Promoting Effect of H+ and Other Factors on NO Removal by Using Acidic NaClO2 Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
3. Results
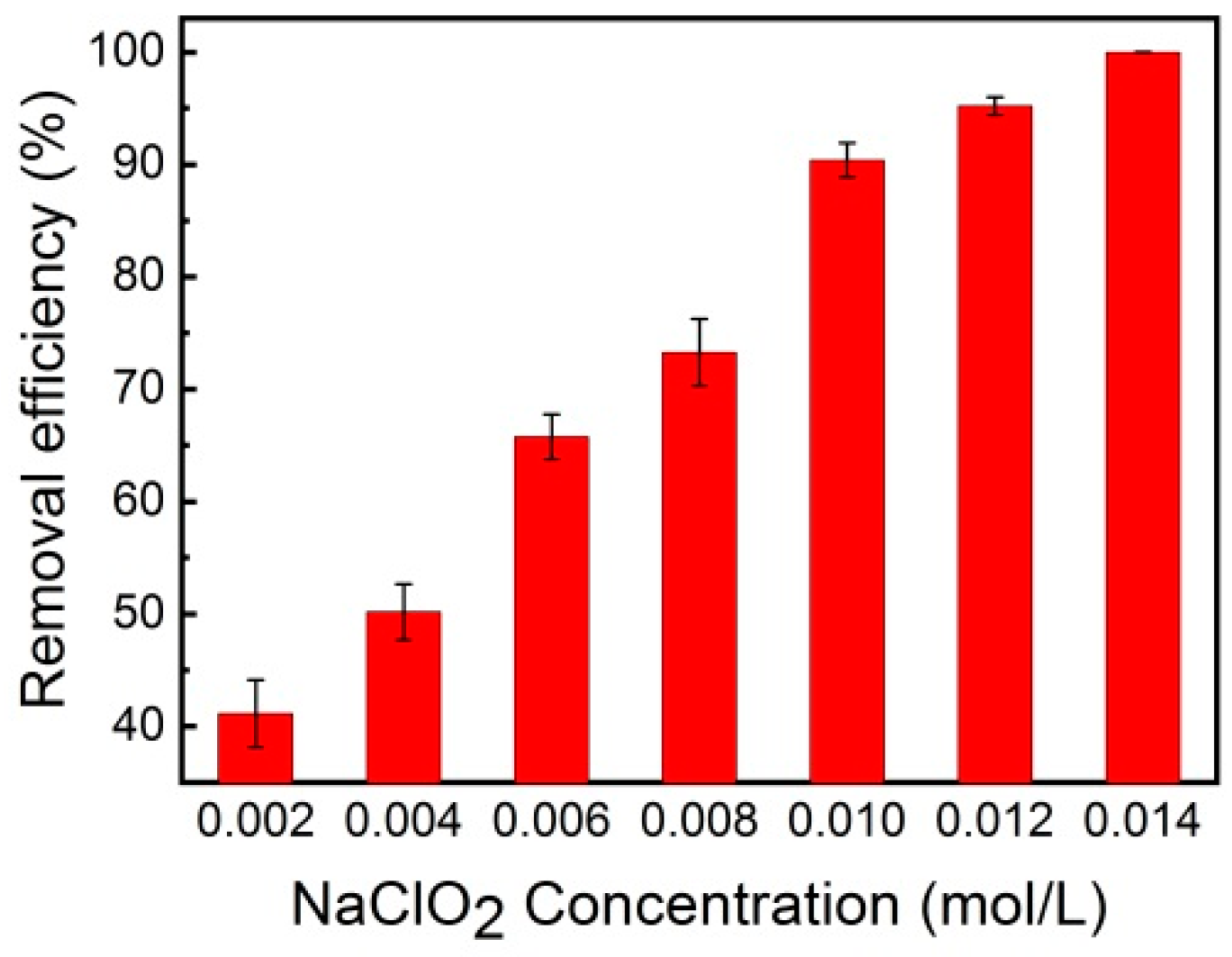
3.1. Effect of NaClO2 Concentration on the NO Removal Efficiency
3.2. Effect of Temperature on the NO Removal Efficiency
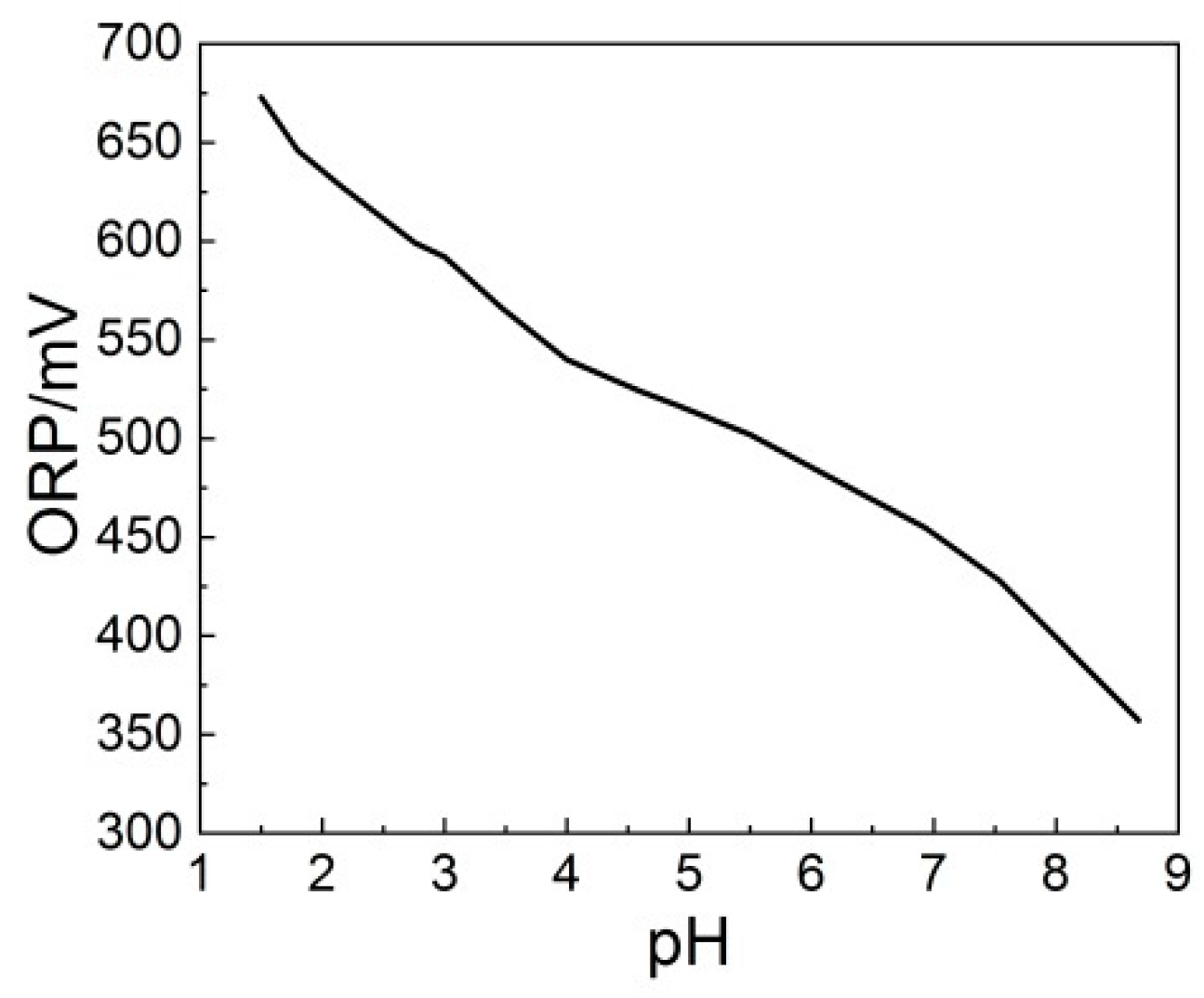
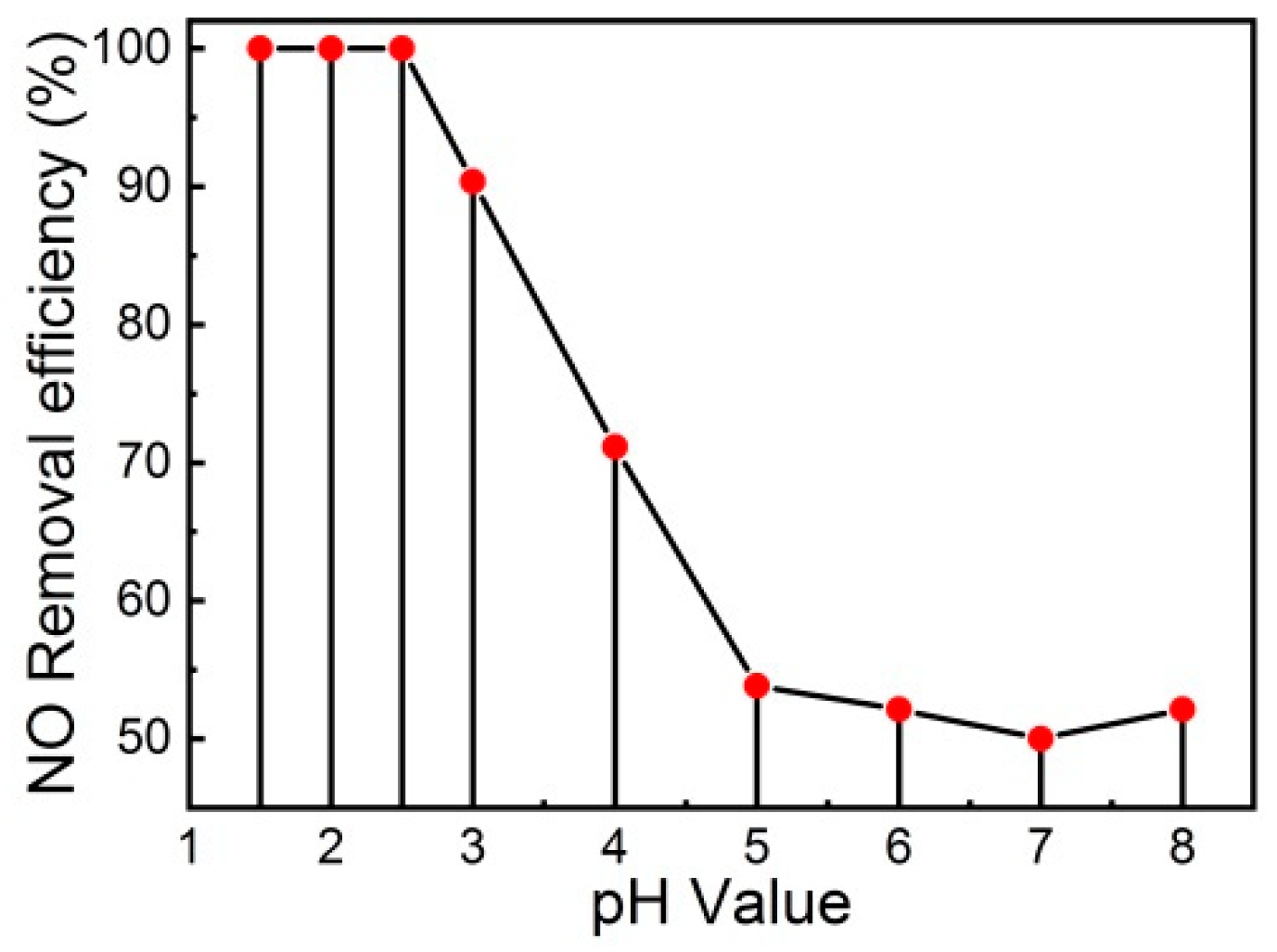
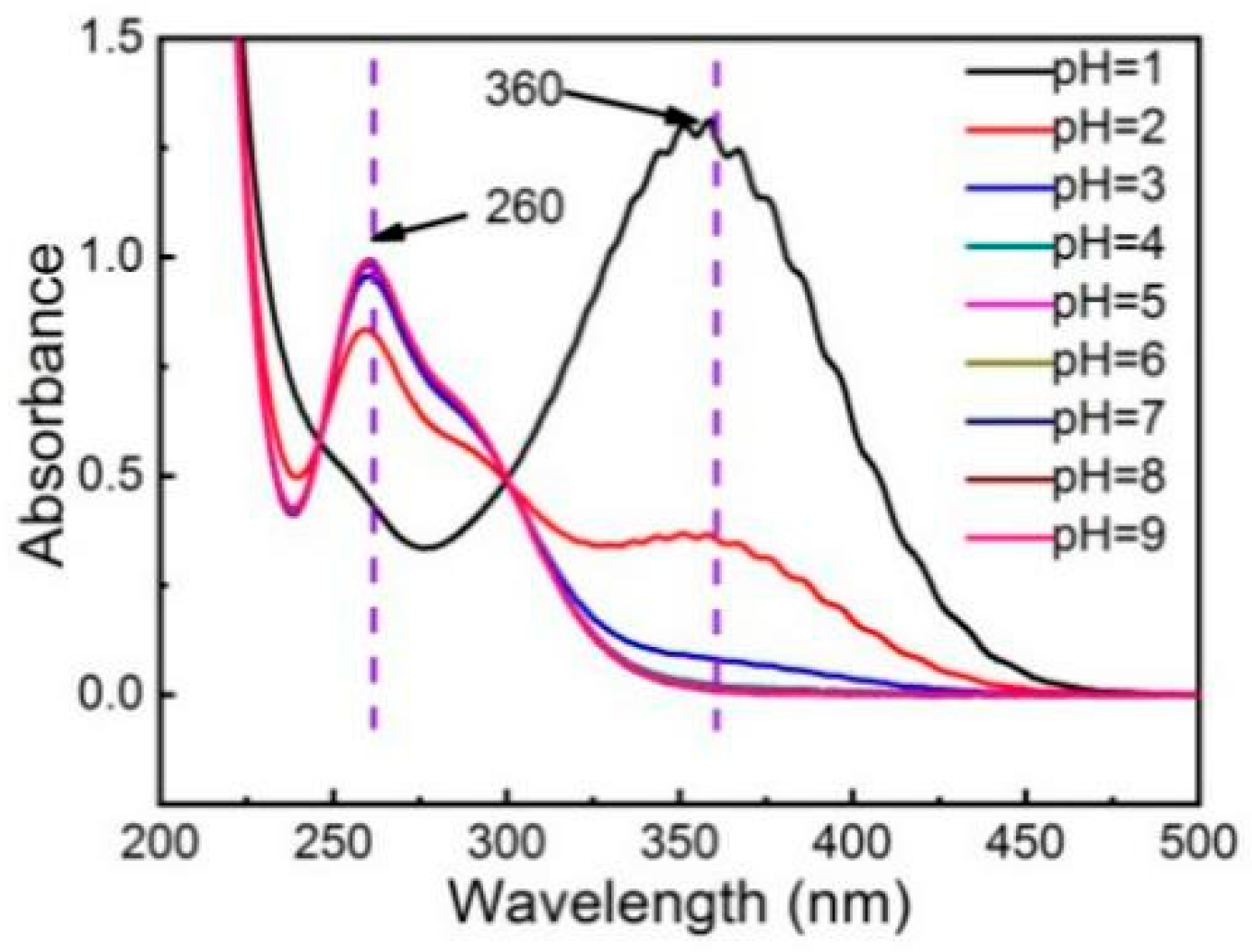
3.3. Effect of Initial pH on the NO Removal Efficiency
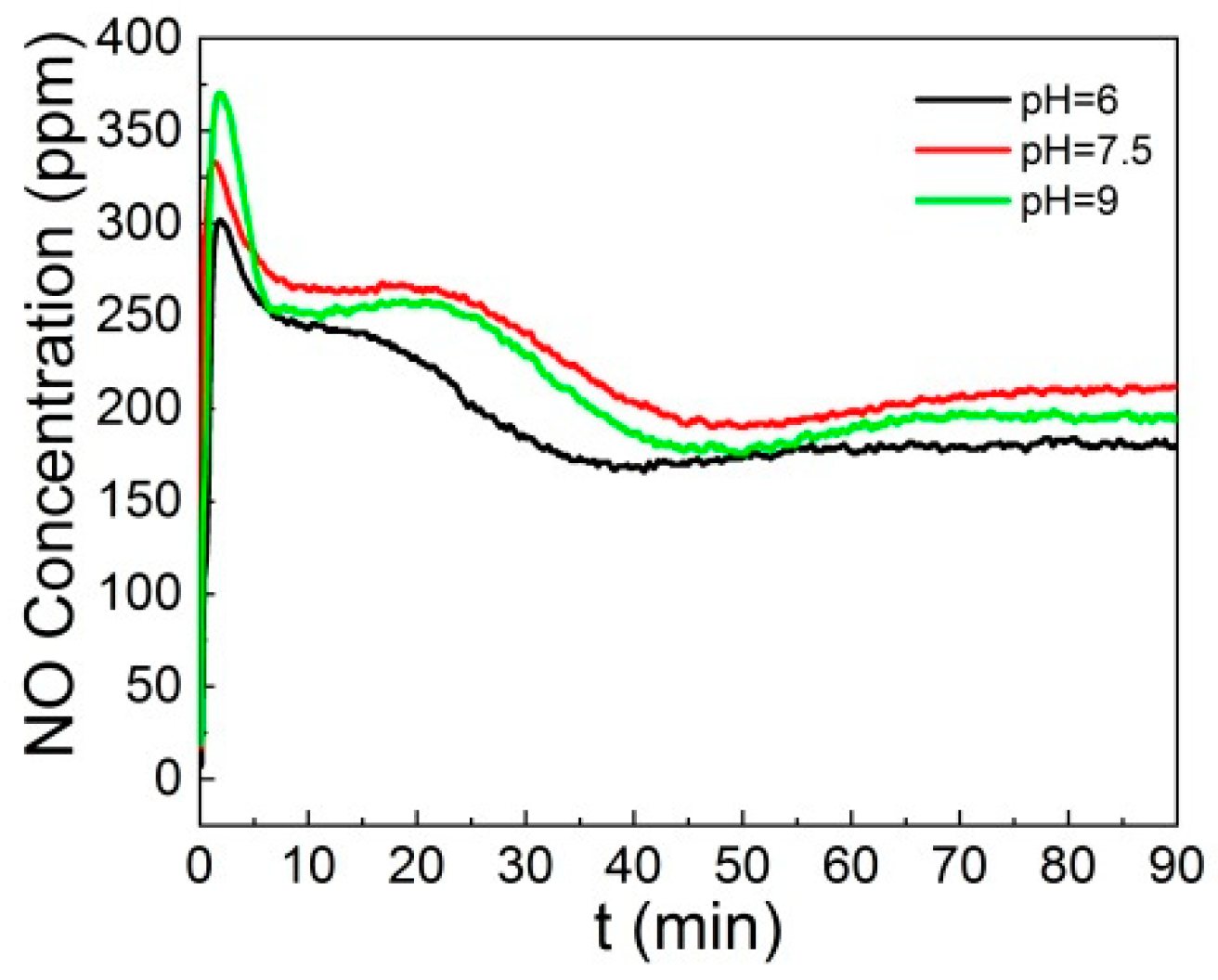
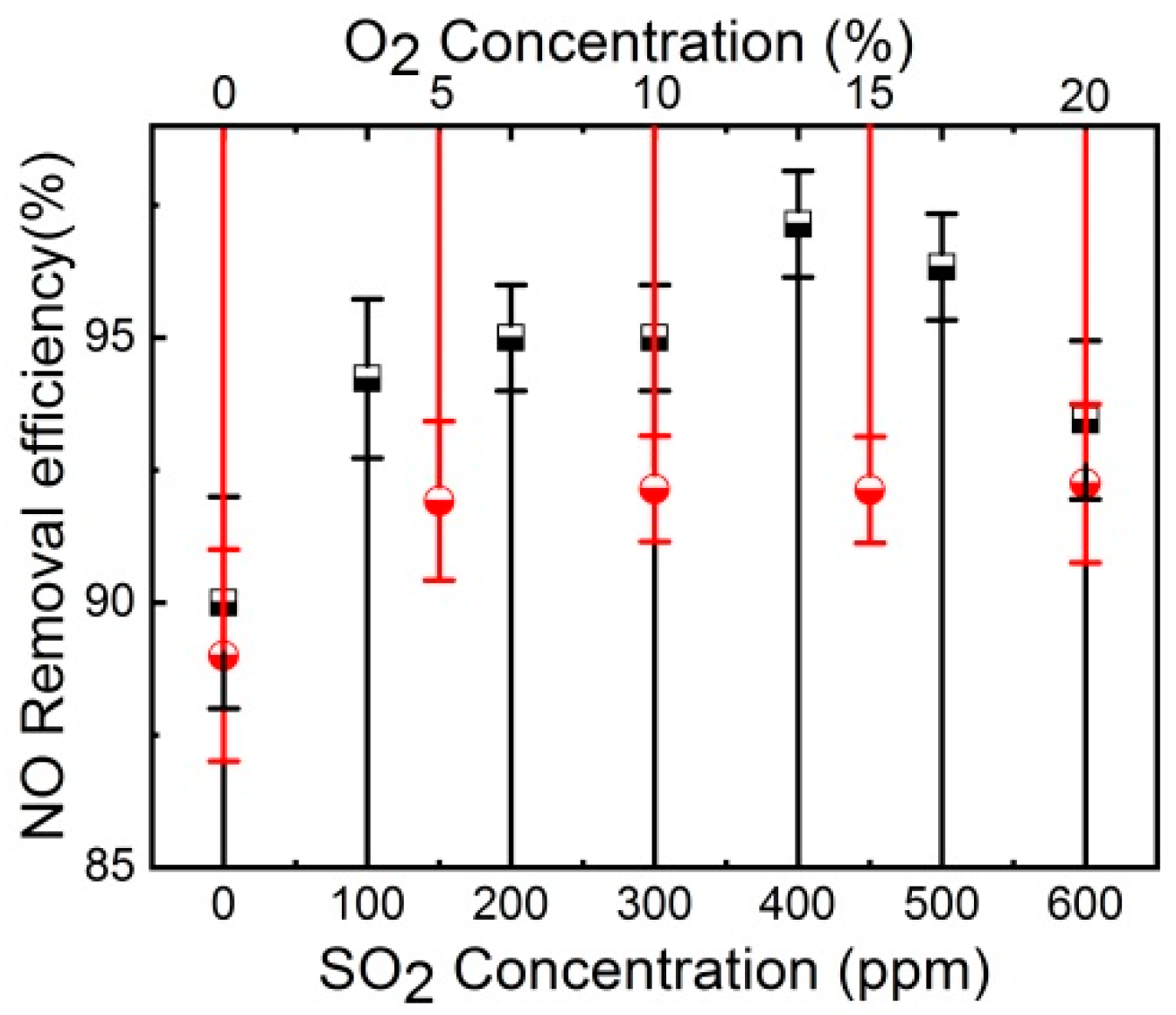
3.4. Effect of Coexistence Gases on the NO Removal Efficiency
4. Reaction Mechanism
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fang, N.; Guo, J.; Shu, S.; Luo, H.; Li, J.; Chu, Y. Effect of calcination temperature on low-temperature NH3-SCR activity and the resistance of SO2 with or without H2O over Fe-Mn-Zr catalyst. J. Taiwan Inst. Chem. E 2019, 93, 277–288. [Google Scholar] [CrossRef]
