Constructing Accurate Equivalent Electrical Circuit Models of Lithium Iron Phosphate and Lead–Acid Battery Cells for Solar Home System Applications
Abstract
1. Introduction
1.1. Selecting the Battery Model
1.2. Importance of Low Current Battery Models for SHS
1.3. Contributions
- developing an accurate battery cell level model especial for a low currents, as the case in SHS;
- proposing a common modelling methodology based on electrical circuit model applicable to both VRLA and LFP batteries; and
- improving the current electrical circuit models for VRLA battery including
- –
- a non-linear relation between and ;
- –
- a 2nd order RC circuit-based EECM model using a Thevenin approach;
- –
- considering the parasitic branch of the EECM in terms of and C-rate-based Coulombic efficiency.
2. Background
2.1. Battery Parameters
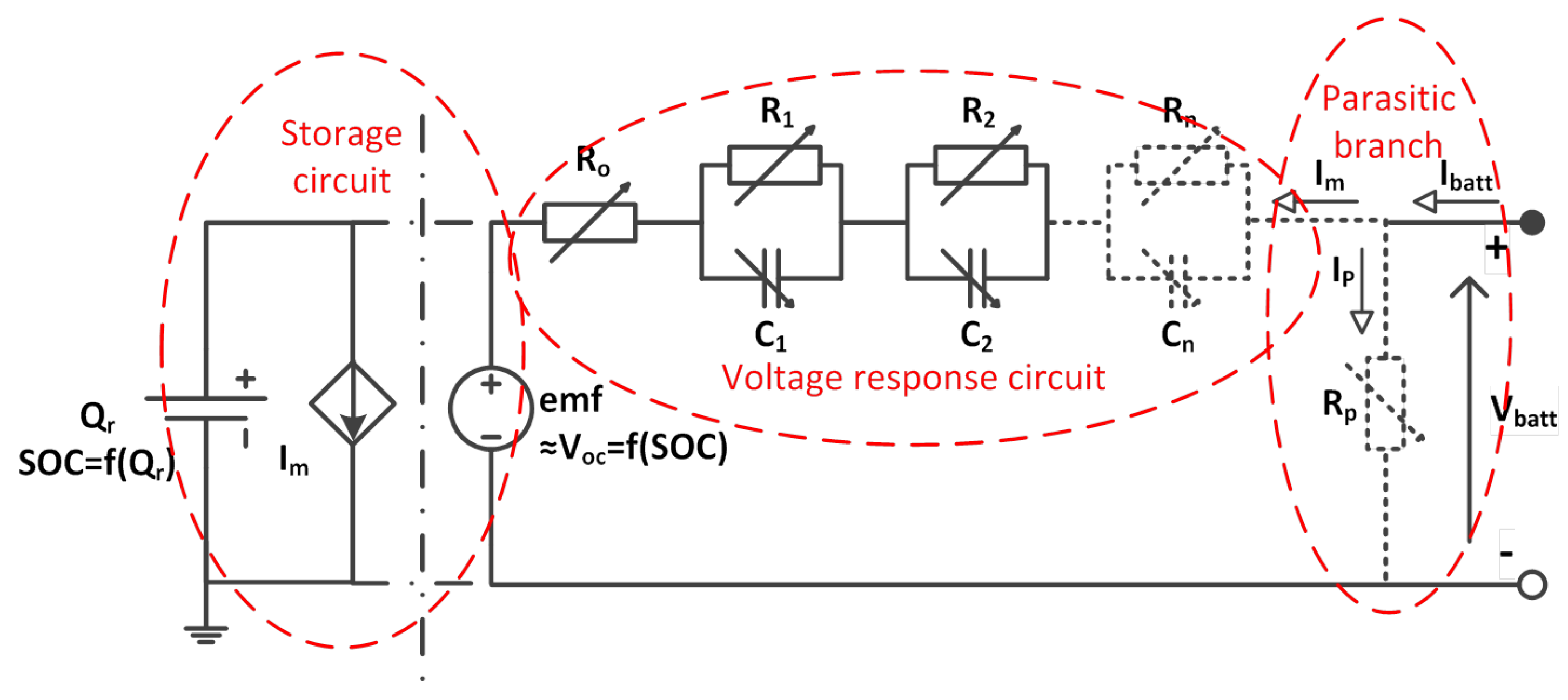
2.2. Construction of the Dynamic Battery Model
2.3. Storage Circuit
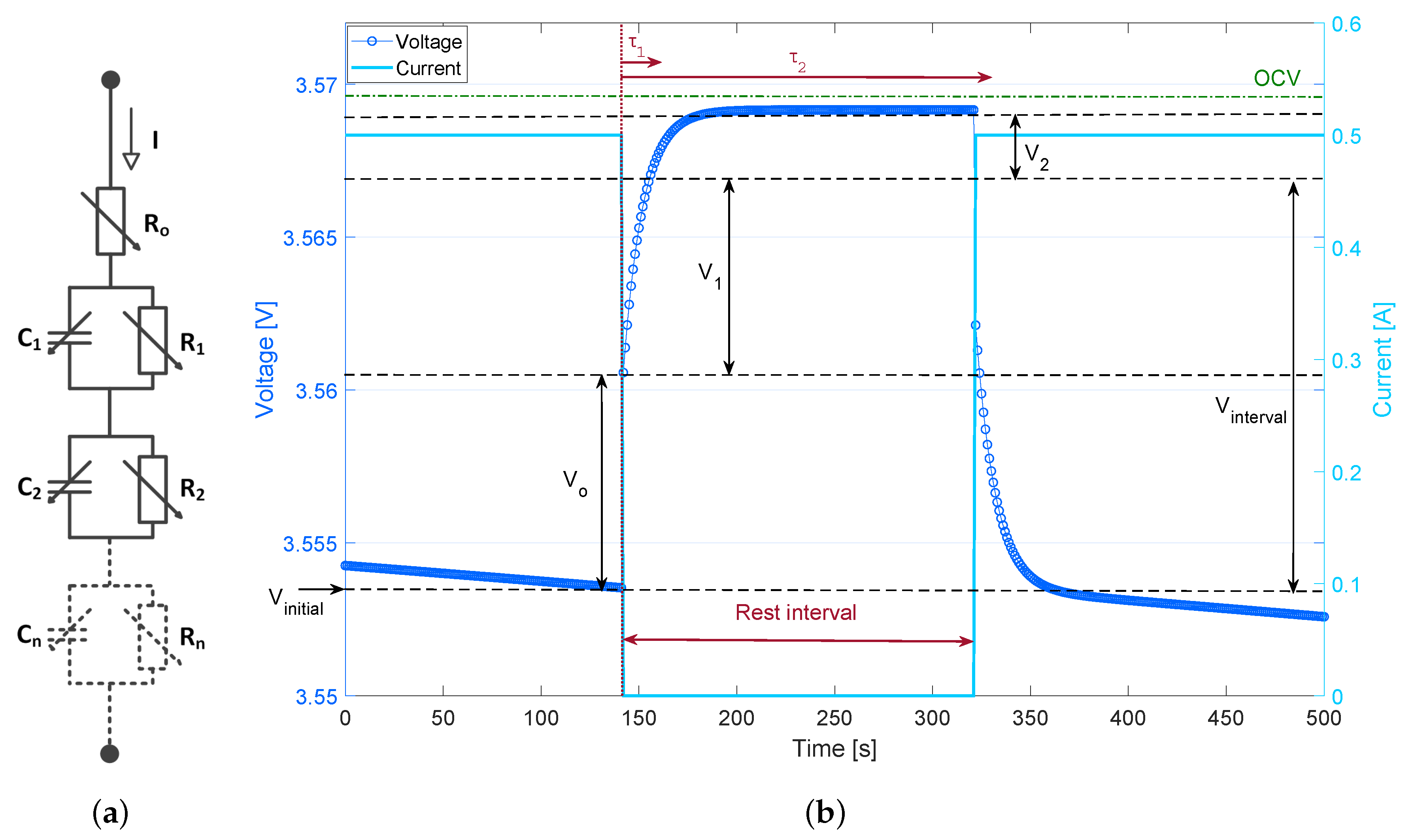
2.4. Electrical Response Circuit
2.5. Parasitic Reaction Circuit
3. Methods and Experiment
3.1. Choice of Operational Variables
3.2. Overall Methodology
- Experimentation. Series of experiments were performed to extract the parameters of each electrical element pertaining to every sub-circuit.
- (a)
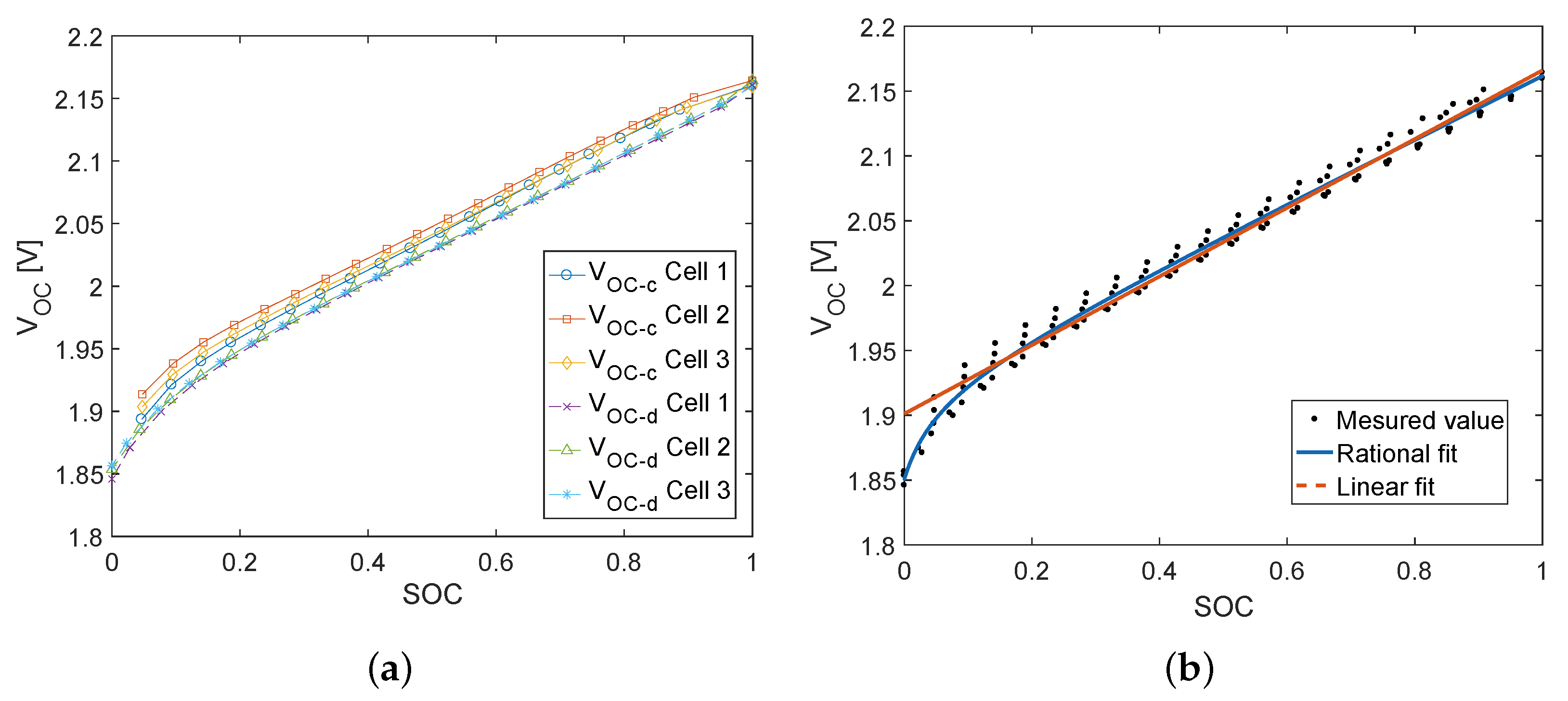
- Storage circuit. The cte-OCV measurement method was performed.
