Optimisation of Tray Drier Microalgae Dewatering Techniques Using Response Surface Methodology
Abstract
1. Introduction
- microalgae cultivation
- dewatering
- lipid extraction
- transesterification.
2. Materials and Methods
2.1. Microalgae Strain and Preparation
2.2. Tray Drier Description
2.3. Experimental Procedure
2.4. Design of Experiments
3. Results and Discussion
3.1. RSM Model Estimation
3.2. Dewatering Efficiency Optimisation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Cheruvu, S.; Van Ginkel, S.; Wei, X.; Zhang, X.; Steiner, D.; Rego De Oliveira, S.H.; Xu, C.; Kalil Duarte, L.H.; Salvi, E.; Hu, Z.; et al. Algae harvesting. Membr. Technol. Biorefining 2016, 309–328. [Google Scholar] [CrossRef]
- Onumaegbu, C.; Alaswad, A.; Rodriguez, C.; Olabi, A. Optimization of Pre-Treatment Process Parameters to Generate Biodiesel from Microalga. Energies 2018, 11, 806. [Google Scholar] [CrossRef]
- Chng, L.M.; Lee, K.T.; Chan, D.J.C. Synergistic effect of pretreatment and fermentation process on carbohydrate-rich Scenedesmus dimorphus for bioethanol production. Energy Convers. Manag. 2017, 141, 410–419. [Google Scholar] [CrossRef]
- González-González, L.M.; Zhou, L.; Astals, S.; Thomas-Hall, S.R.; Eltanahy, E.; Pratt, S.; Jensen, P.D.; Schenk, P.M. Biogas production coupled to repeat microalgae cultivation using a closed nutrient loop. Bioresour. Technol. 2018, 263, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Lin, M. Effects of algae and live food density on the feeding ability, growth and survival of miiuy croaker during early development. Aquaculture 2014, 428–429, 284–289. [Google Scholar] [CrossRef]