- Han, Z.H.; Zhao, D.; Zheng, D.; Pan, X.; Liu, B.; Han, Z.H.; Gao, Y.; Wang, J.; Yan, Z.H. NO Removal from simulated flue gas with a NaClO2 mist generated using the ultrasonic atomization method. Energies 2018, 11, 1043. [Google Scholar] [CrossRef]
- Xiang, K.; Liu, H.; Yang, B.; Zhang, C.; Yang, S.; Liu, Z.; Liu, C.; Xie, X.; Chai, L.; Min, X. Selenium catalyzed Fe(III)-EDTA reduction by Na2SO3: A reaction-controlled phase transfer catalysis. Environ. Sci. Pollut. Res. 2016, 23, 8113–8119. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, S.; Yang, S.; Zhao, S.; Ma, Y.; Du, W.; Shang, G.; Hao, Z. Technical progress of the control of NOx emission in carbon based fuel combustion process. Appl. Chem. 2018, 47, 2279–2286. [Google Scholar]
- You, Y.; Chang, H.; Zhu, T.; Zhang, T.; Li, X.; Li, J. The poisoning effects of phosphorus on CeO2-MoO3/TiO2 DeNOx catalysts: NH3-SCR activity and the formation of N2O. Mol. Catal. 2017, 439, 15–24. [Google Scholar] [CrossRef]
- Li, C.; Shen, M.; Yu, T.; Wang, J.; Wang, J.; Zhai, Y. The mechanism of ammonium bisulfate formation and decomposition over V/WTi catalysts for NH3-selective catalytic reduction at various temperatures. Phys. Chem. Chem. Phys. 2017, 19, 15194–15206. