Copper-Tin Alloys for the Electrocatalytic Reduction of CO2 in an Imidazolium-Based Non-Aqueous Electrolyte
Abstract
:1. Introduction
2. Experimental
2.1. Film Characterization
2.2. Electrochemistry
3. Results and Discussion
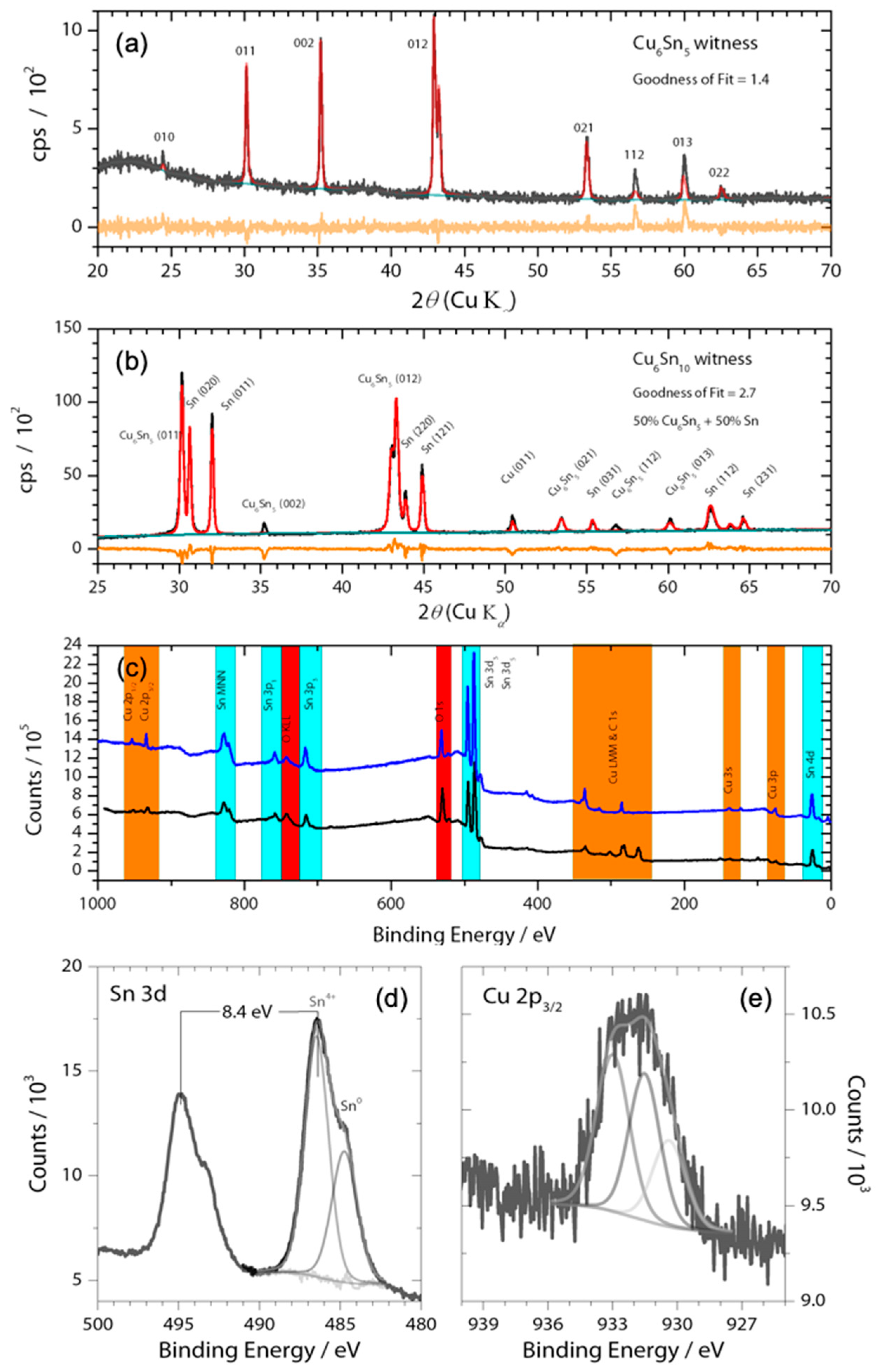
3.1. Catalyst Characterization
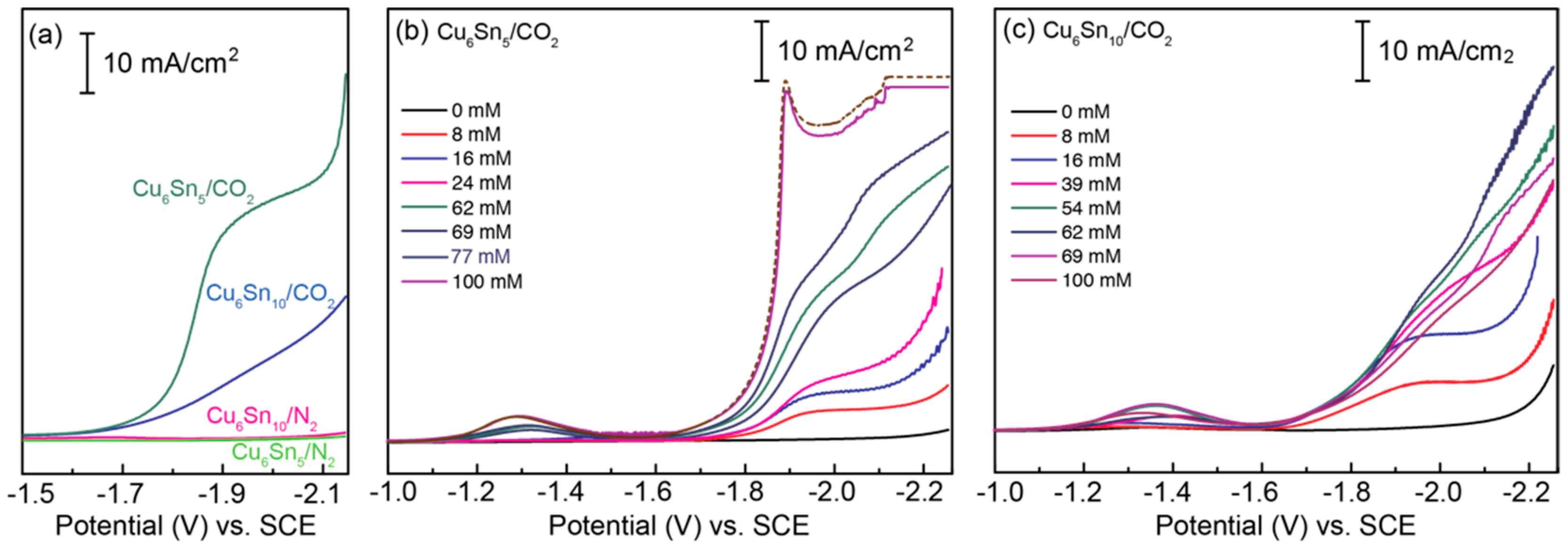
3.2. Catalyst Activity
3.3. Adsorption Pre-Catalysis Peak
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| [BMIM]+ | n-butyl-N′-methyl imidazolium |
| CPE | controlled potential electrolysis |
| DC | direct current |
| FE | Faradaic efficiency |
| GC | gas chromatography |
| NMR | nuclear magnetic resonance |
| TBA+ | tetrabutylammonium |
| OTf | triflate |
| SCE | standard calomel electrode |
| XPS | X-ray photoelectron spectroscopy |
References
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- US Energy Information Administration. Monthly Energy Review—May 2018; U.S. Energy Information Administration: Washington, DC, USA, 2018.