- (b)
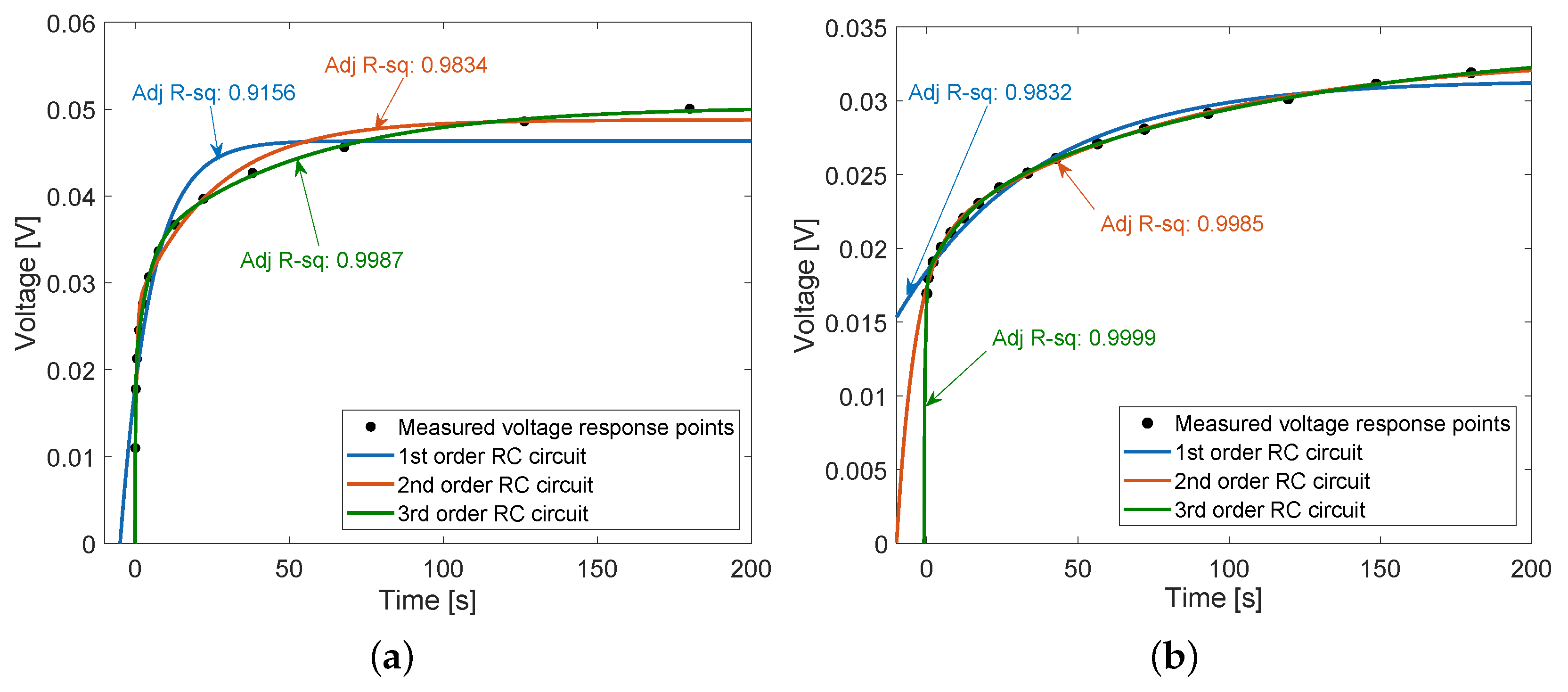
- Voltage response circuit. Voltage relaxation (also called step response) method was used.
- (c)
- Parasitic branch. The differential recharge efficiency measurement method was used.
These measurements were applied on each featured and with selected current rate, as further described in Table 1. - Parameter extraction. The parameters of each component in the EEMC were extracted and analyzed based on the experiments. The values of those parameters were then summarized into equations, and those equations can represent each of the electrical element in the electrical circuit.
- EECM construction and model verification. All parts of the electrical elements were put together to construct the full EECM. Finally, the constructed EECM of each battery was simulated and compared with the experimental results.
3.3. Equipment and Materials
3.4. Storage Circuit
3.5. Voltage Response Circuit
3.6. Parasitic Branch
- Fully charge and discharge the battery to measure the initial battery capacity and the overall Coulombic efficiency.