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Zhan, R.; Lin, H.; Huang, Z. Review of state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl. Eng. 2014, 66, 395–414. [Google Scholar]
- Sun, W.; Zhou, J.; Chen, J. Removal of nitric oxide using combined FeIIEDTA and coal slurry in the presence of SO2. Sep. Purif. Technol. 2017, 188, 134–139. [Google Scholar] [CrossRef]
- You, Y.; Chang, H.; Ma, L.; Guo, L.; Qin, X.; Li, J.; Li, J. Enhancement of N2O decomposition performance by N2O pretreatment over Ce-Co-O catalyst. Chem. Eng. J. 2017, 439, 15–24. [Google Scholar] [CrossRef]
- Wang, J. Application and comparison of SCR, SNCR and SNCR/SCR flue gas denitration technology. Electr. Power Technol. Environ. Prot. 2018, 40, 71–73. [Google Scholar]
- Hao, R.; Wang, X.; Mao, X.; Tian, B.; Zhao, Y.; Yuan, B.; Tao, Z.; Shen, Y. An integrated dual-reactor system for simultaneous removal of SO2 and NO: Factors assessment, reaction mechanism and application prospect. Fuel 2018, 220, 240–247. [Google Scholar] [CrossRef]
- Xi, H.; Zhou, S.; Zhou, J. New experimental results of NO removal from simulated marine engine exhaust gases by Na2S2O8/urea solutions. Chem. Eng. J. 2019, 362, 12–20. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.J.; Zhang, G.; Wang, Z.Y.; Zhu, P. Effects of slurry properties on simultaneous removal of SO2 and NO by ammonia-Fe(II)EDTA absorption in sintering plants. J. Environ. Manag. 2016, 183, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Deng, X.; Chen, M. Kinetics of FeIIIEDTA complex reduction with iron powder under aerobic conditions. Rsc. Adv. 2016, 6, 38416–38423. [Google Scholar] [CrossRef]
- Qian, D.; Zhao, H.; Ming, L.; Gao, J.; Zhao, G.; Wu, S. Experimental investigation on the kinetics of NO complex absorption through FeIIEDTA solvent in a double-stirred reactor. Ind. Eng. Chem. Res. 2011, 50, 4425–4431. [Google Scholar]
- Pan, W.; Zhang, X.; Guo, R.; Zhou, Y.; Jin, Q.; Ren, J. A thermodynamic study of simultaneous removal of SO2 and NO by a KMnO4/ammonia solution. Energy Sources Part A Recovery Util. Environ. Eff. 2015, 37, 721–726. