Thermal Transformation of Carbon and Oxygen-Containing Organic Compounds in Sewage Sludge During Pyrolysis Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermo-Gravimetric Fourier Transform Infrared (TG-FTIR) Spectroscopy
2.3. X-ray photoelectron spectroscopy (XPS) Analysis
2.4. Pyrolysis-Gas-Chromatography/Mass Spectrometry (Py-GC/MS) Technology
3. Results and Discussion
3.1. Distribution of Organic Carbon and Oxygen-Containing Groups in Sewage Sludge
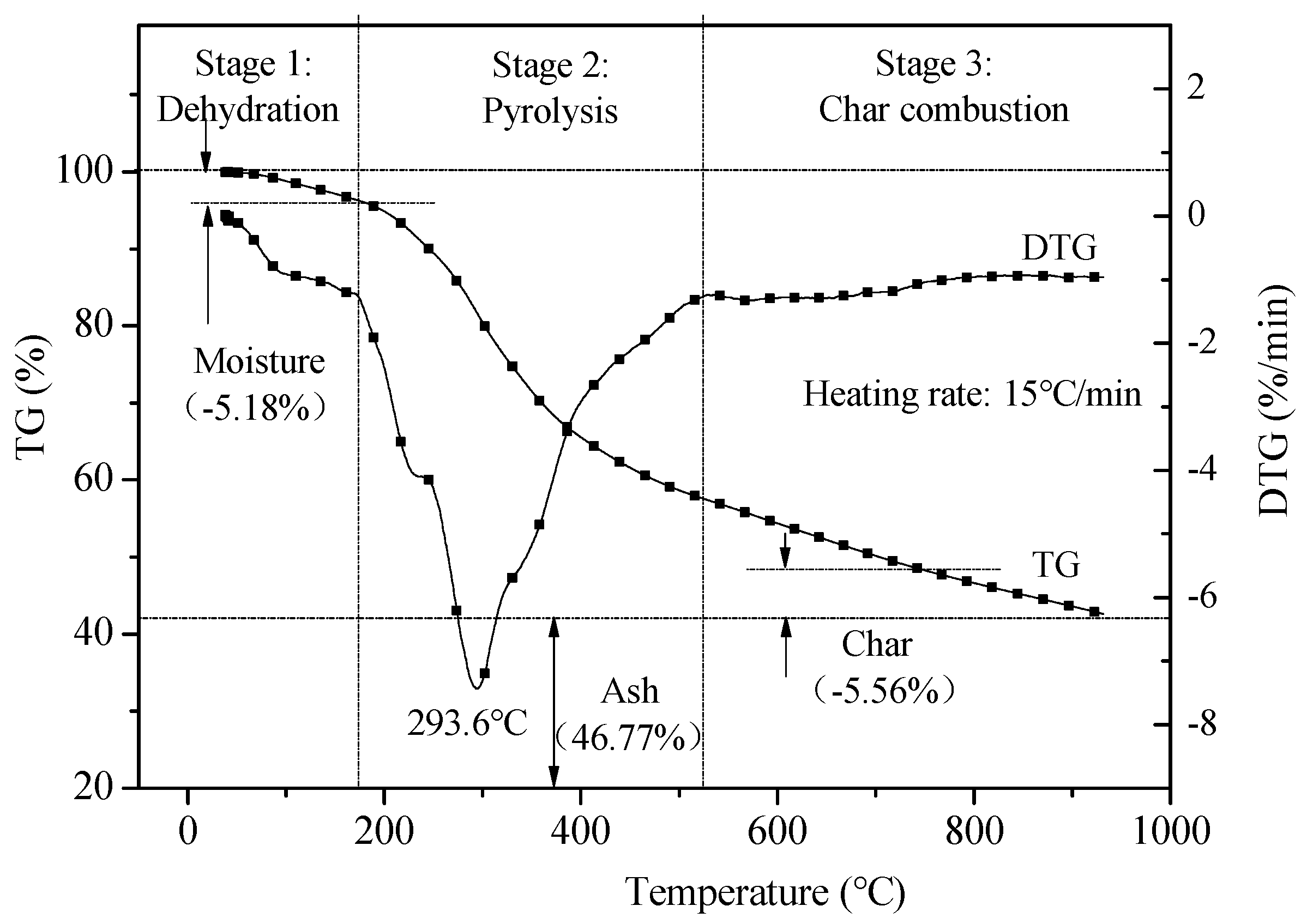
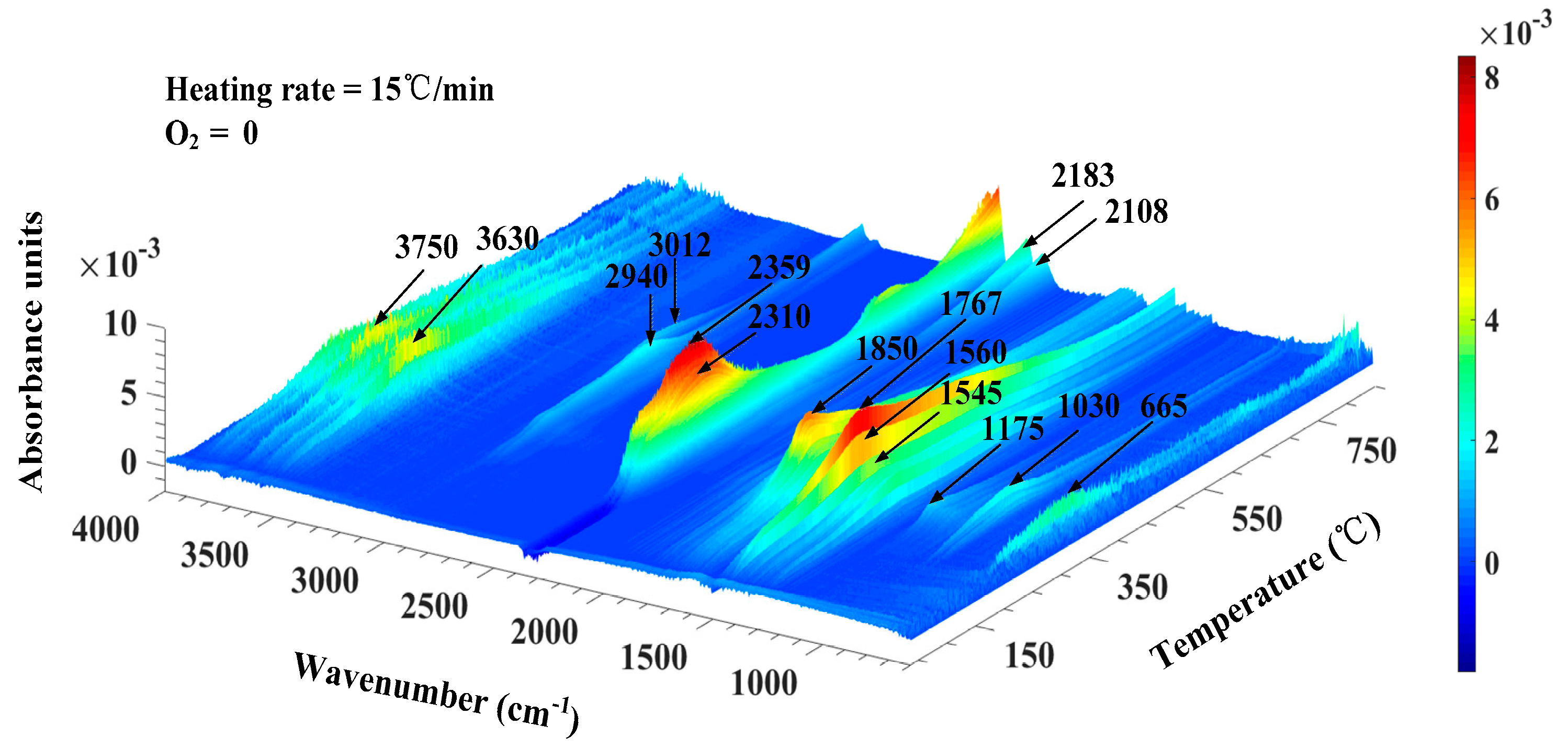
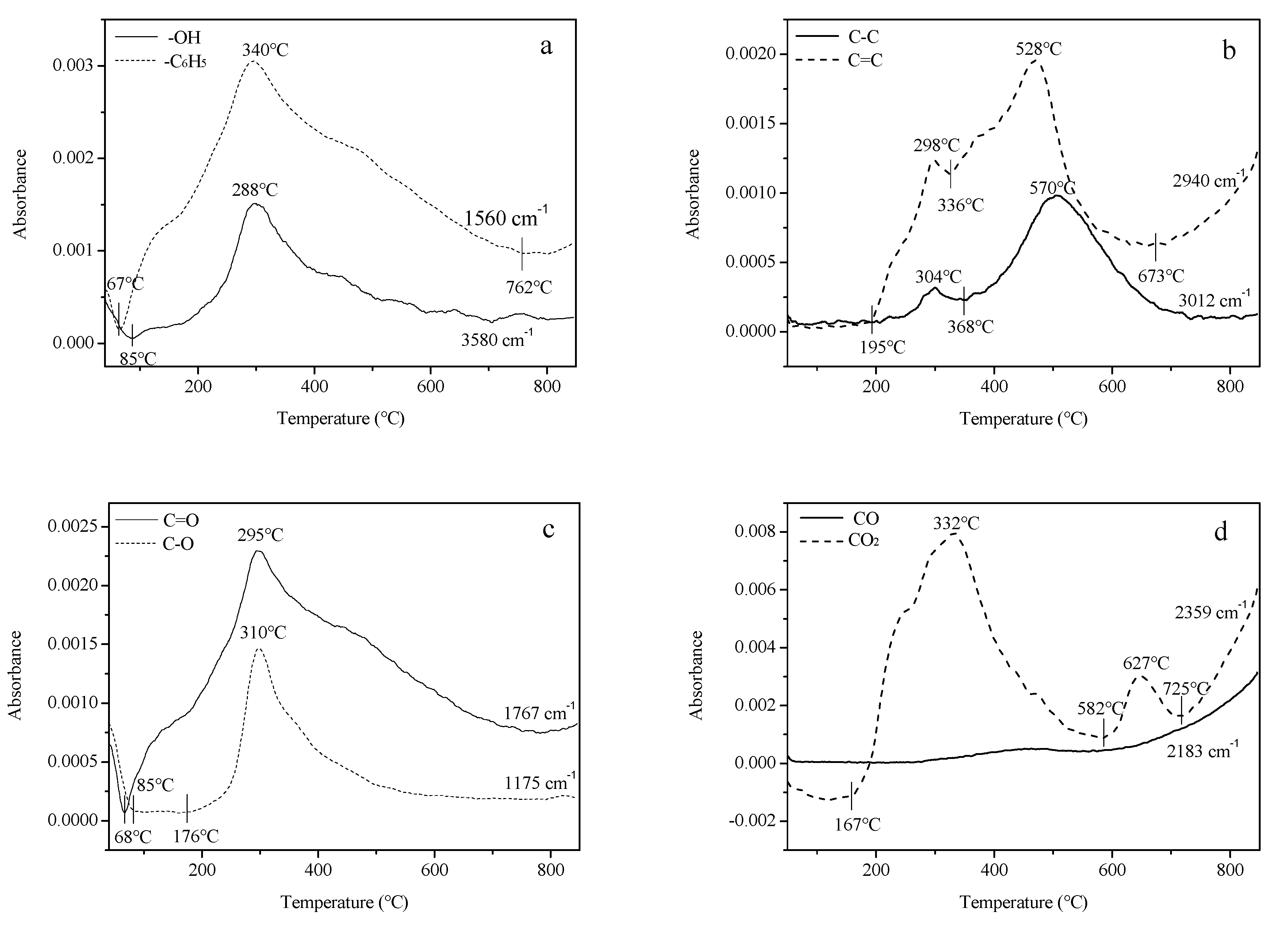
3.2. Thermo-Gravimetric Fourier Transform Infrared Spectroscopy (TG-FTIR) Analysis
3.3. Py-GC/MS Analysis
3.4. Pyrolysis Evolution Pathway of Organic Carbon and Oxygen-Containing Compounds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Song, S.; Sheng, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Alonso, M.; Díaz, R.M. Influence of sewage sludge treatment on pyrolysis and combustion of dry sludge. Energy 2013, 55, 426–435. [Google Scholar] [CrossRef]
- Zhou, H.B.; Ma, C.; Gao, D.; Chen, T.B.; Zheng, G.D.; Chen, J.; Pan, T.H. Application of a recyclable plastic bulking agent for sewage sludge composting. Bioresour. Technol. 2014, 152, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Li, C.X.; Zhang, B.; Lin, J.J.; Chi, Q.Q.; Wang, Y. Migration and risk assessment of heavy metals in sewage sludge during hydrothermal treatment combined with pyrolysis. Bioresour. Technol. 2016, 221, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.P.; Wang, J.Y. Characterization of sludge for pyrolysis conversion process based on biomass composition analysis and simulation of pyrolytic properties. Waste Manag. 2018, 72, 274–286. [Google Scholar] [CrossRef]
- He, C.; Chen, C.L.; Giannis, A.; Yang, Y.H.; Wang, J.Y. Hydrothermal gasification of sewage sludge and model compounds for renewable hydrogen production: A review. Renew. Sustain. Energy Rev. 2014, 39, 1127–1142. [Google Scholar] [CrossRef]
