of duplicate experiments.
 ) NO2− (▲). Empty symbols show the results of duplicate experiments.
) NO2− (▲). Empty symbols show the results of duplicate experiments.


 , nitrate) and (d) the leaching of Ru during these different periods with contact times (tcs) of (1) 0.99 h gcata gTOC−1, (2) 1.98 h gcata gTOC−1, (3) 2.97 h gcata gTOC−1, and (4) the initial conditions of period (1). X: reaction was stopped because of a leak in the liquid pump.
, nitrate) and (d) the leaching of Ru during these different periods with contact times (tcs) of (1) 0.99 h gcata gTOC−1, (2) 1.98 h gcata gTOC−1, (3) 2.97 h gcata gTOC−1, and (4) the initial conditions of period (1). X: reaction was stopped because of a leak in the liquid pump.
 , nitrate) and (d) the leaching of Ru during these different periods with contact times (tcs) of (1) 0.99 h gcata gTOC−1, (2) 1.98 h gcata gTOC−1, (3) 2.97 h gcata gTOC−1, and (4) the initial conditions of period (1). X: reaction was stopped because of a leak in the liquid pump.
, nitrate) and (d) the leaching of Ru during these different periods with contact times (tcs) of (1) 0.99 h gcata gTOC−1, (2) 1.98 h gcata gTOC−1, (3) 2.97 h gcata gTOC−1, and (4) the initial conditions of period (1). X: reaction was stopped because of a leak in the liquid pump.
 , nitrate; ▲, nitrite), and (d) leaching of Pt during these different periods; contact time (tc) of (1) 0.99 h gcata gTOC−1, (2) 1.98 h gcata gTOC−1, (3) 2.97 h gcata gTOC−1.
, nitrate; ▲, nitrite), and (d) leaching of Pt during these different periods; contact time (tc) of (1) 0.99 h gcata gTOC−1, (2) 1.98 h gcata gTOC−1, (3) 2.97 h gcata gTOC−1.
 , nitrate; ▲, nitrite), and (d) leaching of Pt during these different periods; contact time (tc) of (1) 0.99 h gcata gTOC−1, (2) 1.98 h gcata gTOC−1, (3) 2.97 h gcata gTOC−1.
, nitrate; ▲, nitrite), and (d) leaching of Pt during these different periods; contact time (tc) of (1) 0.99 h gcata gTOC−1, (2) 1.98 h gcata gTOC−1, (3) 2.97 h gcata gTOC−1.
| Parameter | Unit | Vinasse Bonne Chauffe (VBC) | Sugarcane Vinasse |
|---|---|---|---|
| pH | 3.15 ± 0.2 | 4.6 ± 0.4 | |
| COD a | g O2 L−1 | 23.5 ± 10 | 70 ± 10 |
| BOD b | g O2 L−1 | 35 ± 5 | |
| Total Nitrogen | mg L−1 | 235 ± 12 | 1260 ± 65 |
| Color | brown | dark brown |
| Support | Supplier | Nature | Surface Area (m2 g−1) | Crystalline Phase | Wetting Volume (mL g−1) a | pHPZC b | Av. Pore Diameter (nm) |
|---|---|---|---|---|---|---|---|
| TiO2 DT51 | Cristal | powder | 92 | anatase | 0.87 | 4.4 | 9 |
| ZrO2 XZO632/18 | Mel Chemicals | powder | 90 | monoclinic | 0.45 | 6.1 | 9 |
| TiO2 G | St Gobain | extrudates | 41 | anatase | 0.40 | nm | 21.5 |
| Time (h) | Pt Leached (%) | TN (mM) | NH4+ (mM) | NO2− (mM) | NO3− (mM) | Inorganic N (mM) |
|---|---|---|---|---|---|---|
| 2 | 10 | 2.6 | 1.4 | 0 | 0 | 1.4 |
| 4 | 11.4 | 2.3 | 0 | 0.1 | 1.2 | 1.3 |
| 7 | 7.6 | 2.3 | 0 | 0.1 | 1.5 | 1.6 |
| 24 | 1.3 | 2.5 | 0 | 0 | 2.1 | 2.1 |
| 32 | 0.2 | 2.2 | 0 | 0 | 2.0 | 2.0 |
| 32 | 0.3 | 2.2 | 0 | 0 | 2.0 | 2.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Phuong, T.; Besson, M. Catalytic Wet Air Oxidation Using Supported Pt and Ru Catalysts for Treatment of Distillery Wastewater (Cognac and Sugarcane Vinasses). Energies 2019, 12, 3974. https://doi.org/10.3390/en12203974
Le Phuong T, Besson M. Catalytic Wet Air Oxidation Using Supported Pt and Ru Catalysts for Treatment of Distillery Wastewater (Cognac and Sugarcane Vinasses). Energies. 2019; 12(20):3974. https://doi.org/10.3390/en12203974
Chicago/Turabian StyleLe Phuong, Thu, and Michèle Besson. 2019. "Catalytic Wet Air Oxidation Using Supported Pt and Ru Catalysts for Treatment of Distillery Wastewater (Cognac and Sugarcane Vinasses)" Energies 12, no. 20: 3974. https://doi.org/10.3390/en12203974