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Ye, Y.; Chen, N.; Li, H. Study on the removal of nitric oxide (NO) by dual oxidant (H2O2/S2O82−) system. Chem. Eng. Sci. 2016, 145, 133–140. [Google Scholar] [CrossRef]
- Mondal, M.; Chelluboyana, V. New experimental results of combined SO2 and NO removal from simulated gas stream by NaClO as low-cost absorbent. Chem. Eng. J. 2013, 217, 48–53. [Google Scholar] [CrossRef]
- Hutson, N.; Krzyzynska, R.; Srivastava, R. Simultaneous removal of SO2, NOx, and Hg from coal flue gas using a NaClO2-enhanced wet scrubber. Ind. Eng. Chem. Res. 2008, 47, 5825–5831. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, F.; Ying, Z.; Wu, X.; Zhu, Q.; Liu, H.; Lu, H. Wet oxidation and absorption procedure for NOx removal. Environ. Technol. Innov. 2018, 11, 41–48. [Google Scholar] [CrossRef]
- Yang, S.; Pan, X.; Han, Z.; Zheng, D.; Yu, J.; Xia, P.; Liu, B.; Yan, Z. Nitrogen oxide removal from simulated flue gas by UV-irradiated sodium chlorite solution in a bench-scale scrubbing reactor. Ind. Eng. Chem. Res. 2017, 56, 3671–3678. [Google Scholar] [CrossRef]