- Piskorz, J.; Scott, D.S.; Westerberg, I.B. Flash pyrolysis of sewage sludge. Ind. Eng. Chem. Res. 1986, 25, 265–270. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lee, M.K.; Chang, J.H.; Su, T.Y.; Chang, Y.M. Characterization of bio-oil from induction-heating pyrolysis of food-processing sewage sludges using chromatographic analysis. Bioresour. Technol. 2009, 100, 2650–2654. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, D.K. An experimental study of oil recovery from sewage sludge by low-temperature pyrolysis in a fluidised-bed. Fuel 2003, 82, 465–472. [Google Scholar] [CrossRef]
- Font, R.; Fullana, A.; Conesa, J. Kinetic models for the pyrolysis and combustion of two types of sewage sludge. J. Anal. Appl. Pyrolysis 2005, 74, 429–438. [Google Scholar] [CrossRef]
- Urban, D.L.; Antal, M.J., Jr. Study of the kinetics of sewage sludge pyrolysis using DSC and TGA. Fuel 1982, 61, 799–806. [Google Scholar] [CrossRef]
- Piatkowski, N.; Steinfeld, A. Reaction kinetics of the combined pyrolysis and steamgasification of carbonaceous waste materials. Fuel 2010, 89, 1133–1140. [Google Scholar] [CrossRef]
- Thipkhunthod, P.; Meeyoo, V.; Rangsunvigit, P.; Rirksomboon, T. Describing sewage sludge pyrolysis kinetics by a combination of biomass fractions decomposition. J. Anal. Appl. Pyrolysis 2007, 79, 78–85. [Google Scholar] [CrossRef]
- Zhou, P.; Xiong, S.J.; Zhang, Y.X.; Jiang, H.; Chi, Y.C.; Lin, L. Study on the nitrogen transformation during the primary pyrolysis of sewage sludge by Py-GC/MS and Py-FTIR. Int. J. Hydrog. Energy 2017, 42, 18181–18188. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, J.; Zuo, W.; Chen, L.; Cui, Y.N.; Tan, T. Nitrogen conversion in relation to NH3 and HCN during microwave pyrolysis of sewage sludge. Environ. Sci. Technol. 2013, 47, 3498–3505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zuo, W.; Tian, Y.; Chen, L.; Yin, L.L.; Zhang, J. Sulfur transformation during microwave and conventional pyrolysis of sewage sludge. Environ. Sci. Technol. 2017, 51, 709–717. [Google Scholar] [CrossRef] [PubMed]