- Karl, T.R.; Trenberth, K.E. Modern global climate change. Science 2003, 302, 1719–1723. [Google Scholar] [CrossRef] [PubMed]
- Olah, G.A.; Prakash, G.K.S.; Goeppert, A. Anthropogenic chemical carbon cycle for a sustainable future. J. Am. Chem. Soc. 2011, 133, 12881–12898. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. technological use of CO2. Chem. Rev. 2013, 11, 1709–1742. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.L.R.; Siang, Y.B. Recent advances in understanding mechanisms for the electrochemical reduction of carbon dioxide. Curr. Opin. Electrochem. 2018, 8, 126–134. [Google Scholar]
- Rosenthal, J. Progress Toward the Electrocatalytic Production of Liquid Fuels from Carbon Dioxide; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 299–338. [Google Scholar]
- Rosen, B.A.; Salehi-Khojin, A.; Thorson, M.R.; Zhu, W.; Whipple, D.T.; Kenis, P.J.A.; Masel, R.I. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science 2011, 334, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chi, M.; Veith, G.M.; Zhang, P.; Lutterman, D.A.; Rosenthal, J.; Overbury, S.H.; Dai, S.; Zhu, H. Rational design of Bi nanoparticles for efficient electrochemical CO2 Reduction: The elucidation of size and surface condition effects. ACS Catal. 2016, 6, 6255–6264. [Google Scholar] [CrossRef]
- Sun, L.; Ramesha, G.K.; Kamat, P.V.; Brennecke, J.F. Switching the reaction course of electrochemical CO2 reduction with ionic liquids. Langmuir 2014, 30, 6302–6308. [Google Scholar] [CrossRef]
- Tanner, E.E.L.; Batchelor-Mcauley, C.; Compton, R.G. Carbon dioxide reduction in room-temperature ionic liquids: the effect of the choice of electrode material, cation, and anion. J. Phys. Chem. C 2016, 120, 26442–26447. [Google Scholar] [CrossRef]
- Medina-Ramos, J.; DiMeglio, J.L.; Rosenthal, J. Efficient reduction of CO2 To CO with high current density using in situ or ex situ prepared Bi-based materials. J. Am. Chem. Soc 2014, 136, 8361–8367. [Google Scholar] [CrossRef] [PubMed]
- Winikoff, S.G.; DiMeglio, J.L.; Chemburkar, A.; Medina-Ramos, J.; Rosenthal, J.; Neurock, M. Cooperativity between bismuth electrodes and ionic liquid electrolytes promotes efficient electrocatalytic reduction of CO2 to CO. Nat. Commun. 2018. submitted. [Google Scholar]
- Matsubara, Y.; Grills, D.C.; Kuwahara, Y. Thermodynamic aspects of electrocatalytic CO2 reduction in acetonitrile and with an ionic liquid as solvent or electrolyte. ACS Catal. 2015, 5, 6440–6452. [Google Scholar] [CrossRef]
- Medina-Ramos, J.; Pupillo, R.C.; Keane, T.P.; DiMeglio, J.L.; Rosenthal, J. Efficient conversion of CO2 to CO using tin and other inexpensive and easily prepared post-transition metal catalysts. J. Am. Chem. Soc. 2015, 137, 5021–5027. [Google Scholar] [CrossRef] [PubMed]
- Atifi, A.; Boyce, D.W.; DiMeglio, J.L.; Rosenthal, J. Directing the outcome of CO2 reduction at bismuth cathodes using varied ionic liquid promoters. ACS Catal. 2018, 8, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- Furuya, N.; Motoo, S. The electrochemical behavior of ad-atoms and their effect on hydrogen evolution: Part I. Order-disorder rearrangement of copper ad-atoms on platinum. J. Electroanal. Chem. 1976, 72, 165–175. [Google Scholar] [CrossRef]