- Discharge the battery to a specific value (x%) and note down the discharged capacity .
- Fully recharge the battery and record the recharged capacity .
- Calculate the recharge efficiency / assuming it is the averaged integral recharge efficiency at the mean between x% and 100%.
- Go back to Step 2 and repeat the steps until enough data points are obtained.
- Test the whole procedure under different C-rates.
3.7. EECM Construction
4. Results and Discussion
4.1. Experimental Results
4.1.1. Cte-OCV as a Function of
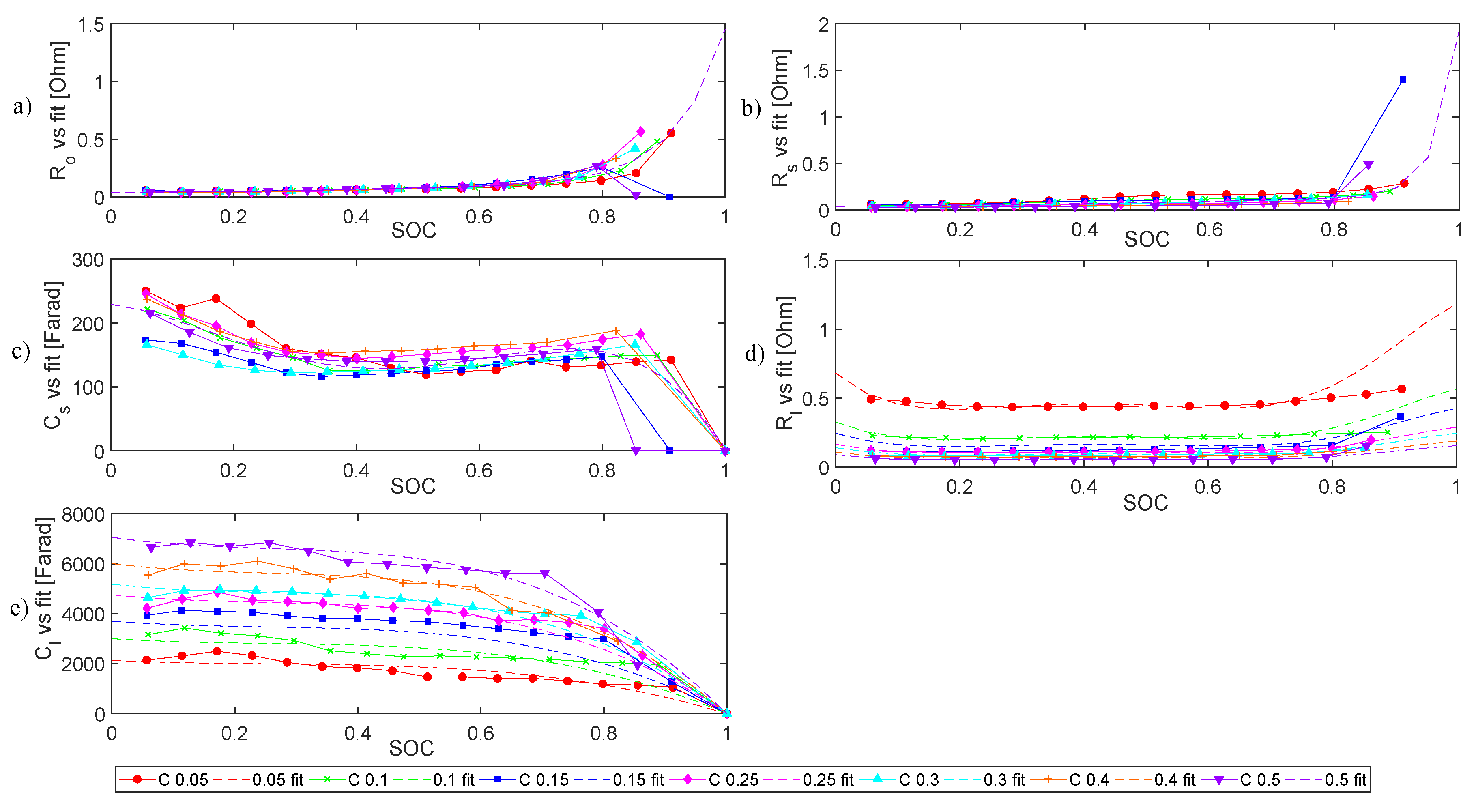
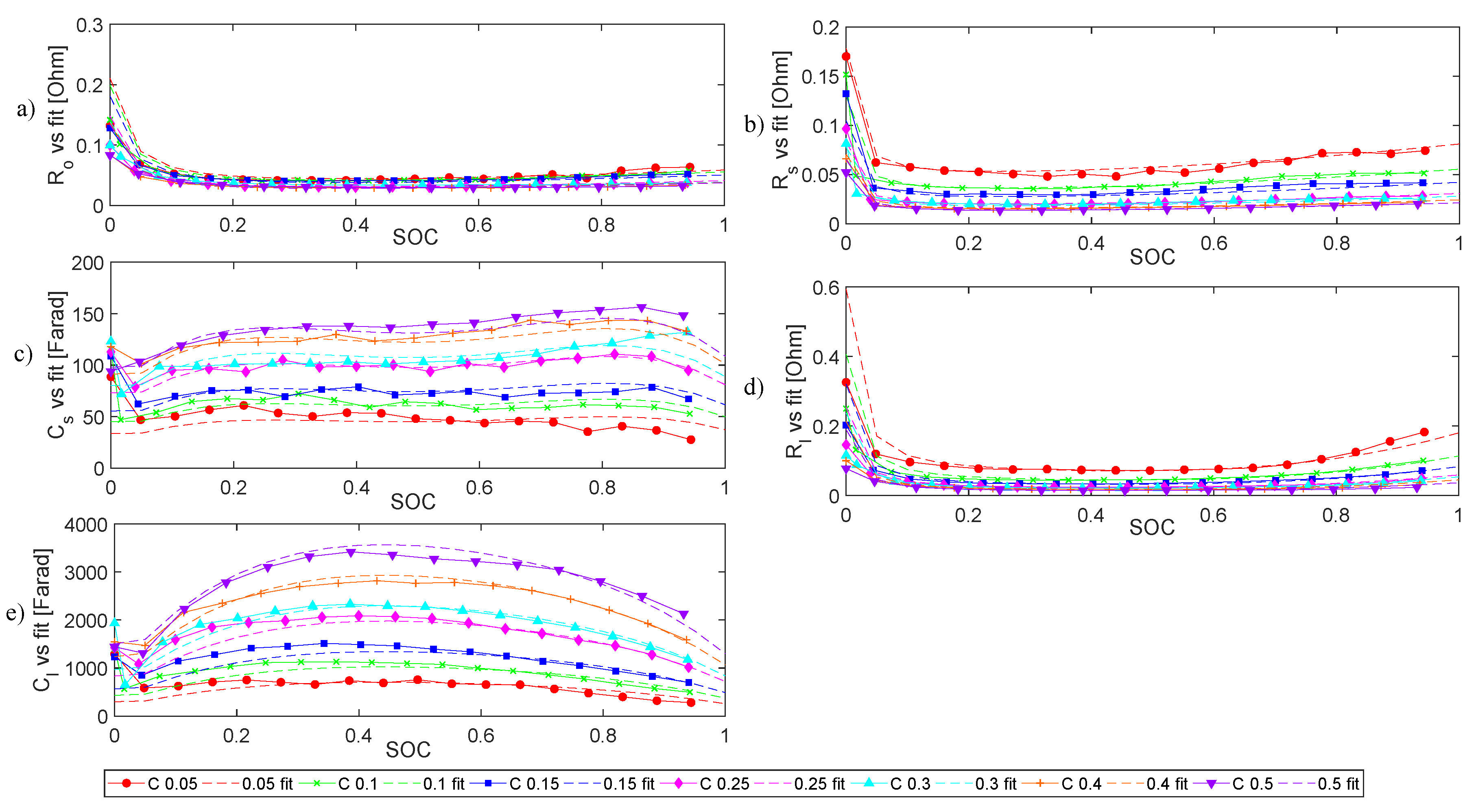
4.1.2. Internal Impedance
- All the electrical elements show a strong dependency on .
- All the elements also show an inverse trend versus in charge stage and discharge stage.
- In terms of current dependency, elements during charge and discharge stages have diverse behaviours. While charging, only and values show a clear operational current dependence. While discharging, all the elements except are evidently influenced by the operational current.
Charge
Discharge
- Except for , all other electrical elements show a strong dependency on SOC.