- Rugnini, L.; Costa, G.; Congestri, R.; Bruno, L. Testing of two different strains of green microalgae for Cu and Ni removal from aqueous media. Sci. Total Environ. 2017, 601–602, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Skjånes, K.; Lindblad, P.; Muller, J. BioCO2—A multidisciplinary, biological approach using solar energy to capture CO2 while producing H2 and high value products. Biomol. Eng. 2007, 24, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Hattab Al, M.; Ghaly, A.; Hammoud, A. Microalgae Harvesting Methods for Industrial Production of Biodiesel: Critical Review and Comparative Analysis. J. Fundam. Renew. Energy Appl. 2015, 5, 1000154. [Google Scholar] [CrossRef]
- Sahoo, N.K.; Gupta, S.K.; Rawat, I.; Ansari, F.A.; Singh, P.; Naik, S.N.; Bux, F. Sustainable dewatering and drying of self-flocculating microalgae and study of cake properties. J. Clean. Prod. 2017, 159, 248–256. [Google Scholar] [CrossRef]
- Hernández Leal, L.; Temmink, H.; Zeeman, G.; Buisman, C.J.N. Bioflocculation of grey water for improved energy recovery within decentralized sanitation concepts. Bioresour. Technol. 2010, 101, 9065–9070. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, R.C.; Rodriguez, C.; Durrant, A.; Olabi, A.G. Micro-Macroalgae Properties and Applications. In Reference Module in Materials Science and Materials Engineering; Hashmi, S., Ed.; Elsevier: New York, NY, USA, 2018. [Google Scholar]