- ISO 11722. Solid Mineral Fuels. Hard Coal. Determination of Moisture in the General Analysis by Drying in Nitrogen; International Organization for Standardization: Geneva, Switzerland, 1999. [Google Scholar]
- ISO 625. Solid Mineral Fuels-Determination of Carbon and Hydrogen-Liebig Method, MOD; International Organization for Standardization: Geneva, Switzerland, 1996. [Google Scholar]
- ISO/TS 11725. Solid Mineral Fuels-Determination of Nitrogen-Semi-Micro Gasification, MOD; International Organization for Standardization: Geneva, Switzerland, 2004. [Google Scholar]
- ISO 351. Solid Mineral Fuels-Determination of Total Sulfur-High Temperature Combustion Method, NEQ; International Organization for Standardization: Geneva, Switzerland, 1996. [Google Scholar]
- ISO 1928. Solid Gross Calorific Value by Calculation of Mineral Fuels-Determination of the Bomb Calorimetric Method and Net Calorific Value, MOD; International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Lin, Y.; Liao, Y.F.; Yu, Z.S.; Fang, S.W.; Ma, X.Q. A study on co-pyrolysis of bagasse and sewage sludge using TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2017, 151, 190–198. [Google Scholar] [CrossRef]
- Jiang, W.K.; Chu, J.Y.; Wu, S.B.; Lucia, L.A. Modeling pyrolytic behavior of pre-oxidized lignin using four representative β-ether-type lignin-like model polymers. Fuel Proc. Technol. 2018, 176, 221–229. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, F.; Li, J.; Li, B.J.; Zhao, C.S. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym. Chem. 2010, 1, 1430–1433. [Google Scholar] [CrossRef]
- Finsgar, M.; Fassbender, S.; Hirth, S.; Milosev, I. Electrochemical and XPS study of polyethyleneimines of different molecular sizes as corrosion inhibitors for AISI 430 stainless steel in near-neutral chloride media. Mater. Chem. Phys. 2009, 116, 198–206. [Google Scholar] [CrossRef]