- Furuya, N.; Motoo, S. The electrochemical behavior of ad-atoms and their effect on hydrogen evolution: Part IV. Tin and lead ad-atoms on platinum. J. Electroanal. Chem. 1979, 98, 195–202. [Google Scholar] [CrossRef]
- DiMeglio, J.L.; Rosenthal, J. Selective conversion of CO2 to CO with high efficiency using an inexpensive bismuth-based electrocatalyst. J. Am. Chem. Soc 2013, 135, 8798–8801. [Google Scholar] [CrossRef]
- Morimoto, M.; Takatsuji, Y.; Yamasaki, R.; Hashimoto, H.; Nakata, I.; Sakakura, T.; Haruyama, T. Electrodeposited Cu-Sn alloy for electrochemical CO2 reduction to CO/HCOO−. Electrocatalysis 2018, 9, 323–332. [Google Scholar] [CrossRef]
- Baggetto, L.; Jumas, J.-C.; Górka, J.; Bridges, C.A.; Veith, G.M. Predictions of particle size and lattice diffusion pathway requirements for sodium-ion anodes using H-Cu6Sn5 thin films as a model system. Phys. Chem. Chem. Phys. 2013, 15, 10885–10894. [Google Scholar] [CrossRef]
- Moulder, J.F. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Naille, S.; Dedryvère, R.; Martinez, H.; Leroy, S.; Lippens, P.E.; Jumas, J.C.; Gonbeau, D. XPS study of electrode/electrolyte interfaces of H-Cu6Sn5 electrodes in li-ion batteries. J. Power Sources 2007, 174, 1086–1090. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Cabaço, M.I.; Besnard, M.; Chávez, F.V.; Pinaud, N.; Sebastião, P.J.; Coutinho, J.A.P.; Danten, Y. Understanding chemical reactions of CO2 and its isoelectronic molecules with 1-butyl-3-methylimidazolium acetate by changing the nature of the cation: The case of CS2 in 1-butyl-1-methylpyrrolidinium acetate studied by NMR spectroscopy and density functional theory calculations. J. Chem. Phys. 2014, 140, 244307. [Google Scholar] [PubMed]
- Hanc-Scherer, F.A.; Montiel, M.A.; Montiel, V.; Herrero, E.; Sánchez-Sánchez, C.M. Surface structured platinum electrodes for the electrochemical reduction of carbon dioxide in imidazolium based ionic liquids. Phys. Chem. Chem. Phys. 2015, 17, 23909–23916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.L.; Unali, G.; Perosa, A. A “By-productless” cellulose foaming agent for use in imidazolium ionic liquids. Chem. Commun. 2011, 47, 2970–2972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Han, Y.; Wang, Y.; Ye, S.; Yan, T. Comparing the differential capacitance of two ionic liquid electrolytes: Effects of specific adsorption. Electrochem. Commun. 2014, 38, 44–46. [Google Scholar] [CrossRef]
- Gore, T.R.; Bond, T.; Zhang, W.; Scott, R.W.J.; Burgess, I.J. Hysteresis in the measurement of double-layer capacitance at the gold–ionic liquid interface. Electrochem. Commun. 2010, 12, 1340–1343. [Google Scholar] [CrossRef]
- Moganty, S.S.; Baltus, R.E.; Roy, D. Electrochemical windows and impedance characteristics of [Bmim+][] and [Bdmim+][] ionic liquids at the surfaces of Au, Pt, Ta and glassy carbon electrodes. Chem. Phys. Lett. 2009, 483, 90–94. [Google Scholar] [CrossRef]
- Fedorov, M.V.; Kornyshev, A.A. Ionic liquids at electrified interfaces. Chem. Rev. 2014, 114, 2978–3036. [Google Scholar] [CrossRef]
- Kirchner, K.; Kirchner, T.; Ivaništšev, V.; Fedorov, M.V. Electrical double layer in ionic liquids: Structural transitions from multilayer to monolayer structure at the interface. Electrochim. Acta 2013, 110, 762–771. [Google Scholar] [CrossRef]
