Optimization of Batch Dark Fermentation of Chlorella sp. Using Mixed-Cultures for Simultaneous Hydrogen and Butyric Acid Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum Preparation
2.2. Bath Experiment for Acidogenesis Stage for Hydrogen and Butyric Acid Production
2.3. Analysis Methods
3. Results
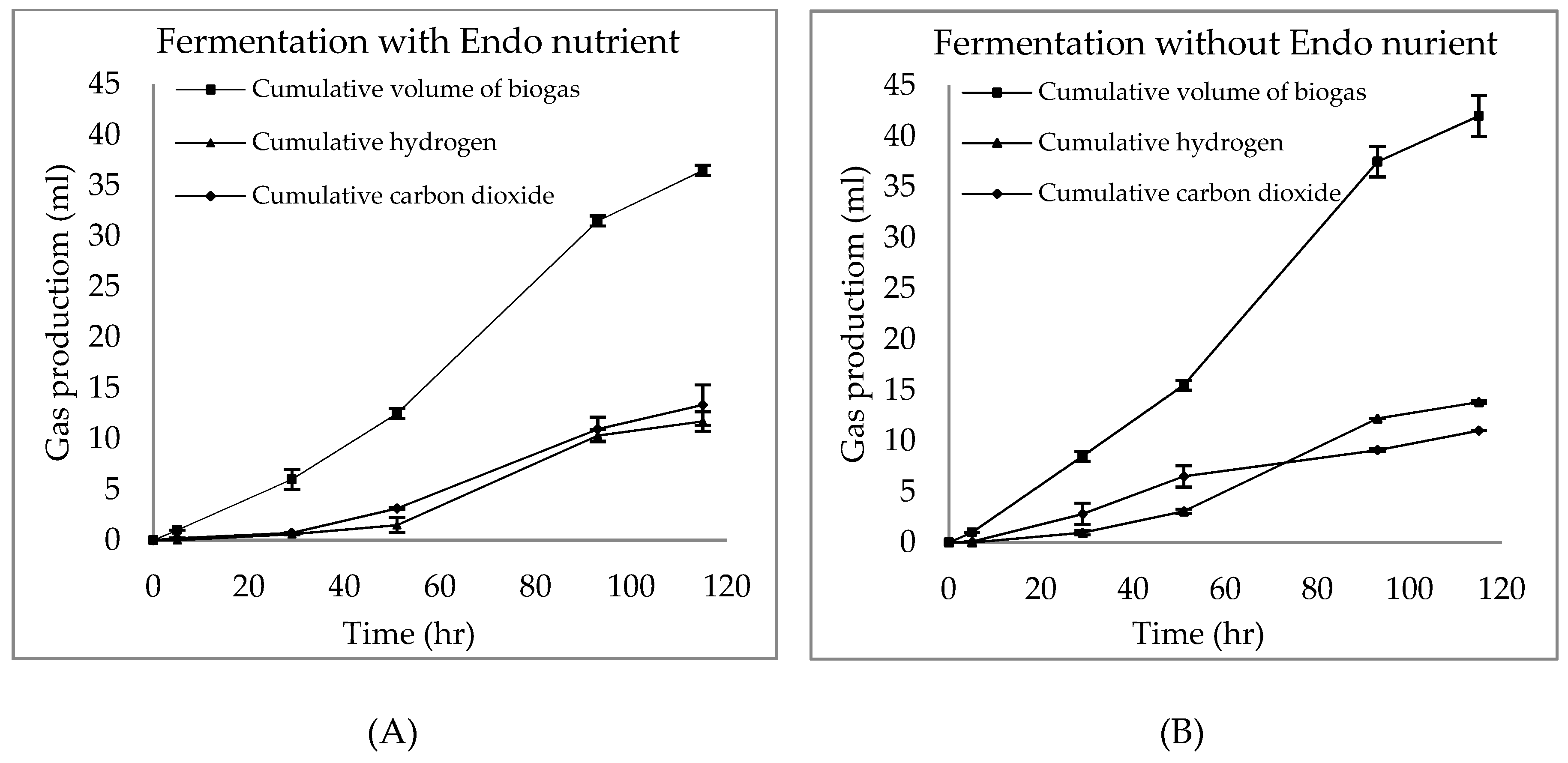
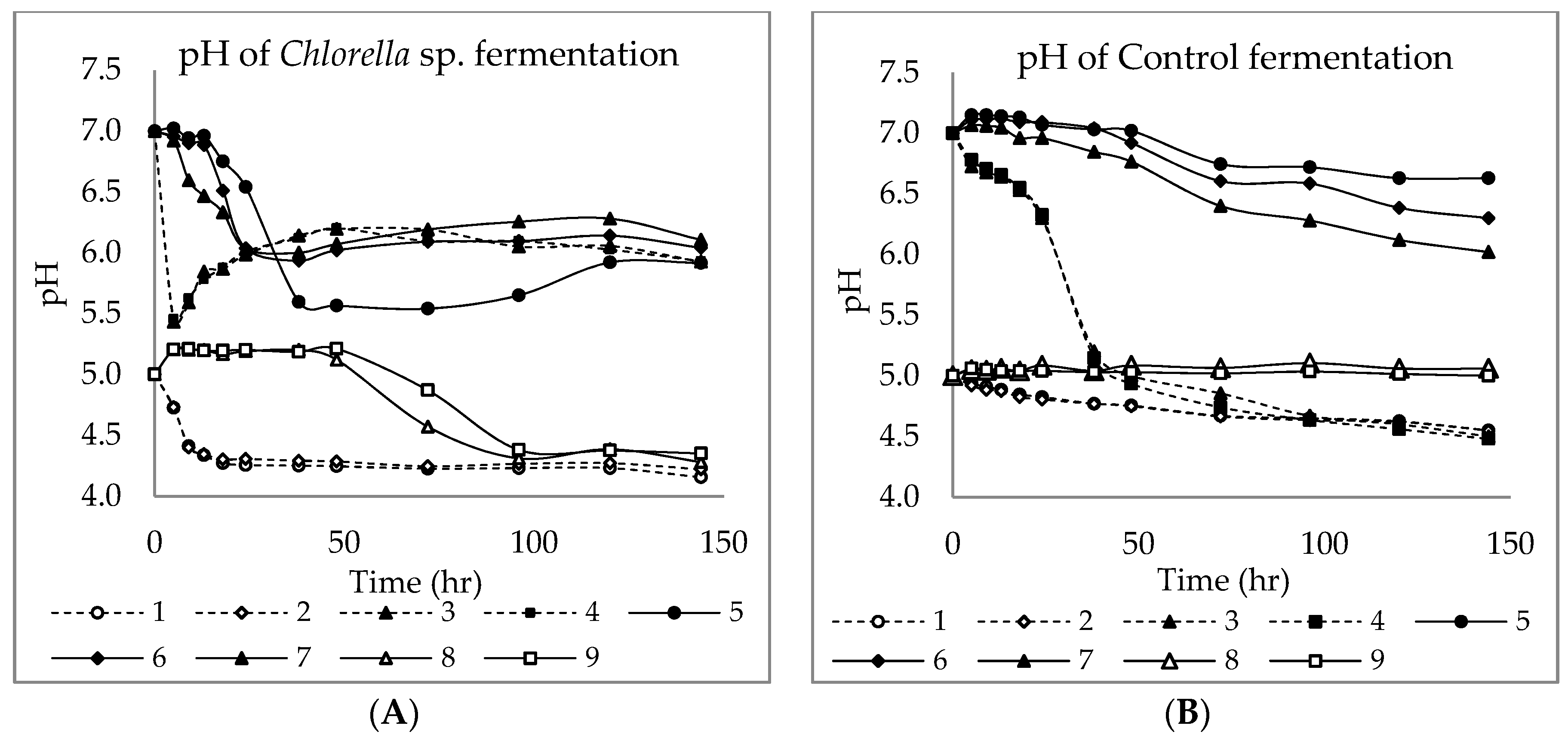