- Yan, Z.L.; Yang, H.M.; Ouyang, J.; Tang, A.D. In situ loading of highly-dispersed CuO nanoparticles on hydroxyl-group-rich SiO2-AlOOH composite nanosheets for CO catalytic oxidation. Chem. Eng. J. 2017, 316, 1035–1046. [Google Scholar] [CrossRef]
- Gao, C.; Yu, X.Y.; Xu, R.X.; Liu, J.H.; Huang, X.J. AlOOH-Reduced graphene oxide nanocomposites: One-pot hydrothermal synthesis and their enhanced electrochemical activity for heavy metal ions. ACS Appl. Mater. Interfaces 2012, 4, 4672–4682. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, Y.; Qiu, R. Parametric study and global sensitivity analysis for co-pyrolysis of rape straw and waste tire via variance-based decomposition. Bioresour. Technol. 2017, 247, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Lin, J.B.; Lee, P.H.; Kokorevicha, S. Evaluation of sewage sludge-based compost by FT-IR spectroscopy. Geoderma 2006, 130, 324–333. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.J.; Huang, G.; Liu, H.R.; Yang, S.L.; Zhang, A.F. Thermal degradation behaviors and reaction mechanism of carbon fibre-epoxy composite from hydrogen tank by TG-FTIR. J. Hazard. Mater. 2018, 357, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Jong, W.D.; Nola, G.D.; Venneker, B.C.H.; Spliethoff, H.; Wojtowicz, M.A. TG-FTIR pyrolysis of coal and secondary biomass fuels: Determination of pyrolysis kinetic parameters for main species and NOx precursors. Fuel 2007, 86, 2367–2376. [Google Scholar] [CrossRef]
- Wang, S.R.; Wang, K.G.; Liu, Q.; Gu, Y.L.; Luo, Z.Y.; Cen, K.F.; Fransson, T. Comparison of the pyrolysis behavior of lignins from different tree species. Biotechnol. Adv. 2009, 27, 562–567. [Google Scholar] [CrossRef]
- Jia, Y.B.; Huang, J.J.; Wang, Y. 2004. Effects of calcium oxide on the cracking of coal tar in the freeboard of a fluidized bed. Energy Fuels 2004, 18, 1625–1632. [Google Scholar] [CrossRef]
- Gu, X.L.; Ma, X.; Li, L.X.; Liu, C.; Chen, K.H.; Li, Z.Z. Pyrolysis of poplar wood sawdust by TG-FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis 2013, 102, 16–23. [Google Scholar] [CrossRef]