- Black, J.M.; Walters, D.; Labuda, A.; Feng, G.; Hillesheim, P.C.; Dai, S.; Cummings, P.T.; Kalinin, S.V.; Proksch, R.; Balke, N. Bias-dependent molecular-level structure of electrical double layer in ionic liquid on graphite. Nano Lett. 2013, 13, 5954–5960. [Google Scholar] [CrossRef] [PubMed]
- Uysal, A.; Zhou, H.; Feng, G.; Lee, S.S.; Li, S.; Cummings, P.T.; Fulvio, P.F.; Dai, S.; McDonough, J.K.; Gogotsi, Y.; et al. Interfacial ionic “liquids”: Connecting static and dynamic structures. J. Phys. Condens. Matter 2014, 27. [Google Scholar] [CrossRef] [PubMed]
- Lauw, Y.; Horne, M.D.; Rodopoulos, T.; Lockett, V.; Akgun, B.; Hamilton, W.A.; Nelson, A.R.J. Structure of [C 4mpyr][NTf 2] room-temperature ionic liquid at charged gold interfaces. Langmuir 2012, 28, 7374–7381. [Google Scholar] [CrossRef] [PubMed]
- García Rey, N.; Dlott, D.D. Structural transition in an ionic liquid controls CO2 electrochemical reduction. J. Phys. Chem. C 2015, 119, 20892–20899. [Google Scholar] [CrossRef]
- Siinor, L.; Siimenson, C.; Ivaništšev, V.; Lust, K.; Lust, E. Influence of cation chemical composition and structure on the double layer capacitance for Bi(111)|room temperature ionic liquid interface. J. Electroanal. Chem. 2012, 668, 30–36. [Google Scholar] [CrossRef]
- Yanson, A.I.; Rodriguez, P.; Garcia-Araez, N.; Mom, R.V.; Tichelaar, F.D.; Koper, M.T.M. Cathodic corrosion: A quick, clean, and versatile method for the synthesis of metallic nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 6346–6350. [Google Scholar] [CrossRef]
- Hersbach, T.J.P.; Yanson, A.I.; Koper, M.T.M. Anisotropic etching of platinum electrodes at the onset of cathodic corrosion. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Tian, Z.; Priest, C.; Chen, L. Recent progress in the theoretical investigation of electrocatalytic reduction of CO2. Adv. Theory Simul. 2018, 1, 1800004–1800023. [Google Scholar] [CrossRef]
- Kunene, T.; Xiong, L.; Rosenthal, J. Solar-powered synthesis of hydrocarbons from carbon dioxide and water. Proc. Natl. Acad. Sci. USA 2019, 116, 9693–9695. [Google Scholar] [CrossRef] [Green Version]



| Film | Electrolyte | FECO | FEH2 | jtot (mA·cm−2) |
|---|---|---|---|---|
| Cu6Sn5 | TBAPF6 | 41.1 ± 3.2% | 4.4 ± 0.3% | 0.44 ± 0.1 |
| TBAPF6 + [BMIM]OTf | 34.4 ± 2.7% | <0.1% | 3.9 ± 0.2 | |
| Cu6Sn10 | TBAPF6 | 22.5 ± 3.8% | 3.9 ± 0.4% | 0.6 ± 0.1 |
| TBAPF6 + [BMIM]OTf | 30.5 ± 3.1% | <0.1% | 2.8 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacci, R.L.; Velardo, S.; Xiong, L.; Lutterman, D.A.; Rosenthal, J. Copper-Tin Alloys for the Electrocatalytic Reduction of CO2 in an Imidazolium-Based Non-Aqueous Electrolyte. Energies 2019, 12, 3132. https://doi.org/10.3390/en12163132
Sacci RL, Velardo S, Xiong L, Lutterman DA, Rosenthal J. Copper-Tin Alloys for the Electrocatalytic Reduction of CO2 in an Imidazolium-Based Non-Aqueous Electrolyte. Energies. 2019; 12(16):3132. https://doi.org/10.3390/en12163132
Chicago/Turabian StyleSacci, Robert L., Stephanie Velardo, Lu Xiong, Daniel A. Lutterman, and Joel Rosenthal. 2019. "Copper-Tin Alloys for the Electrocatalytic Reduction of CO2 in an Imidazolium-Based Non-Aqueous Electrolyte" Energies 12, no. 16: 3132. https://doi.org/10.3390/en12163132