- Anyanwu, R.C.; Rodriguez, C.; Durrant, A.; Olabi, A.G. Microalgae Cultivation Technologies. Modul. Mater. Sci. Mater. Eng. 2018. [Google Scholar] [CrossRef]
- Jakob, G.; Stephens, E.; Feller, R.; Oey, M.; Hankamer, B.; Ross, I.L. Triggered exocytosis of the protozoan Tetrahymena as a source of bioflocculation and a controllable dewatering method for efficient harvest of microalgal cultures. Algal Res. 2016, 13, 148–158. [Google Scholar] [CrossRef]
- Kim, D.G.; La, H.J.; Ahn, C.Y.; Park, Y.H.; Oh, H.M. Harvest of Scenedesmus sp. with bioflocculant and reuse of culture medium for subsequent high-density cultures. Bioresour. Technol. 2011, 102, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Ummalyma, S.B.; Gnansounou, E.; Sukumaran, R.K.; Sindhu, R.; Pandey, A.; Sahoo, D. Bioflocculation: An alternative strategy for harvesting of microalgae—An overview. Bioresour. Technol. 2017, 242, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Van De Staey, G.; Smits, K.; Smets, I. An experimental study on the impact of bioflocculation on activated sludge separation techniques. Sep. Purif. Technol. 2015, 141, 94–104. [Google Scholar] [CrossRef]