- Debono, O.; Villot, A. Nitrogen products and reaction pathway of nitrogen compounds during the pyrolysis of various organic wastes. J. Anal. Appl. Pyrolysis 2015, 114, 222–234. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y.M.; Uzoejinwa, B.B.; Cao, B.; He, Z.X.; Wang, Q.; Xu, S.N. Pyrolysis mechanisms of typical seaweed polysaccharides. J. Anal. Appl. Pyrolysis 2016, 124, 373–383. [Google Scholar] [CrossRef]
- Kim, Y.M.; Han, T.U.; Lee, B.; Watanabe, A.; Teramae, N.; Kim, J.H.; Park, Y.K.; Park, H.; Kim, S. Analytical pyrolysis reaction characteristics of Porphyra tenera. Algal Res. 2018, 32, 60–69. [Google Scholar] [CrossRef]
- Gierer, J. Chemical aspects of kraft pulping. Wood Sci. Technol. 1980, 14, 241–266. [Google Scholar] [CrossRef]
- Wei, A.F.; Cao, J.P.; Zhao, X.Y.; Ren, J.; Gu, B.; Wei, X.Y. Formation of aromatics and removal of nitrogen in catalytic fast pyrolysis of sewage sludge: A study of sewage sludge and model amino acids. Fuel 2018, 218, 148–154. [Google Scholar] [CrossRef]




| Proximate Analysis (wt.%) | Mad (%) | Aad (%) | Vad (%) | FCad (%) | H (MJ·kg−1) |
|---|---|---|---|---|---|
| 2.10 | 41.07 | 54.69 | 2.14 | 13.5 | |
| Ultimate analysis (wt.%) | C | H | O | S | N |
| 35.7 | 5.34 | 12.81 | 1.75 | 3.44 |
| No. | Retention Time(R.T)/(min) | Compounds | Formula | Peak Area/% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 250 °C | 350 °C | 450 °C | 550 °C | 650 °C | 750 °C | 850 °C | ||||
| 1 | 1.595 | butanal, 3-methyl- | C5H10O | - | - | - | - | 2.51 | - | - |
| 2 | 1.634 | benzene | C6H6 | - | - | - | - | - | 4.48 | 6.48 |
| 3 | 1.644 | 1-propanol,2-amino-, (.+/-.)- | C3H9NO | 3.28 | - | - | - | - | - | - |
| 4 | 2.047 | toluene | C7H8 | - | - | - | 1.3 | 2.46 | 4.33 | 5.9 |
| 5 | 2.103 | 1-octene | C8H16 | - | - | - | - | 1.17 | - | - |
| 6 | 2.449 | 2-furanmethanol | C5H6O2 | - | - | 0.7 | 0.99 | - | - | - |
| 7 | 2.586 | ethylbenzene | C8H10 | - | - | - | - | 1.27 | 1.62 | 1.46 |
| 8 | 2.747 | 1-nonene | C9H18 | - | - | - | 0.25 | 0.62 | 0.47 | - |