3.1. Chlorella sp. Characterization and Batch Fermentation of Chlorella sp. with and without Endo Nutrients
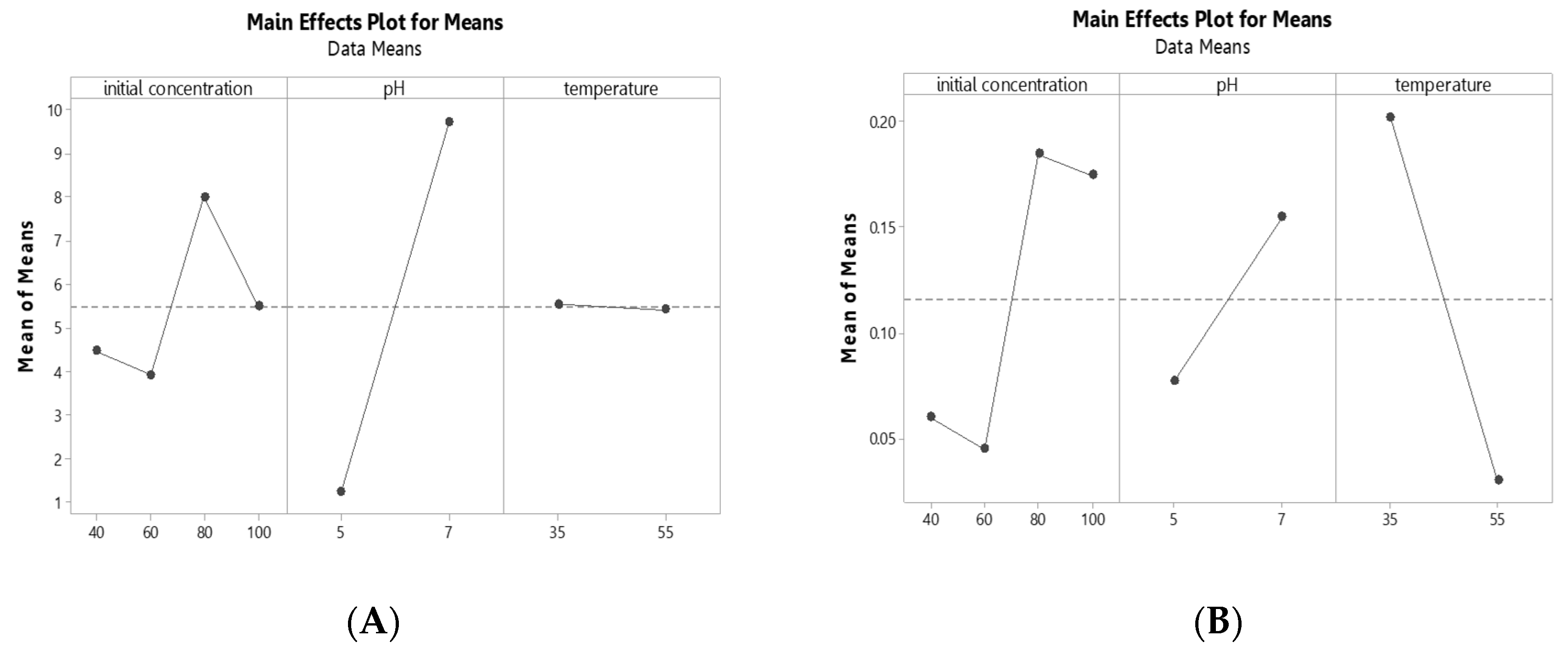
3.2. Hydrogen Production
3.3. Butyric Acid Production
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moon, H.; Park, S.Y.; Jeong, C.; Lee, J. Forecasting electricity demand of electric vehicles by analyzing consumers’ charging patterns. Transp. Res. D Transp. Environ. 2018, 62, 64–79. [Google Scholar] [CrossRef]
- Conti, J.; Holtberg, P.; Diefenderfer, J.; LaRose, A.; Turnure, J.T.; Westfall, L. International Energy Outlook 2016 with Projections to 2040; DOE/EIA-0484(2016) United States 10.2172/1296780. DOEEIA English; Office of Energy Analysis, USDOE Energy Information Administration (EIA): Washington, DC, USA, 2016; 290p. Available online: www.eia.gov/forecasts/ieo (accessed on 30 June 2016).