- All electrical elements exhibit an inverse behaviour versus in charge and discharge.
- The operational current influences , and in both charge and discharge processes.
Charge
Discharge
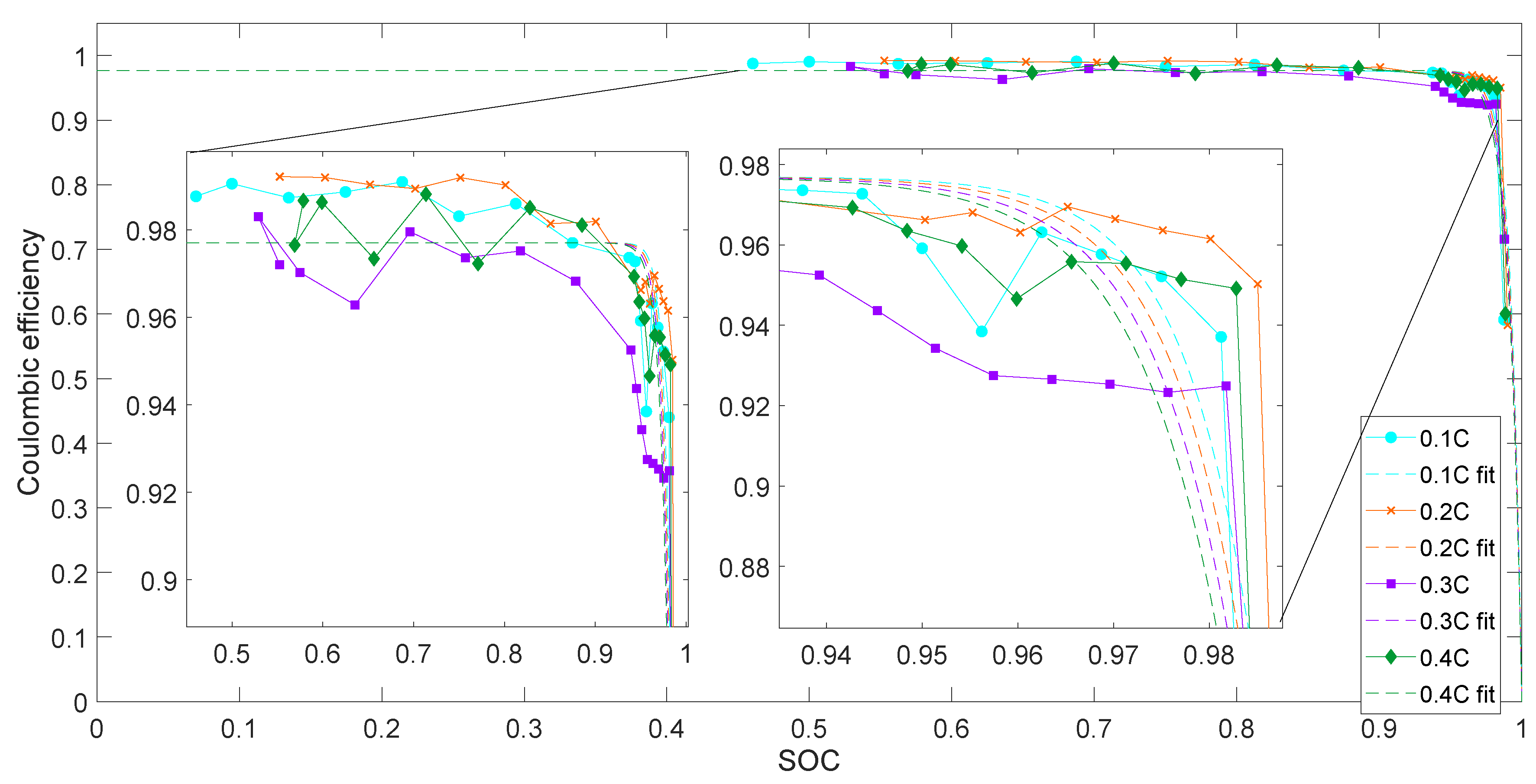
4.1.3. Coulombic Efficiency
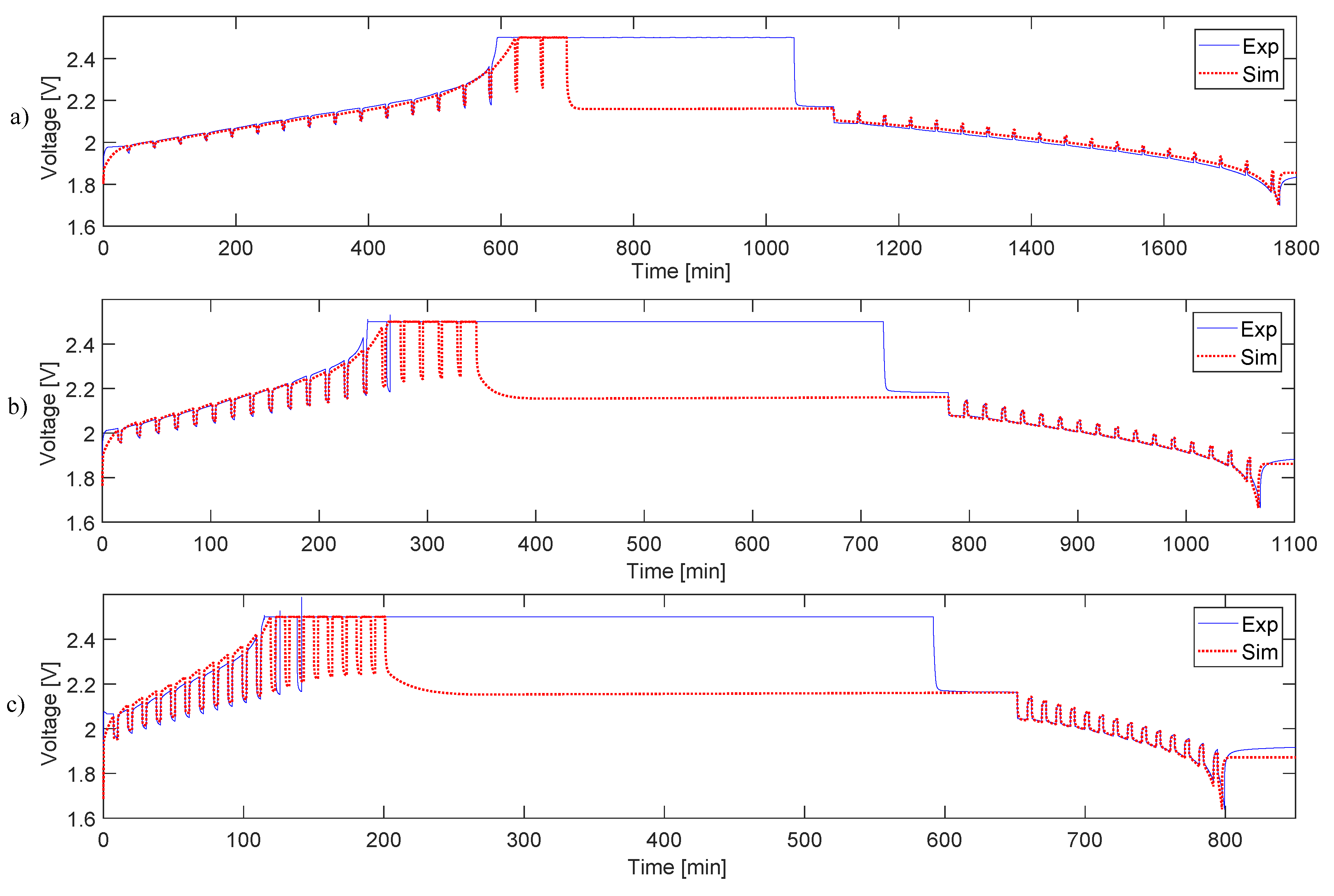
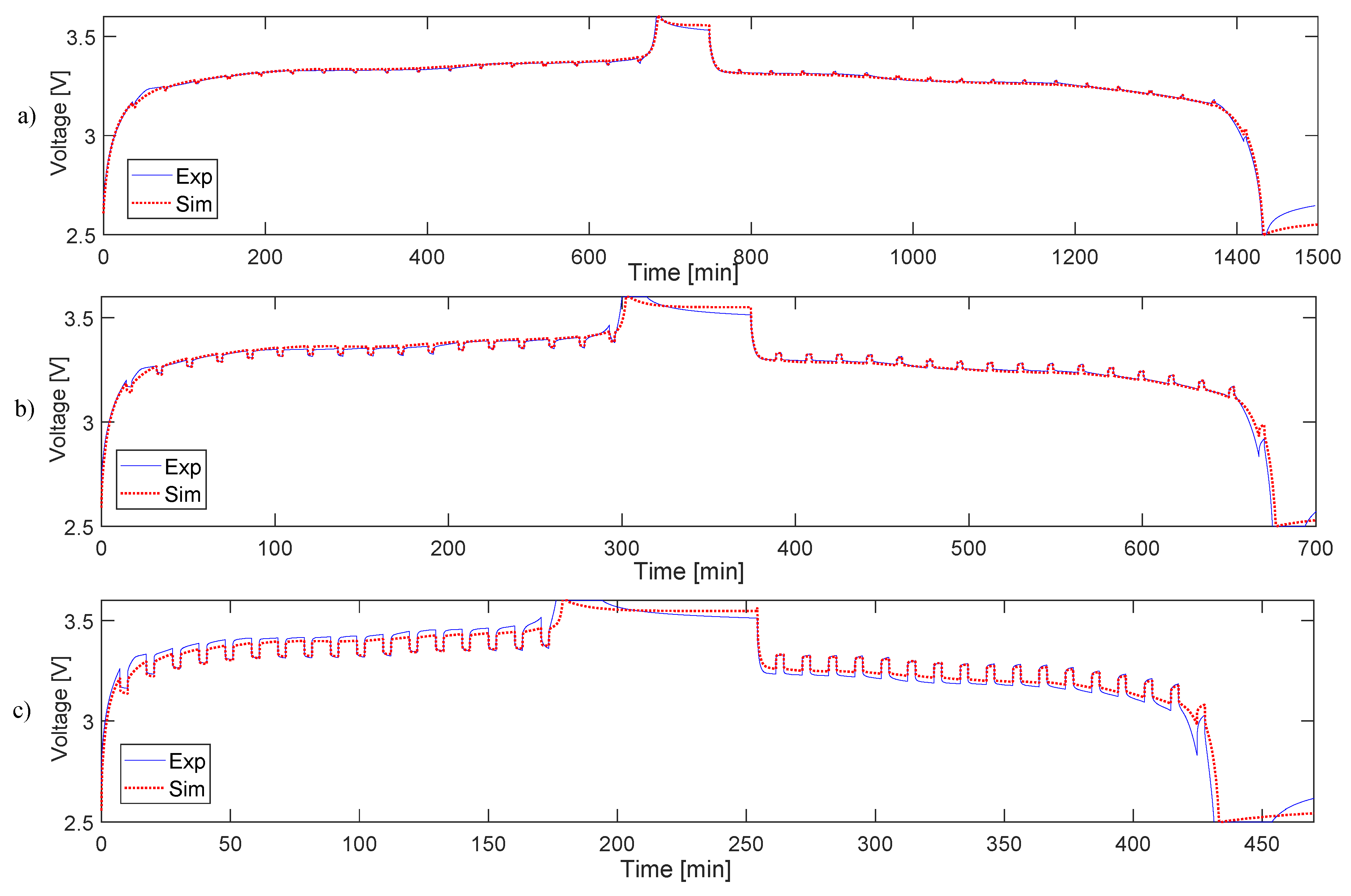
4.2. Simulation and Validation
5. Conclusions
Recommendations and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Open Circuit Voltage | |
| SHS | Solar home systems |
| Battery actual capacity | |
| VRLA | Valve Regulated Lead–Acid Battery |
| Capacity that be extracted since the full battery stage | |
| LFP | LiFePO Battery |
| Main branch current | |
| Battery current | |
| SOC | State of charge |
| EECM | Equivalent Electrical Circuit Model |
| Battery voltage | |
| emf | Electromotive Force |
| Side reaction Current | |
| OCV | Open Circuit Voltage |
| C-rate | |
| cte-OCV | close-to-equilibrium Open Circuit Voltage |
| Nominal battery capacity | |
| CC-CV | Constant current-constant voltage charge/discharge scheme |
| Side reaction resistance | |
| EODV | End of discharge voltage |
| Ohmic resistance | |
| Voltage response from Ohmic resistance | |
| ref | Reference |
| sim | Simulation |
| Voltage response during the relaxation time | |
| exp | Experiment |
| Capacity that remaining in the battery | |
| Time interval in kth order circuit model | |
| Resistance in kth order circuit model | |
| Capacitance in kth order circuit model | |
| Initial voltage before relaxation | |
| Voltage response in kth order circuit model | |
| Coulombic efficiency | |
| Test current | |
| Reference current | |
| / | Charged/discharged battery capacity |
| / | Short/Long term resistance |
| / | Short/Long term capacitance |
References
- Organization for Economic Cooperation and Development, International Energy Agency. From Poverty to Prosperity, 1st ed.; IEA: Paris, France, 2017; p. 144. [Google Scholar]
- Narayan, N.; Papakosta, T.; Vega-Garita, V.; Qin, Z.; Popovic-Gerber, J.; Bauer, P.; Zeman, M. Estimating battery lifetimes in Solar Home System design using a practical modelling methodology. Appl. Energy 2018, 228, 1629–1639. [Google Scholar] [CrossRef]
- Leadbetter, J.; Swan, L.G. Selection of battery technology to support grid-integrated renewable electricity. J. Power Sources 2012, 216, 376–386. [Google Scholar] [CrossRef]
- Moseley, P.; Rand, D. The Valve-regulated Battery—A Paradigm Shift in Lead-Acid Technology. In Valve-Regulated Lead-Acid Batteries; Rand, D., Garche, J., Moseley, P., Parker, C., Eds.; Elsevier: Amsterdam, The Nederlands, 2004; Chapter 1; pp. 1–14. [Google Scholar]
- Hudak, N.S. 4-Nanostructured Electrode Materials for Lithium-Ion Batteries. In Lithium-Ion Batteries; Pistoia, G., Ed.; Elsevier: Amsterdam, The Nederlands, 2014; pp. 57–82. [Google Scholar]