- Cho, K.; Hur, S.P.; Lee, C.H.; Ko, K.; Lee, Y.J.; Kim, K.N.; Kim, M.S.; Chung, Y.H.; Kim, D.; Oda, T. Bioflocculation of the oceanic microalga Dunaliella salina by the bloom-forming dinoflagellate Heterocapsa circularisquama, and its effect on biodiesel properties of the biomass. Bioresour. Technol. 2016, 202, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Ndikubwimana, T.; Zeng, X.; He, N.; Xiao, Z.; Xie, Y.; Chang, J.-S.; Lin, L.; Lu, Y. Microalgae biomass harvesting by bioflocculation-interpretation by classical DLVO theory. Biochem. Eng. J. 2015, 101, 160–167. [Google Scholar] [CrossRef]
- Zhou, W.; Min, M.; Hu, B.; Ma, X.; Liu, Y.; Wang, Q.; Shi, J.; Chen, P.; Ruan, R. Filamentous fungi assisted bio-flocculation: A novel alternative technique for harvesting heterotrophic and autotrophic microalgal cells. Sep. Purif. Technol. 2013, 107, 158–165. [Google Scholar] [CrossRef]
- Buckwalter, P.; Embaye, T.; Gormly, S.; Trent, J.D. Dewatering microalgae by forward osmosis. Desalination 2013, 312, 19–22. [Google Scholar] [CrossRef]
- Son, J.; Sung, M.; Ryu, H.; Oh, Y.-K.; Han, J.-I. Microalgae dewatering based on forward osmosis employing proton exchange membrane. Bioresour. Technol. 2017, 244, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Choi, J.; Hong, S. Evaluation on suitability of osmotic dewatering through forward osmosis (FO) for xylose concentration. Sep. Purif. Technol. 2018, 191, 225–232. [Google Scholar] [CrossRef]
- Lee, S.; Shon, H.K.; Hong, S. Dewatering of activated sludge by forward osmosis (FO) with ultrasound for fouling control. Desalination 2017, 421, 79–88. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Culture medium optimization for enhanced β-carotene and biomass production by Dunaliella salina in mixotrophic culture. Biocatal. Agric. Biotechnol. 2016, 7, 217–223. [Google Scholar] [CrossRef]