| 9 | 2.791 | styrene | C8H8 | - | - | 0.48 | 1.36 | 2.16 | 2.19 | 3.26 |
| 10 | 2.864 | 1,3,5,7-cyclooctatetraene | C8H8 | - | - | 0.87 | - | - | - | - |
| 11 | 3.547 | 1-decene | C10H20 | - | - | - | - | 1.53 | - | - |
| 12 | 3.908 | benzene, 1-propenyl- | C9H10 | - | - | - | - | 0.41 | 1.02 | 0.75 |
| 13 | 3.942 | d-limonene | C10H16 | - | - | - | 0.66 | - | - | |
| 14 | 4.108 | indene | C9H8 | - | - | - | - | - | - | 1.69 |
| 15 | 4.181 | phenol, 2-methyl- | C7H8O | - | - | - | - | - | 0.76 | 0.54 |
| 16 | 4.43 | p-cresol | C7H8O | - | - | 2.24 | 2.4 | 1.99 | 3.38 | 2.36 |
| 17 | 4.996 | 2-methylindene | C10H10 | - | - | - | - | - | 1.07 | 0.88 |
| 18 | 5.05 | 1h-indene, 1-methyl- | C10H10 | - | - | - | - | - | 1.2 | - |
| 19 | 5.226 | 1-dodecene | C12H24 | - | - | - | - | - | 0.61 | - |
| 20 | 5.309 | naphthalene | C10H8 | - | - | - | - | - | 1.75 | 3.11 |
| 21 | 5.992 | 1-methylnaphthalene | C11H10 | - | - | - | - | - | - | 2.29 |
| 22 | 5.996 | 1-tridecene | C13H26 | - | - | - | 0.37 | 0.73 | - | - |
| 23 | 6.289 | naphthalene,2-methyl- | C11H10 | - | - | - | - | - | 1.07 | - |
| 24 | 6.562 | 1,4-benzenediol, 2-methyl- | C7H8O2 | - | - | - | 0.65 | - | - | - |
| 25 | 6.709 | 1-tetradecene | C14H28 | - | - | - | 0.5 | 1.25 | 0.57 | - |
| 26 | 6.758 | biphenyl | C12H10 | - | - | - | - | - | 0.58 | 0.66 |
| 27 | 7.192 | 1-undecene,5-methyl- | C12H24 | - | - | - | 0.52 | - | - | - |
| 28 | 7.265 | 3-allyl-6-methoxyphenol | C10H12O2 | - | 0.45 | 1.04 | 0.77 | - | - | - |
| 29 | 7.363 | acenaphthylene | C12H8 | - | - | - | - | - | - | 0.8 |
| 30 | 7.489 | chloroethyl ester | C15H30 | - | - | - | 0.5 | 0.69 | 0.51 | - |
| 31 | 8.163 | dodecanoic acid | C12H24O | 1.53 | 2.4 | 3.55 | 1.78 | 1.2 | - | - |
| 32 | 8.622 | fluorene | C13H10 | - | - | - | - | - | - | 1.84 |
| 33 | 8.812 | 13-tetradecenal | C14H26O | - | - | - | 0.31 | - | - | - |
| 34 | 10.685 | tetradecanoic acid | C14H28O2 | 2.15 | 1.9 | 1.45 | 1.96 | 0.54 | - | - |