- Lee, H.; Deshwal, B.; Yoo, K. Simultaneous removal of SO2 and NO by sodium chlorite solutionin wetted-wall column. Korean J. Chem. Eng. 2005, 22, 208–213. [Google Scholar] [CrossRef]
- Chien, T.; Chu, H. Removal of SO2 and NO from flue gas by wet scrubbing using an aqueous NaClO2 solution. J. Hazard. Mater. 2000, 80, 43–57. [Google Scholar] [CrossRef]
- Suchecki, T.; Mathews, B.; Augustyniak, A.; Kumazawa, H. Applied kinetics aspects of ferric EDTA complex reduction with metal powder. Ind. Eng. Chem. Res. 2014, 53, 14234–14240. [Google Scholar] [CrossRef]
- Yan, B.; Yang, J.; Guo, M.; Zhu, S.; Yu, W.; Ma, S. Experimental study on FeIICit enhanced absorption of NO in (NH4)2SO3 solution. J. Ind. Eng. Chem. 2015, 21, 476–482. [Google Scholar] [CrossRef]
- Brogren, C.; Karlsson, H.; Bjerle, I. Absorption of NO in an aqueous solution of NaClO2. Chem. Eng. Technol. 1998, 21, 61–70. [Google Scholar] [CrossRef]
- Sada, E.; Kumazawa, H. Individual and simultaneous absorption of dilute NO and SO2 in aqueous slurries of MgSO3 with FeII-EDTA. Ind. Eng. Chem. Process Des. Dev. 1980, 19, 377–382. [Google Scholar] [CrossRef]