- Tiroutchelvame, D. Studies on the drying characteristics of ginger flakes. Master’s Thesis, Indira Gandhi Agricultural University, Chhattisgarh, India, 2000. [Google Scholar]
- Ekpeni, L.E.N.; Benyounis, K.Y.; Nkem-Ekpeni, F.F.; Stokes, J.; Olabi, A.G. Underlying factors to consider in improving energy yield from biomass source through yeast use on high-pressure homogenizer (hph). Energy 2015, 81, 74–83. [Google Scholar] [CrossRef]
- Sarrai, A.E.; Hanini, S.; Merzouk, N.K.; Tassalit, D.; Szabó, T.; Hernádi, K.; Nagy, L. Using central composite experimental design to optimize the degradation of Tylosin from aqueous solution by Photo-Fenton reaction. Materials 2016, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Marrero Barroso, T.; Olabi, A.G. Optimization of mechanical pre-treatment of Laminariaceae spp. biomass-derived biogas. Renew. Energy 2014, 62, 527–534. [Google Scholar] [CrossRef]
- Zakir Hossain, S.M.; Mansour, N.; Sultana, N. Design of a laboratory experiment for the performance analysis of a retrofitted tray dryer unit. Educ. Chem. Eng. 2017, 18, 35–44. [Google Scholar] [CrossRef]
- Ghasemkhani, H.; Keyhani, A.; Aghbashlo, M.; Rafiee, S.; Mujumdar, A.S. Improving exergetic performance parameters of a rotating-tray air dryer via a simple heat exchanger. Appl. Therm. Eng. 2016, 94, 13–23. [Google Scholar] [CrossRef]