- Gonzales, R.R.; Sivagurunathan, P.; Parthiban, A.; Kim, S.-H. Optimization of substrate concentration of dilute acid hydrolyzate of lignocellulosic biomass in batch hydrogen production. Int. Biodeterior. Biodegrad. 2016, 113, 22–27. [Google Scholar] [CrossRef]
- Kamiński, W.; Tomczak, E.; Gorak, A. Biobutanol-production and purification methods. Atmosphere 2011, 2, 3. [Google Scholar]
- Green, E.M. Fermentative production of butanol—The industrial perspective. Curr. Opin. Biotechnol. 2011, 22, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Bioproduction of butanol from biomass: From genes to bioreactors. Curr. Opin. Biotechnol. 2007, 18, 220–227. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, J.H.; Jang, S.H.; Nielsen, L.K.; Kim, J.; Jung, K.S. Fermentative butanol production by Clostridia. Biotechnol. Bioeng. 2008, 101, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y.; Takeda, K.; Kobayashi, G.; Sonomoto, K.; Ishizaki, A.; Yoshino, S. High butanol production by Clostridium saccharoperbutylacetonicum N1-4 in fed-batch culture with pH-stat continuous butyric acid and glucose feeding method. J. Biosci. Bioeng. 2004, 98, 263–268. [Google Scholar] [CrossRef]
- Li, J.; Chi, X.; Zhang, Y.; Wang, X. Enhanced coproduction of hydrogen and butanol from rice straw by a novel two-stage fermentation process. Int. Biodeterior. Biodegrad. 2018, 127, 62–68. [Google Scholar] [CrossRef]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef]
- Kongjan, P.; O-Thong, S.; Kotay, M.; Min, B.; Angelidaki, I. Biohydrogen production from wheat straw hydrolysate by dark fermentation using extreme thermophilic mixed culture. Biotechnol. Bioeng. 2010, 105, 899–908. [Google Scholar] [CrossRef]
- Stein, U.H.; Wimmer, B.; Ortner, M.; Fuchs, W.; Bochmann, G. Maximizing the production of butyric acid from food waste as a precursor for ABE-fermentation. Sci. Total Environ. 2017, 598, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Yossan, S.; Sompong, O.; Prasertsan, P. Effect of initial pH, nutrients and temperature on hydrogen production from palm oil mill effluent using thermotolerant consortia and corresponding microbial communities. Int. J. Hydrogen Energy 2012, 37, 13806–13814. [Google Scholar] [CrossRef]
- Marone, A.; Izzo, G.; Mentuccia, L.; Massini, G.; Paganin, P.; Rosa, S.; Varrone, C.; Signorini, A. Vegetable waste as substrate and source of suitable microflora for bio-hydrogen production. Renew. Energy 2014, 68, 6–13. [Google Scholar] [CrossRef]
- Angenent, L.T.; Wrenn, B.A. Optimizing Mixed-Culture Bioprocessing to Convert Wastes into Bioenergy; American Society of Microbiology: Dulles, VA, USA, 2008; pp. 179–194. [Google Scholar]
- Kleerebezem, R.; van Loosdrecht, M.C. Mixed culture biotechnology for bioenergy production. Curr. Opin. Biotechnol. 2007, 18, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Al-Shorgani, N.K.N.; Ali, E.; Kalil, M.S.; Yusoff, W.M.W. Bioconversion of butyric acid to butanol by Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564) in a limited nutrient medium. Bioenergy Res. 2012, 5, 287–293. [Google Scholar] [CrossRef]
- Kim, N.-J.; Li, H.; Jung, K.; Chang, H.N.; Lee, P.C. Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Bioresour. Technol. 2011, 102, 7466–7469. [Google Scholar] [CrossRef]