- Hao, R.; Wang, X.; Liang, Y.; Lu, Y.; Cai, Y.; Mao, X.; Yuan, B.; Zhao, Y. Reactivity of NaClO2 and HA-Na in air pollutants removal: Active species identification and cooperative effect revelation. Chem. Eng. J. 2017, 330, 1279–1288. [Google Scholar] [CrossRef]
- Guo, R.; Gao, X.; Pan, W.; Ren, J.; Wu, J.; Zhang, X. Absorption of NO into NaClO3/NaOH solutions in a stirred tank reactor. Fuel 2010, 89, 3431–3435. [Google Scholar] [CrossRef]
- Fang, Y. Study on the Preparation of Chlorine Dioxide with Sodium Chlorite; Nanjing University of Science and Technology: Nanjing, China, 2013. [Google Scholar]
- Zhao, Y.; Guo, T.; Chen, Z.; Du, Y. Simultaneous removal of SO2 and NO using M/NaClO2 complex absorbent. Chem. Eng. J. 2010, 160, 42–47. [Google Scholar] [CrossRef]
- Hao, R.; Mao, X.; Wang, Z.; Zhao, Y.; Wang, T.; Sun, Z.; Yuan, B.; Li, Y. A novel method of ultraviolet/NaClO2-NH4OH for NO removal: Mechanism and kinetics. J. Hazard. Mater. 2019, 368, 234–242. [Google Scholar] [CrossRef]