- Nykvist, B.; Nilsson, M. Rapidly falling costs of battery packs for electric vehicles. Nat. Clim. Chang. 2015, 5, 329–332. [Google Scholar] [CrossRef]
- Zhang, C.; Li, K.; McLoone, S.; Yang, Z. Battery modelling methods for electric vehicles—A review. In Proceedings of the 2014 European Control Conference, Strasbourg, France, 24–27 June 2014; pp. 2673–2678. [Google Scholar]
- Mohanty, P.; Gujar, M. PV Component Selection for Off-Grid Applications. In Solar Photovoltaic System Applications: A Guidebook for Off-Grid Electrification; Mohanty, P., Muneer, T., Kolhe, M., Eds.; Springer International Publishing: Berlin, Germany, 2016; pp. 85–106. [Google Scholar]
- Deutsche Gesellshaft fur Sonnenenergie. Planning and Installing Photovoltaic Systems; Earthscan: London, UK, 2005; p. 304. [Google Scholar]
- Stroe, A.; Stroe, D.; Swierczynski, M.; Teodorescu, R.; Kær, S.K. Lithium-ion battery dynamic model for wide range of operating conditions. In Proceedings of the 2017 International Conference on Optimization of Electrical and Electronic Equipment (OPTIM) & 2017 International Aegean Conference on Electrical Machines and Power Electronics (ACEMP), Fundata, Romania, 25–27 May 2017; pp. 660–666. [Google Scholar]
- Liaw, B.Y.; Nagasubramanian, G.; Jungst, R.G.; Doughty, D.H. Modeling of lithium ion cells—A simple equivalent-circuit model approach. Solid State Ionics 2004, 175, 835–839. [Google Scholar]
- Tian, S.; Hong, M.; Ouyang, M. An experimental study and nonlinear modeling of discharge I-V behavior of valve-regulated lead-acid batteries. IEEE Trans. Energy Convers. 2009, 24, 452–458. [Google Scholar] [CrossRef]
- Pop, V.; Bergveld, H.J.; Danilov, D.; Regtien, P.P.; Notten, P.H. Introduction. In Battery Management Systems: Accurate State-of-Charge Indication for Battery-Powered Applications; Springer: Dordrecht, The Netherlands, 2008; pp. 1–9. [Google Scholar]
- Crompton, T. 1—Introduction to battery technology. In Battery Reference Book, 3rd ed.; Crompton, T., Ed.; Newnes: Oxford, UK, 2000; pp. 1–64. [Google Scholar]
- Vincent, C.A. 2–Theoretical background. In Modern Batteries, 2nd ed.; Vincent, C., Scrosati, B., Eds.; Butterworth-Heinemann: Oxford, UK, 1997; pp. 18–64. [Google Scholar]
- Dubarry, M.; Svoboda, V.; Hwu, R.; Yann Liaw, B. Incremental Capacity Analysis and Close-to-Equilibrium OCV Measurements to Quantify Capacity Fade in Commercial Rechargeable Lithium Batteries. Electrochem. Solid-State Lett. 2006, 9, A454–A457. [Google Scholar] [CrossRef]
- Root, M. The TAB Battery Book An In-Depth Guide to Construction, Design and Use; McGraw-Hill Companies: New York, NY, USA, 2010; p. 85. [Google Scholar]
- Doerffel, D. Testing and Characterisation of Large High-Energy Lithium-Ion Batteries for Electric and Hybrid Electric Vehicles. Ph.D. Thesis, University of Southampton, Southampton, UK, 2007. [Google Scholar]