- Misha, S.; Mat, S.; Ruslan, M.H.; Sopian, K.; Salleh, E. The Prediction of Drying Uniformity in Tray Dryer System using CFD Simulation. Int. J. Mach. Learn. Comput. 2013, 3, 419–423. [Google Scholar] [CrossRef]
- Shalini, R.; Ranjan, A.; Kumar, N. Studies on the Drying Characteristics of apple pomace on Tray Drier. In Proceedings of the 16th International Drying Symposium (IDS 2008), Hyderabad, India, 9–12 November 2008; pp. 1636–1640. [Google Scholar]
- Mahn, A.; Martin, C.; Reyes, A.; Saavedra, A. Evolution of sulforaphane content in sulforaphane-enriched broccoli during tray drying. J. Food Eng. 2016, 186, 27–33. [Google Scholar] [CrossRef]
- Colak, N.; Hepbasli, A. Performance analysis of drying of green olive in a tray dryer. J. Food Eng. 2007, 80, 1188–1193. [Google Scholar] [CrossRef]

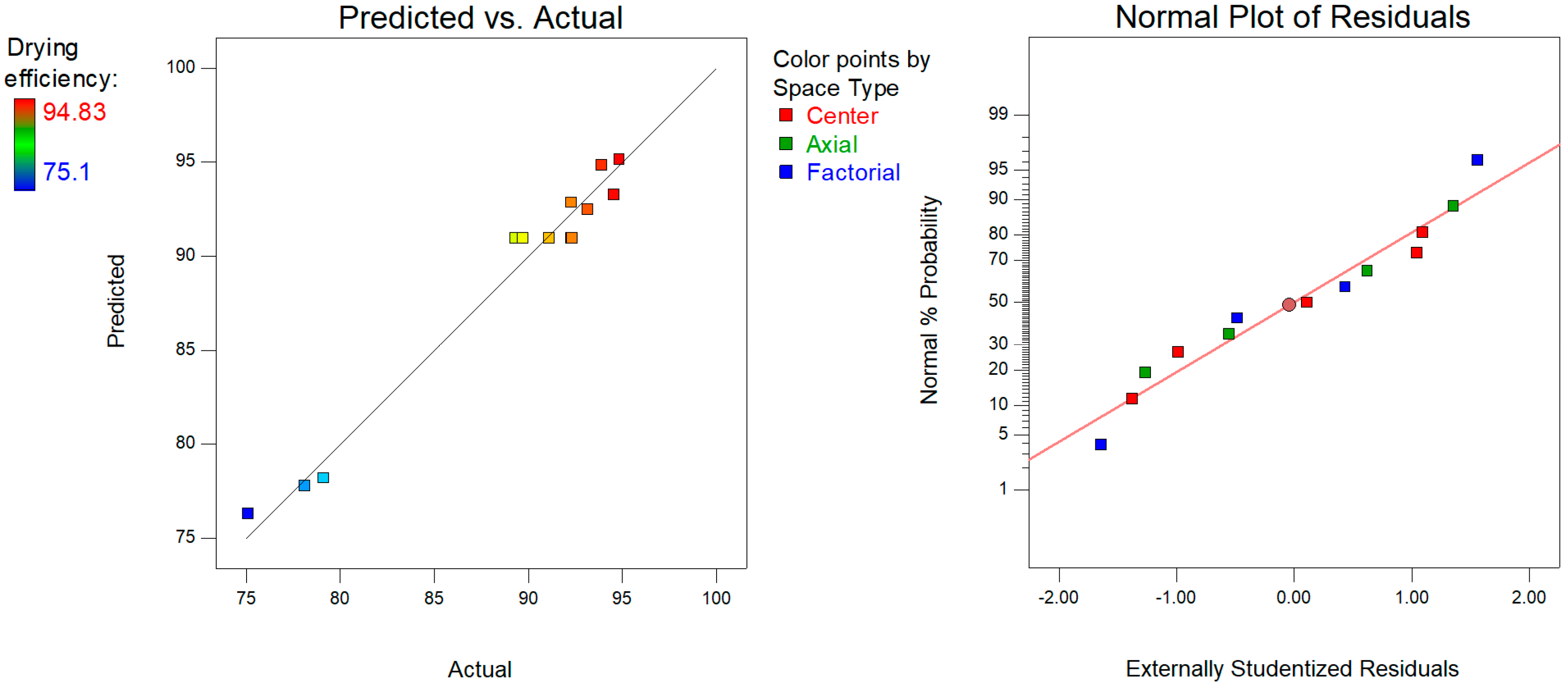
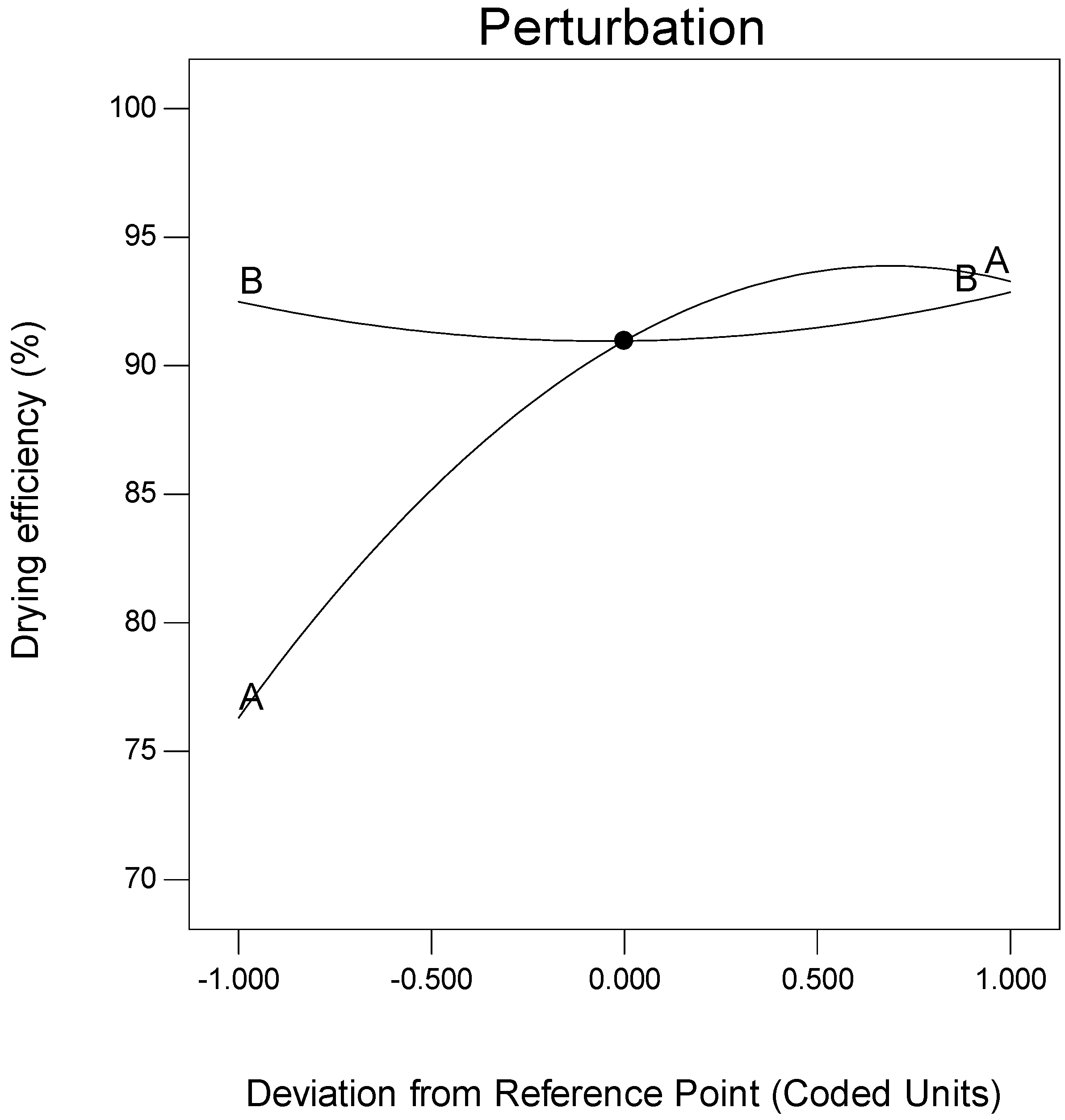
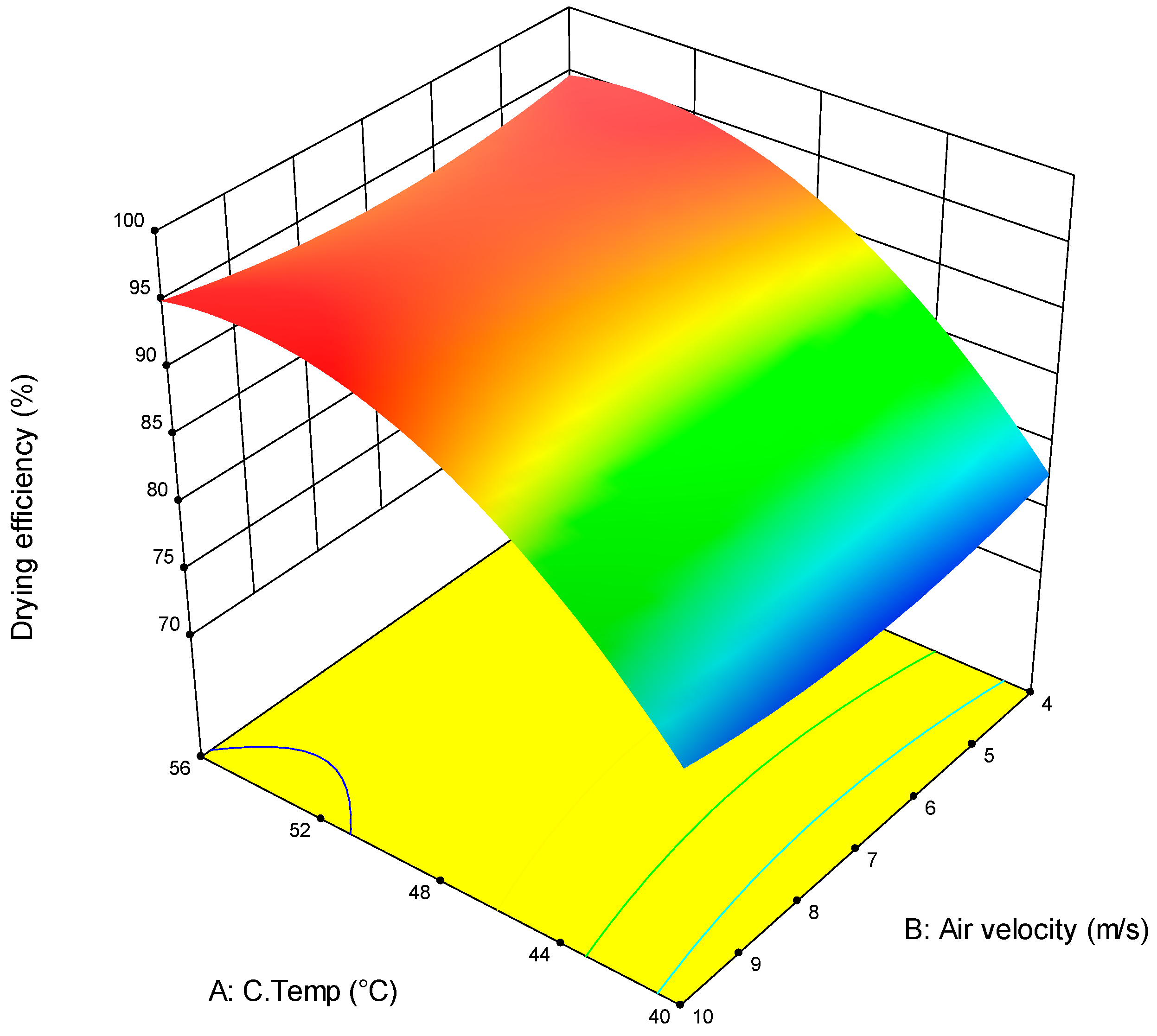
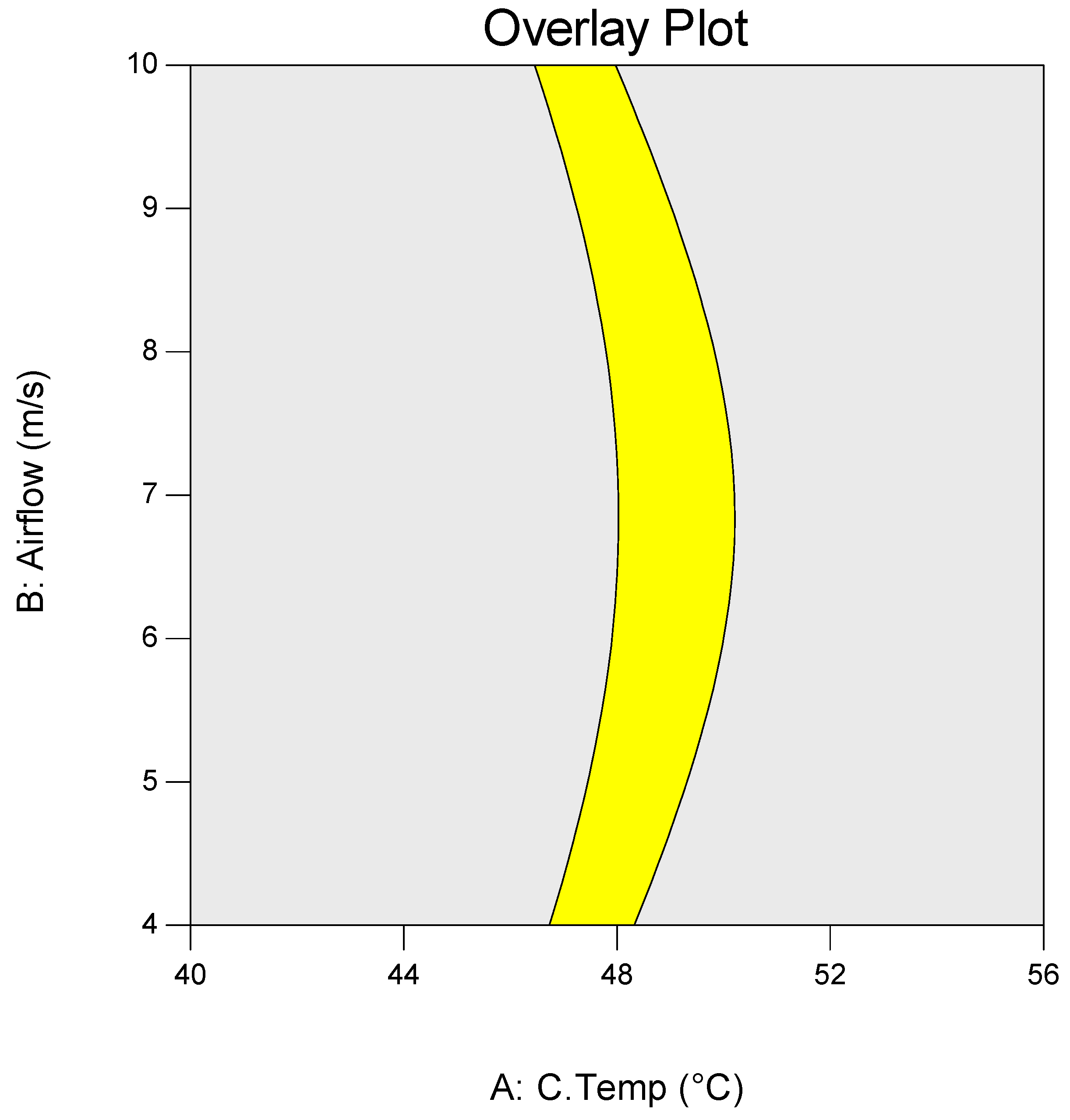
| Independent Variable | −1 | 0 | 1 |
| A: Temperature (°C) | 40 | 48 | 56 |
| B: Air Velocity (m/s) | 4 | 7 | 10 |
| Run | Factor A Temperature (°C) | Factor B Air Velocity (m/s) | Response Dewatering Efficiency (%) |
|---|---|---|---|
| 1 | 40 | 7 | 75.10 ± 0.17 |
| 2 | 56 | 4 | 93.89 ± 2.08 |
| 3 | 40 | 10 | 79.12 ± 0.14 |
| 4 | 48 | 4 | 93.14 ± 0.06 |
| 5 | 40 | 4 | 78.11 ± 0.01 |
| 6 | 48 | 7 | 92.33 ± 0.01 |
| 7 | 48 | 7 | 91.11 ± 0.81 |