- Banerjee, S.; Ramaswamy, S. Dynamic process model and economic analysis of microalgae cultivation in open raceway ponds. Algal Res. 2017, 26, 330–340. [Google Scholar] [CrossRef]
- Demirbas, M.F. Biofuels from algae for sustainable development. Appl. Energy 2011, 88, 3473–3480. [Google Scholar] [CrossRef]
- Davis, R.; Aden, A.; Pienkos, P.T. Techno-economic analysis of autotrophic microalgae for fuel production. Appl. Energy 2011, 88, 3524–3531. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zhao, X.-Q.; Yen, H.-W.; Ho, S.-H.; Cheng, C.-L.; Lee, D.-J.; Bai, F.-W.; Chang, J.-S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Phanduang, O.; Lunprom, S.; Salakkam, A.; Reungsang, A. Anaerobic solid-state fermentation of bio-hydrogen from microalgal Chlorella sp. biomass. Int. J. Hydrogen Energy 2017, 42, 9650–9659. [Google Scholar] [CrossRef]
- Endo, G.; Noike, T.; Matsumoto, J. Characteristics of Cellulose and Glucose Decomposition in Acidogenic Phase of Anaerobic Digestion. Proc. Jpn. Soc. Civ. Eng. 1982, 61–68. [Google Scholar] [CrossRef]
- De la Rubia, M.; Riau, V.; Raposo, F.; Borja, R. Thermophilic anaerobic digestion of sewage sludge: Focus on the influence of the start-up. A review. Crit. Rev. Biotechnol. 2013, 33, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.; Kumar, C.G.; Prakasham, R.S.; Hobbs, P.J. The Taguchi methodology as a statistical tool for biotechnological applications: A critical appraisal. Biotechnol. J. 2008, 3, 510–523. [Google Scholar] [CrossRef] [PubMed]
- APhA, A. WEF (American Public Health Association, American Water Works Association, and Water Environment Federation). In Standard Methods for the Examination of Water and Wastewater; WEF: Washington, DC, USA, 1998; Volume 19. [Google Scholar]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Lee, O.K.; Oh, Y.-K.; Lee, E.Y. Bioethanol production from carbohydrate-enriched residual biomass obtained after lipid extraction of Chlorella sp. KR-1. Bioresour. Technol. 2015, 196, 22–27. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, X.; Shi, X.; Chu, C.; Guo, R.; Kong, H. Fermentation of Chlorella sp. for anaerobic bio-hydrogen production: Influences of inoculum–substrate ratio, volatile fatty acids and NADH. Bioresour. Technol. 2011, 102, 10480–10485. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Bhatnagar, M.; Chinnasamy, S.; Das, K. Chlorella minutissima—A promising fuel alga for cultivation in municipal wastewaters. Appl. Biochem. Biotechnol. 2010, 161, 523–536. [Google Scholar] [CrossRef]
- Phukan, M.M.; Chutia, R.S.; Konwar, B.; Kataki, R. Microalgae Chlorella as a potential bio-energy feedstock. Appl. Energy 2011, 88, 3307–3312. [Google Scholar] [CrossRef]
- Wieczorek, N.; Kucuker, M.A.; Kuchta, K. Fermentative hydrogen and methane production from microalgal biomass (Chlorella vulgaris) in a two-stage combined process. Appl. Energy 2014, 132, 108–117. [Google Scholar] [CrossRef]
- Abu-Ruwaida, A.; Banat, I.; Haditirto, S.; Khamis, A. Nutritional requirements and growth characteristics of a biosurfactant-producingRhodococcus bacterium. World J. Microbiol. Biotechnol. 1991, 7, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Li, C.; Fang, H.H. Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Crit. Rev. Env. Sci. Technol. 2007, 37, 1–39. [Google Scholar] [CrossRef]