| 35 | 11.315 | phenanthrene | C14H10 | - | - | - | - | - | 0.28 | 0.49 |
| 36 | 11.486 | anthracene | C14H10 | - | - | - | - | - | - | 0.13 |
| 37 | 11.778 | pentadecanoic acid | C15H30O2 | 3.57 | 2.18 | 1.38 | 1.53 | 0.58 | - | - |
| 38 | 14.482 | palmitoleic acid | C16H30O2 | 9.27 | 3.04 | 0.13 | 2.26 | 0.86 | - | - |
| 39 | 14.852 | n-hexadecanoic acid | C16H32O2 | 25.02 | 4.78 | 4.24 | 3.03 | 1.86 | 0.47 | - |
| 40 | 17.282 | fluoranthene | C16H10 | - | - | - | - | - | - | 0.2 |
| 41 | 19.542 | oleic acid | C18H34O2 | 7.45 | 2.1 | - | 1.72 | 0.61 | - | - |
| 42 | 19.971 | 6-octadecenoic acid, (z)- | C18H34O2 | - | - | 2.13 | 0.34 | - | - | - |
| 43 | 20.22 | octadecanoic acid | C18H36O2 | 3.57 | 0.85 | 0.84 | 0.6 | 0.51 | - | - |
| 44 | 24.48 | 2-octylcyclopropaneoctanal | C19H36O | - | 0.13 | 0.11 | - | - | - | - |
| 45 | 38.181 | .gamma.-tocopherol | C28H48O2 | - | 0.15 | - | 0.11 | - | - | - |
| 46 | 38.458 | cholestan-3-ol | C27H48O | 4.28 | - | - | - | - | - | - |
| 47 | 38.488 | cholestanol | C27H48O | - | 0.66 | 0.29 | 0.35 | 0.21 | - | - |
| 48 | 39.537 | cholesterol | C27H46O | 1.79 | - | 0.2 | 0.23 | - | - | - |
| 49 | 43.109 | stigmastanol | C29H52O | - | 0.24 | 0.1 | 0.18 | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Lu, Y.; Ma, W.; Wang, L.; Wibowo, H.; Huang, Z.; Yu, F. Thermal Transformation of Carbon and Oxygen-Containing Organic Compounds in Sewage Sludge During Pyrolysis Treatment. Energies 2019, 12, 2258. https://doi.org/10.3390/en12122258
Hu Y, Lu Y, Ma W, Wang L, Wibowo H, Huang Z, Yu F. Thermal Transformation of Carbon and Oxygen-Containing Organic Compounds in Sewage Sludge During Pyrolysis Treatment. Energies. 2019; 12(12):2258. https://doi.org/10.3390/en12122258
Chicago/Turabian StyleHu, Yanjun, Yanjun Lu, Wenchao Ma, Linjie Wang, Haryo Wibowo, Zhicheng Huang, and Fan Yu. 2019. "Thermal Transformation of Carbon and Oxygen-Containing Organic Compounds in Sewage Sludge During Pyrolysis Treatment" Energies 12, no. 12: 2258. https://doi.org/10.3390/en12122258