| 8 | 48 | 7 | 89.71 ± 0.10 |
| 9 | 56 | 7 | 94.55 ± 0.12 |
| 10 | 56 | 10 | 94.83 ± 0.22 |
| 11 | 48 | 7 | 89.32 ± 0.16 |
| 12 | 48 | 10 | 92.28 ± 0.04 |
| 13 | 48 | 7 | 92.28 ± 0.04 |
| Experiment | Temperature (°C) | Air Velocity (m/s) | DE (%) | |
|---|---|---|---|---|
| 1 | 42 | 10 | Actual | 79.45 |
| Predicted | 83.02 | |||
| Error (%) | −4.05 | |||
| 2 | 50 | 6 | Actual | 93.89 |
| Predicted | 92.81 | |||
| Error (%) | 1.15 | |||
| 3 | 55 | 4 | Actual | 93.64 |
| Predicted | 95.19 | |||
| Error (%) | −1.66 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anyanwu, R.C.; Rodriguez, C.; Durrant, A.; Olabi, A.G. Optimisation of Tray Drier Microalgae Dewatering Techniques Using Response Surface Methodology. Energies 2018, 11, 2327. https://doi.org/10.3390/en11092327
Anyanwu RC, Rodriguez C, Durrant A, Olabi AG. Optimisation of Tray Drier Microalgae Dewatering Techniques Using Response Surface Methodology. Energies. 2018; 11(9):2327. https://doi.org/10.3390/en11092327
Chicago/Turabian StyleAnyanwu, Ruth Chinyere, Cristina Rodriguez, Andy Durrant, and Abdul Ghani Olabi. 2018. "Optimisation of Tray Drier Microalgae Dewatering Techniques Using Response Surface Methodology" Energies 11, no. 9: 2327. https://doi.org/10.3390/en11092327
APA StyleAnyanwu, R. C., Rodriguez, C., Durrant, A., & Olabi, A. G. (2018). Optimisation of Tray Drier Microalgae Dewatering Techniques Using Response Surface Methodology. Energies, 11(9), 2327. https://doi.org/10.3390/en11092327