- Sangyoka, S.; Reungsang, A.; Lin, C.-Y. Optimization of biohydrogen production from sugarcane bagasse by mixed cultures using a statistical method. Sustain. Environ. Res. 2016, 26, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.-M.; Jung, K.-W.; Kim, D.-H.; Oh, Y.-K.; Shin, H.-S. Microalgal biomass as a feedstock for bio-hydrogen production. Int. J. Hydrogen Energy 2012, 37, 15533–15539. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lin, C.-Y.; Lin, M.-C. Acid–base enrichment enhances anaerobic hydrogen production process. Appl. Microbiol. Biotechnol 2002, 58, 224–228. [Google Scholar] [PubMed]
- Fabiano, B.; Perego, P. Thermodynamic study and optimization of hydrogen production by Enterobacter aerogenes. Int. J. Hydrogen Energy 2002, 27, 149–156. [Google Scholar] [CrossRef]
- Jo, J.H.; Lee, D.S.; Park, D.; Choe, W.-S.; Park, J.M. Optimization of key process variables for enhanced hydrogen production by Enterobacter aerogenes using statistical methods. Bioresour. Technol. 2008, 99, 2061–2066. [Google Scholar] [CrossRef]
- Qiu, C.; Yuan, P.; Sun, L.; Wang, S.; Lo, S.; Zhang, D. Effect of fermentation temperature on hydrogen production from xylose and the succession of hydrogen-producing microflora. J. Chem. Technol. Biotechnol. 2017, 92, 1990–1997. [Google Scholar] [CrossRef]
- Valdez-Vazquez, I.; Rios-Leal, E.; Esparza-Garcia, F.; Cecchi, F.; Poggi-Varaldo, H.M. Semi-continuous solid substrate anaerobic reactors for H2 production from organic waste: Mesophilic versus thermophilic regime. Int. J. Hydrogen Energy 2005, 30, 1383–1391. [Google Scholar] [CrossRef]
- Yokoyama, H.; Waki, M.; Moriya, N.; Yasuda, T.; Tanaka, Y.; Haga, K. Effect of fermentation temperature on hydrogen production from cow waste slurry by using anaerobic microflora within the slurry. Appl. Microbiol. Biotechnol. 2007, 74, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.K.; Chen, W.-H.; Li, L.; Sung, S. Biological hydrogen production: Effects of pH and intermediate products. Int. J. Hydrogen Energy 2004, 29, 1123–1131. [Google Scholar] [CrossRef]
- Hu, Z.-H.; Yu, H.-Q.; Zhu, R.-F. Influence of particle size and pH on anaerobic degradation of cellulose by ruminal microbes. Int. Biodeterior. Biodegrad. 2005, 55, 233–238. [Google Scholar] [CrossRef]
- De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Biohydrogen production from dark fermentation of cheese whey: Influence of pH. Int. J. Hydrogen Energy 2014, 39, 20930–20941. [Google Scholar] [CrossRef]
- Wang, J.L.; Wan, W. Factors influencing fermentative hydrogen production: A review. Int. J. Hydrogen Energy 2009, 34. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Liu, H. Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour. Technol. 2002, 82, 87–93. [Google Scholar] [CrossRef]
- Lay, J.J. Modeling and optimization of anaerobic digested sludge converting starch to hydrogen. Biotechnol. Bioeng. 2000, 68, 269–278. [Google Scholar] [CrossRef]
- Al-Shorgani, N.K.N.; Kalil, M.S.; Yusoff, W.M.W.; Hamid, A.A. Impact of pH and butyric acid on butanol production during batch fermentation using a new local isolate of Clostridium acetobutylicum YM1. Saudi J. Biol. Sci. 2018, 25, 339–348. [Google Scholar] [CrossRef]