- Hao, R.; Zhang, Y.; Wang, Z.; Li, Y.; Bo, Y.; Mao, X.; Yi, Z. An advanced wet method for simultaneous removal of SO2 and NO from coal-fired flue gas by utilizing a complex absorbent. Chem. Eng. J. 2017, 307, 562–571. [Google Scholar] [CrossRef]
- Ishizuka, T.; Kabashima, H.; Yamaguchi, T.; Tanabe, K.; Hattori, H. Initial step of flue gas desulfurization-An IR study of the reaction of SO2 with NOx on CaO. Environ. Sci. Technol. 2000, 34, 2799–2803. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, P.; Li, X. Promoting Effect of H+ and Other Factors on NO Removal by Using Acidic NaClO2 Solution. Energies 2019, 12, 2966. https://doi.org/10.3390/en12152966
Gong P, Li X. Promoting Effect of H+ and Other Factors on NO Removal by Using Acidic NaClO2 Solution. Energies. 2019; 12(15):2966. https://doi.org/10.3390/en12152966
Chicago/Turabian StyleGong, Pijian, and Xinxue Li. 2019. "Promoting Effect of H+ and Other Factors on NO Removal by Using Acidic NaClO2 Solution" Energies 12, no. 15: 2966. https://doi.org/10.3390/en12152966