- Waag, W.; Käbitz, S.; Sauer, D.U. Experimental investigation of the lithium-ion battery impedance characteristic at various conditions and aging states and its influence on the application. Appl. Energy 2013, 102, 885–897. [Google Scholar] [CrossRef]
- Lam, L.; Bauer, P.; Kelder, E. A practical circuit-based model for Li-ion battery cells in electric vehicle applications. In Proceedings of the 2011 IEEE 33rd International Telecommunications Energy Conference (INTELEC), Amsterdam, The Netherlands, 9–13 October 2011; pp. 1–9. [Google Scholar]
- Zhan, C.J.; Wu, X.; Kromlidis, S.; Ramachandaramurthy, V.; Barnes, M.; Jenkins, N.; Ruddell, A. Two electrical models of the lead–acid battery used in a dynamic voltage restorer. IEEE Proc. Gener. Trans. Distrib. 2003, 150, 175. [Google Scholar] [CrossRef]
- Ceraolo, M. New Dynamical Models of Lead—Acid Batteries. IEEE Trans. Power Syst. 2000, 15, 1184–1190. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Peng, H. A comparative study of equivalent circuit models for Li-ion batteries. J. Power Sources 2012, 198, 359–367. [Google Scholar] [CrossRef]
- Panchal, S.; Mcgrory, J.; Kong, J.; Fraser, R.; Fowler, M.; Dincer, I.; Agelin-Chaab, M. Cycling degradation testing and analysis of a LiFePO4 battery at actual conditions. Int. J. Energy Res. 2017, 41, 2565–2575. [Google Scholar] [CrossRef]
- Jones, W.E.M.; Feder, D.O. Behavior of VRLA cells on long term float. II. The effects of temperature, voltage and catalysis on gas evolution and consequent water loss. In Proceedings of the Intelec’96—International Telecommunications Energy Conference, Boston, MA, USA, 6–10 October 1996; pp. 358–366. [Google Scholar]
- Buller, S. Impedance-based simulation models for energy storage devices in advanced automotive power systems. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2003. [Google Scholar]
- Kaushik, R.; Mawston, I.G. Coulombic efficiency of lead/acid batteries, particularly in remote-area power-supply (RAPS) systems. J. Power Sources 1991, 35, 377–383. [Google Scholar] [CrossRef]
- Andre, D.; Meiler, M.; Steiner, K.; Wimmer, C.; Soczka-Guth, T.; Sauer, D.U. Characterization of high-power lithium-ion batteries by electrochemical impedance spectroscopy. I. Experimental investigation. J. Power Sources 2011, 196, 5334–5341. [Google Scholar] [CrossRef]
- MACCOR. Series 4000 Automated Test System. Available online: http://www.maccor.com/ProductDocs/Data%20Sheet%20for%20Series%204000%20Test%20System.pdf (accessed on 10 August 2018).
- Enersys. Application Manual of CYCLONE AGM Single Cell. Available online: http://www.enersys-emea.com/reserve/pdf/EN-CYC-AM-007_1208.pdf (accessed on 10 August 2018).
- A123 Systems. Nanophosphate High Power Lithium Ion Cell ANR26650M1-B. Available online: https://www.batteryspace.com/prod-specs/6610.pdf (accessed on 10 August 2018).