- Giang, T.T.; Lunprom, S.; Liao, Q.; Reungsang, A.; Salakkam, A. Enhancing Hydrogen Production from Chlorella sp. Biomass by Pre-Hydrolysis with Simultaneous Saccharification and Fermentation (PSSF). Energies 2019, 12, 908. [Google Scholar] [CrossRef]
- Maaroff, R.M.; Jahim, J.M.; Azahar, A.M.; Abdul, P.M.; Masdar, M.S.; Nordin, D.; Nasir, M.A.A. Biohydrogen production from palm oil mill effluent (POME) by two stage anaerobic sequencing batch reactor (ASBR) system for better utilization of carbon sources in POME. Int. J. Hydrogen Energy 2018. [Google Scholar] [CrossRef]
- Cappai, G.; De Gioannis, G.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Effect of inoculum to substrate ratio (ISR) on hydrogen production through dark fermentation of food waste. In Proceedings of the Fifteenth International Waste Management and Landfill Symposium, Cagliari, Italy, 5–9 October 2015. [Google Scholar]



| Batch Run | Conc. (g-VS/L) A | pH B | Temp. (°C) C |
|---|---|---|---|
| 1 | 40 | 5 | 35 |
| 2 | 60 | 5 | 35 |
| 3 | 80 | 7 | 35 |
| 4 | 100 | 7 | 35 |
| 5 | 40 | 7 | 55 |
| 6 | 60 | 7 | 55 |
| 7 | 80 | 7 | 55 |
| 8 | 80 | 5 | 55 |
| 9 | 100 | 5 | 55 |
| Character of Chlorella sp. | Value | Character of Chlorella sp. | Value |
|---|---|---|---|
| TS % (w/w) | 97.7 | Mg (mg/kg) | 2458.66 |
| VS % (w/w TS) | 91.0 | Ca (mg/kg) | 1919.06 |
| Ash % (w/w TS) | 6.7 | As (mg/kg) | ND |
| Total Sugar % (w/w) | 6.1 | Cr (mg/kg) | 5.22 |
| COD (g-COD/g-VS) | 1.43 | Cd (mg/kg) | ND |
| Oil and grease % (w/w) | 2.6 | Cu (mg/kg) | 7.4 |
| TKN % (w/w) | 4.6 | Pb (mg/kg) | 5.22 |
| Na (mg/kg) | 248.04 | Zn (mg/kg) | 110.1 |
| K (mg/kg) | 7267.19 | Mn (mg/kg) | 48.74 |
| Fe (mg/kg) | 574.41 |
| Run | Conc. (g-VS/L) | pH | T (°C) | Acetic â (g/g-VS) | Propionic â (g/g-VS) | Butyric â (g/g-VS) | Total â (g/g-VS) | H2 Yield (mL/g-VS) | % COD Removal |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 5 | 35 | 0.03 | 0.00 | 0.016 | 0.05 | 0.0 | 0.0 |
| 2 | 60 | 5 | 35 | 0.17 | 0.00 | 0.013 | 0.18 | 0.0 | 0.0 |
| 3 | 80 | 7 | 35 | 0.47 | 0.00 | 0.053 | 0.56 | 22.2 | 1.0 |
| 4 | 100 | 7 | 35 | 0.34 | 0.00 | 0.038 | 0.38 | 22.0 | 1.0 |
| 5 | 40 | 7 | 55 | 0.24 | 0.27 | 0.002 | 0.80 | 17.9 | 0.7 |
| 6 | 60 | 7 | 55 | 0.36 | 0.28 | 0.000 | 0.81 | 15.7 | 0.7 |
| 7 | 80 | 7 | 55 | 0.33 | 0.08 | 0.001 | 0.46 | 9.8 | 0.4 |
| 8 | 80 | 5 | 55 | 0.03 | 0.00 | 0.004 | 0.03 | 0.1 | 0.0 |
| 9 | 100 | 5 | 55 | 0.03 | 0.02 | 0.003 | 0.07 | 0.0 | 0.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usmanbaha, N.; Jariyaboon, R.; Reungsang, A.; Kongjan, P.; Chu, C.-Y. Optimization of Batch Dark Fermentation of Chlorella sp. Using Mixed-Cultures for Simultaneous Hydrogen and Butyric Acid Production. Energies 2019, 12, 2529. https://doi.org/10.3390/en12132529
Usmanbaha N, Jariyaboon R, Reungsang A, Kongjan P, Chu C-Y. Optimization of Batch Dark Fermentation of Chlorella sp. Using Mixed-Cultures for Simultaneous Hydrogen and Butyric Acid Production. Energies. 2019; 12(13):2529. https://doi.org/10.3390/en12132529
Chicago/Turabian StyleUsmanbaha, Nikannapas, Rattana Jariyaboon, Alissara Reungsang, Prawit Kongjan, and Chen-Yeon Chu. 2019. "Optimization of Batch Dark Fermentation of Chlorella sp. Using Mixed-Cultures for Simultaneous Hydrogen and Butyric Acid Production" Energies 12, no. 13: 2529. https://doi.org/10.3390/en12132529