- Yan, J.H.; Chen, H.Y.; Li, W.S.; Wang, C.I.; Zhan, Q.Y. A study on quick charging method for small VRLA batteries. J. Power Sources 2006, 158, 1047–1053. [Google Scholar] [CrossRef]
- Aylor, J.H.; Thieme, A.; Johnson, B.W. A Battery State-of-Charge Indicator for Electric Wheelchairs. IEEE Trans. Ind. Electron. 1992, 39, 398–409. [Google Scholar] [CrossRef]
- Pang, S.P.S.; Farrell, J.; Du, J.D.J.; Barth, M. Battery state-of-charge estimation. In Proceedings of the 2001 American Control Conference, Arlington, VA, USA, 25–27 June 2001; Volume 2, pp. 1644–1649. [Google Scholar]
- Hu, Y.; Yurkovich, S.; Guezennec, Y.; Yurkovich, B.J. Electro-thermal battery model identification for automotive applications. J. Power Sources 2011, 196, 449–457. [Google Scholar] [CrossRef]





| Battery Brand | A123systems® APR26650M1B | Cyclon® AGM D Single Cell | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Battery capacity | 2.5 Ah | 2.5 Ah | |||||||
| Nominal voltage | 3.3 V | 2 V | |||||||
| End of charge | CC-CV: 3.6V until I ≤ 0.01C | CC-CV: 2.5V until I ≤ 0.002 C | |||||||
| End of discharge | CC-CV: 2.5V until I ≤ 0.01 C | Depending on C-rate and EODV: | |||||||
| C-rate | 0.05 | 0.1 | 0.2 | 0.4 | 1 | 2 | >5 | ||
| EODV | 1.75 | 1.7 | 1.67 | 1.65 | 1.6 | 1.55 | 1.5 | ||
| High | Low | |
|---|---|---|
| SOC value | 90% to 99%; 1% steps | 10% to 90%; 10% steps |
| C-rate | 0.1 C, 0.2 C, 0.3 C, 0.4 C | 0.1 C, 0.2 C, 0.3 C, 0.4 C |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Narayan, N.; Vega-Garita, V.; Popovic-Gerber, J.; Qin, Z.; Wagemaker, M.; Bauer, P.; Zeman, M. Constructing Accurate Equivalent Electrical Circuit Models of Lithium Iron Phosphate and Lead–Acid Battery Cells for Solar Home System Applications. Energies 2018, 11, 2305. https://doi.org/10.3390/en11092305
Yu Y, Narayan N, Vega-Garita V, Popovic-Gerber J, Qin Z, Wagemaker M, Bauer P, Zeman M. Constructing Accurate Equivalent Electrical Circuit Models of Lithium Iron Phosphate and Lead–Acid Battery Cells for Solar Home System Applications. Energies. 2018; 11(9):2305. https://doi.org/10.3390/en11092305
Chicago/Turabian StyleYu, Yunhe, Nishant Narayan, Victor Vega-Garita, Jelena Popovic-Gerber, Zian Qin, Marnix Wagemaker, Pavol Bauer, and Miro Zeman. 2018. "Constructing Accurate Equivalent Electrical Circuit Models of Lithium Iron Phosphate and Lead–Acid Battery Cells for Solar Home System Applications" Energies 11, no. 9: 2305. https://doi.org/10.3390/en11092305
APA StyleYu, Y., Narayan, N., Vega-Garita, V., Popovic-Gerber, J., Qin, Z., Wagemaker, M., Bauer, P., & Zeman, M. (2018). Constructing Accurate Equivalent Electrical Circuit Models of Lithium Iron Phosphate and Lead–Acid Battery Cells for Solar Home System Applications. Energies, 11(9), 2305. https://doi.org/10.3390/en11092305







